Abstract
Endothelial cells (ECs) are present throughout blood vessels and have variable roles in both physiological and pathological settings. EC fate is altered and regulated by several key factors in physiological or pathological conditions. Reactive nitrogen species and reactive oxygen species derived from NAD(P)H oxidases, mitochondria, or nitric oxide-producing enzymes are not only cytotoxic but also compose a signaling network in the redox system. The formation, actions, key molecular interactions, and physiological and pathological relevance of redox signals in ECs remain unclear. We review the identities, sources, and biological actions of oxidants and reductants produced during EC function or dysfunction. Further, we discuss how ECs shape key redox sensors and examine the biological functions, transcriptional responses, and post-translational modifications evoked by the redox system in ECs. We summarize recent findings regarding the mechanisms by which redox signals regulate the fate of ECs and address the outcome of altered EC fate in health and disease. Future studies will examine if the redox biology of ECs can be targeted in pathophysiological conditions.
Keywords: Redox homeostasis, Endothelial cell fate, Atherosclerosis, Hypertension, Cancer, Obesity, Diabetes
Introduction
Endothelial cells (ECs) are continuously exposed to circulating blood, which maintains the structure and function of blood vessels [1]. EC fate significantly affects the initiation and progression of diseases, including atherosclerosis [2], hypertension [3], aging [4], inflammation [5], tumor angiogenesis [6], and obesity [7]. The redox system is responsible for EC function and dysfunction [8, 9]. Indeed, modest and controlled generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in ECs is required for numerous vital signaling pathways involved in cell survival, proliferation, activation, stress response, cell motility, vasodilation, and angiogenesis. High levels of oxidants or reductants in ECs have detrimental effects, such as promoting oxygen toxicity. Thus, it is critical to address the important roles of redox homeostasis in the regulation of EC fate, including EC survival, apoptosis, proliferation, migration, senescence, and transdifferentiation. We will also review several pivotal ROS/RNS-sensitive molecules, such as adenosine monophosphate-activated protein kinase (AMPK), phosphatase and tensin homolog deleted from chromosome 10 (PTEN), and hypoxia-inducible factors (HIF), which are involved in redox-regulated EC fate. Specifically, all of the aforementioned molecules can act as ‘redox sensors and modulators’ due to redox modifications of their cysteine residues, which are critically important for the regulation of protein function.
Redox biology of blood endothelial cells
Executors in the redox system
Key oxidants in ECs: O·−2, H2O2, ·OH, NO, and ONOO−
The redox state is characterized by the reduction–oxidation reaction. The redox system includes oxidants and reductants. Reactive oxidants include ROS and RNS. ROS are key components of EC oxidants and are implicated in stress-related conditions and diseases. ROS have recently been shown to be influential players in the biology of ECs [10]. The major ROS in ECs are superoxide anions (O·−2), hydrogen peroxide (H2O2) [11], and hydroxyl radicals (·OH) (Fig. 1). Among these ROS, H2O2 is a freely diffusible and stable ROS, whereas O·−2 is a short-lived and highly reactive oxidant. The half-life of the O·−2 ranges from a few nanoseconds to milliseconds.
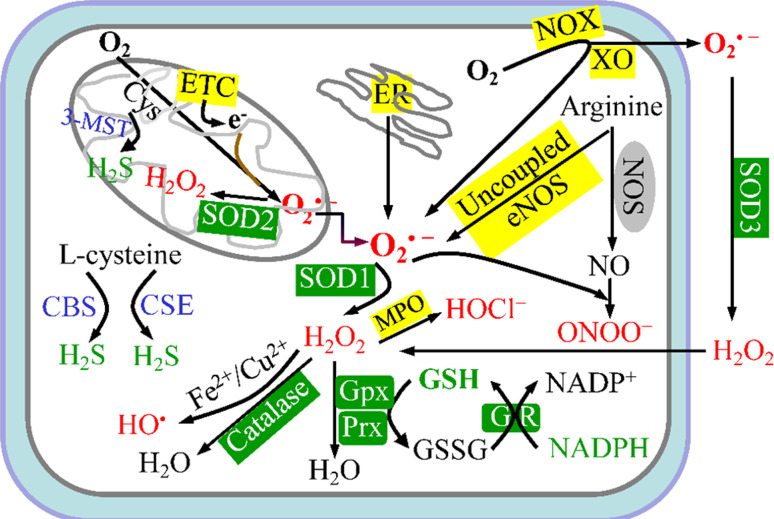
Fig. 1.
Redox homeostasis in the endothelial cell. Mitochondrial electron-transport chain (ETC), membrane-bound NADPH-oxidase (NOX) complex, uncoupled endothelial nitric-oxide synthase (eNOS), endoplasmic-reticulum (ER) stress, and xanthine oxidase (XO) are the five major intracellular sources (shown in yellow background) of reactive oxygen species (ROS). Superoxide anion (O·−2) is the predominant ROS and is rapidly converted into hydrogen peroxide (H 2 O 2) by superoxide dismutases (SODs). Alternatively, O·−2 can generate peroxynitrite (ONOO −) by reacting with nitric oxide (NO). H2O2 can be catalyzed to ·OH in the presence of Fe2+ or Cu2+ ions or may be converted to H2O and O2 through a reaction catalyzed by catalase, glutathione peroxidase (Gpx), or peroxiredoxins (Prx). To maintain redox homeostasis, living cells engage powerful scavenger antioxidant enzyme systems (shown in green background) to eliminate intracellular oxidants (shown in red ). Major reductants are shown in green. Endogenous H2S is produced from l-cysteine (Cys) by three enzymes, which are cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST). GR glutathione reductase, GSH reduced glutathione, GSSG oxidized glutathione
Reactive nitrogen species in ECs include nitric oxide (NO), nitric dioxide (NO2), and the much more powerful oxidant, peroxynitrite anion (ONOO−). ONOO− is generated from a rapid reaction between NO, which is highly diffusible and has a short half-life of 3–4 s, and O·−2 [12, 13] (Fig. 1). ONOO− has a half-life of approximately 1 s at physiological conditions and reacts with other molecules to form additional RNS, such as NO2 and N2O3. RNS in ECs can react with functional proteins, including protacyclin synthase (PGIS) [14, 15]. The effects of RNS are diverse and may be similar to or opposite from those of ROS.
Key reductants in ECs: H2S, GSH
Hydrogen sulfide (H2S) is the third most recognized endogenous gaseous signaling molecule, after NO and carbon monoxide (CO) [16] [17]. H2S gas is primarily generated from l-cysteine in mammals by the three enzymes, cystathionine γ-lyase (CSE, EC 4.4.1.1) [18, 19], cystathionine β-synthase (CBS, EC 4.2.1.22) [20, 21], and 3-mercaptopyruvate sulfurtransferase (3-MST, EC 2.8.1.2) [22–24] (Fig. 1). Both CSE and CBS predominantly exist in the cytosol, whereas 3-MST is mainly localized in mitochondria and the cytoplasm [25]. CBS was also recently shown to localize in the mitochondrial outer membrane of the colon cancer-derived epithelial cell line HCT116 [20]. CSE is predominantly expressed in the endothelial layer of blood vessels [26] and in the heart [27], whereas CBS is highly concentrated in the brain [28]. CSE can be physiologically activated by calcium-calmodulin [26]. In addition, lipophilic atorvastatin, but not hydrophilic pravastatin, enhances net production of H2S in perivascular adipose tissue by inhibiting H2S mitochondrial oxidation [29].
H2S exists in solution as both H2S (about 20 %) and hydrosulfide anion (HS−) (about 80 %) at pH 7.4. In contrast, the completely deprotonated sulfide (S2−) is found at exceptionally lower levels at physiological pH [30]. Thus, the term H2S will be employed to refer to the total sulfide pool (H2S + HS− + S2−) [31, 32]. H2S serves as an electron donor [22] and functions as a reductant [33], altering the cellular redox status. Emerging data suggest that H2S is an antioxidant signaling molecule at lower physiological concentrations [34, 35]. However, H2S is a lethal toxin at higher concentrations due to the inhibition of cytochrome-c oxidase in mitochondrial complex IV and prevention of cellular respiration [36]. Similar to NO, high concentrations (100–250 μM) of H2S promote oxidative stress and reduced survival of ECs and vascular smooth muscle cells (VSMCs) [39]. In contrast, low concentrations (about 30 μM) of H2S protect ECs against various stressors, such as H2O2 [38], high glucose [46], and hyper-homocysteinemia [34]. Low concentrations of H2S exert distinct physiological functions [35, 47], including vasodilation [26, 41], EC migration and proliferation [20, 41], inhibition of inflammation [48], and stimulation of cellular bioenergetics [20, 22] (Table 1). There are several mechanisms involved in H2S function [49]. For example, H2S that is released from ECs can parallel and complement NO [50]. Recently, it was reported that cytoprotective function of H2S is eNOS-NO dependent [51]. Moreover, H2S is an endothelium-derived hyperpolarizing factor that mediates endothelium-dependent vasorelaxation [45]. H2S promotes Nrf2 localization to the nucleus, which induces expression of multiple cellular antioxidants. The predominant function of H2S in ECs appears to be sulfhydration of target proteins. Sulfhydration is the conversion of cysteinyl thiolates (Cys-SH) to cysteinyl persulfide (Cys-S-SH) by the addition of H2S-derived sulfur [52, 53] (Fig. 2). H2S acts as a prominent physiological endothelium-derived hyperpolarizing factor by covalently sulfhydrating the ATP-sensitive potassium channel to induce vessel relaxation [44]. H2S regulates the activity of vascular endothelial growth factor receptor 2 (VEGFR2) and several other molecules by breaking intrinsic inhibitory disulfide bonds, such as that between Cys1045 and Cys1024 of VEGFR2 [40]. H2S also S-sulfhydrates the C226 and C613 residues in Kelch-like ECH-associated protein-1 (Keap1), which is a redox-sensitive ubiquitin ligase substrate adaptor that represses Nrf2. This activity may reduce the C226-C613 disulfide bridge formed by H2O2 [54]. H2S was recently demonstrated to reversibly oxidize free cysteine thiols, but not disulfide bonds, in PTEN. In addition, H2S inactivates PTEN via polysulfide formation [31], although it is not clear if this modification occurs in ECs. Therefore, H2S may oxidize free cysteine thiols by sulfhydration at high concentration, while reducing disulfide bonds at low doses (Fig. 2).
Table 1.
Hydrogen sulfide functions in endothelial cells
| Donor or knockout model | H2S function | Mechanisms | References |
|---|---|---|---|
| Na2S (IK-1001) | Vasorelaxation | Improves EC function | [37] |
| NaHS | Anti-oxidant | Increases expression of SOD, CAT, GPx, and GST | [38] |
| Increases Trx-1; decreases NOX4 | [34] | ||
| Pro-angiogenic effect | Activates Akt; breaks the Cys1045-Cys1024 disulfide bond in VEGFR2 | [39, 40] | |
| Angiogenesis and vasorelaxation | Increases pAkt-Ser473, peNOS-Ser1177, pVEGFR2, pVASP-Ser239 |
[41] [42] |
|
| GYY-4137 | Vasodilator, anti-hypertension | Opens ATP-sensitive K+ channels in VSMC | [32] |
| Pro-angiogenesis | Inhibits soluble fms-like tyrosine kinase-1 and endoglin | [18] | |
| DATS | Attenuates LV dys-function following HF | Decreases angiostatin, increases VEGF, peNOS, and consequent angiogenesis | [43] |
| CSE−/− | Anti-hypertension | Endothelium-dependent vasorelaxation; hyperpolarization by sulfhydrating ATP-sensitive K+ channels; | |
| Anti-atherosclerosis | Decreases levels of total cholesterol, LDL/HDL-cholesterol; increases GSH level, SOD activity, inhibits H2O2 production, NF-κB activation, and ICAM-1 expression in aorta or VSMC in response to atherogenic diet; suppresses proliferation of intimal cells in aortic root | [19] | |
| CBS knock down/inhibition | Angiogenesis | Increases cell proliferation and migration of tumor cells and ECs | [20] |
CBS cystathionine-β-synthase, CSE cystathionine γ-lyase, DATS diallyl trisulfide, GYY4137 morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate, Trx-1 thioredoxin-1, LV left ventricular, HF heart failure
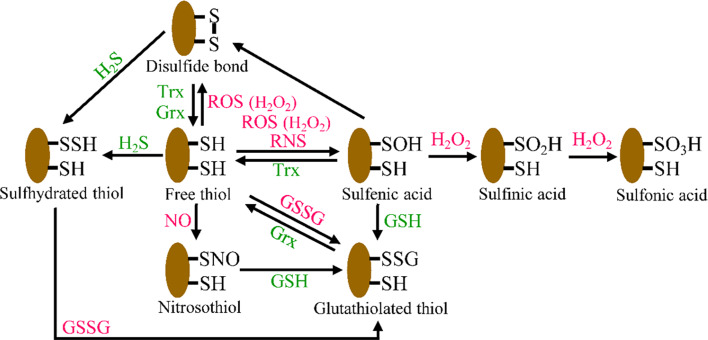
Fig. 2.
Reversible and irreversible redox modifications of protein cysteines in ECs. Oxidation of cysteine thiol (RSH) by ROS or RNS leads to the generation of highly reactive sulfenic acid (RSOH), which can react with another thiol to produce a disulfide bond (RSSR) or with GSH to become S-glutathionylated (RSSG). This oxidative modification is reversible, and reduction is catalyzed by the Trx or Grx system. Further oxidation of RSOH to sulfinic acid (RSO 2 H) and sulfonic acid (RSO 3 H) is thought to be generally irreversible in vivo. Free thiols can also be modified by sulfhydration or S-nitrosylation
Another critical low-molecular-weight reductant in ECs is reduced glutathione (γ-glutamyl-cysteinyl-glycine, GSH). The glutathione/glutathione disulfide (GSH/GSSG) molecules represent the most abundant thiol-redox system in ECs [55] (Fig. 1). Intracellular GSH is differentially distributed in various subcellular compartments of the cytosol, mitochondria, ER, and nucleus. The cytosol contains more than 70 % of total cellular GSH. The redox state of a cell is generally indicated by the ratio of GSH to GSSG. One versatile property of GSH is its antioxidant function, which maintains redox balance. Interestingly, GSH regulates EC fate and functions, including EC apoptosis [56], angiogenesis [57], and EC-dependent vasodilation [58]. The major molecular mechanisms by which GSH regulates redox modification of redox-sensitive cysteines are thiol-disulfide exchange and protein S-glutathiolation [59]. These modifications control a variety of activities, including EC differentiation, proliferation, and apoptosis. For example, S-glutathiolation of Cys118 in p21Ras causes activation of p21Ras and downstream phosphorylation of Erk and Akt in ECs [60].
Redox homeostasis in ECs
Endothelial cells possess modest levels of intracellular oxidants and reductants. The EC redox status is balanced by oxidant-generating systems and antioxidant systems. Five major systems are responsible for the generation of vascular endothelial ROS, the mitochondrial electron-transport chain (ETC) [10, 61–63], the membrane-bound NAD(P)H-oxidase (NOX) complex [64, 65], uncoupled endothelial nitric-oxide synthase (eNOS) [66–68], endoplasmic-reticulum (ER) stress [69, 70], and xanthine oxidase (XO) [71, 72] (Fig. 1). The electrons from the mitochondrial ETC can be captured by O2 to form the superoxide anion (O·−2) [73]. Moreover, the mitochondrial ETC produces O·−2 primarily at complexes I and III [74]. Then, O·−2 can be rapidly converted into H2O2 by superoxide dismutase 2 (SOD2, Mn-SOD) in mitochondria. H2O2 diffuses out of the mitochondrion in human coronary arteriolar endothelial cells and elicits vasodilation in response to shear stress [75]. Complex III is the main site of ROS production in human umbilical vein endothelial cells (HUVECs) during hypoxia-reoxygenation [76].
Nitric oxide in ECs is mainly produced by eNOS and inducible nitric oxide synthase (iNOS). eNOS function is altered by several oxidants, including ONOO− [66] and hypochlorous acid (HOCl), which is the major oxidant of myeloperoxidase (MPO) [77]. Normal eNOS function requires the essential co-factor, tetrahydrobiopterin (BH4), which is synthesized by GTP-cyclohydrolase I (GTPCH I) in a rate-limiting step. ONOO− elevated by high levels of glucose activates the 26S proteasome [78–80] or directly oxidizes and releases zinc ions from GTPCH I. In addition, ONOO− enhances the ubiquitination and degradation of GTPCH I, which leads to BH4 deficiency [81] and subsequent eNOS uncoupling and dysfunction.
During steady-state cellular conditions, intracellular oxidants levels are tightly regulated by intricate antioxidative systems to maintain redox homeostasis. Major antioxidants in ECs include low-molecular-antioxidants, such as H2S, reduced GSH, vitamin C, and vitamin E, noncatalytic antioxidant proteins, such as thioredoxin (Trx) [82], glutaredoxin (Grx), peroxiredoxins (Prx) [83], and metallothioneins (MTs) [84], and antioxidant enzymatic defense systems, including SODs, catalase (CAT), glutathione peroxidase (Gpx), and glutathione reductase (GR) (Fig. 1). For example, aged GPx-1 −/− mice demonstrate elevated oxidant formation as compared with their wild-type littermates, leading to decreased NO bioavailability due to eNOS uncoupling and consequent EC dysfunction [85]. Recent data also indicate that H2S is a powerful reducing agent [86] that plays a pivotal role in maintaining redox homeostasis through the following distinct mechanisms: (1) H2S directly scavenges endogenous oxidant species, including ONOO− [87], O·−2, and H2O2 [38]; and (2) H2S increases the expression of antioxidative enzymes, including SOD, CAT, and Gpx [38]. In addition, GSH maintains the intracellular redox balance by scavenging ROS that are involved in Gpx-mediated hydroperoxide metabolism and GR-catalyzed and NADPH-dependent GSH regeneration (Fig. 1). ROS production within mitochondria can be prevented by uncoupling protein (UCP) 2. For example, the AMPK activator, 5-amino-4-imidazole carboxamide riboside (AICAR), upregulates AMPK-mediated mitochondrial UCP2 via p38 signaling, which attenuates O·−2 and prostacyclin synthase nitration in aortic ECs of diabetic mice [88]. In general, oxidized/reduced GSH, oxidized/reduced Trx, oxidized/reduced Grx, and oxidized/reduced Prx are important redox-buffering systems [83].
General mechanisms of redox-mediated regulation of ECs
Key transcription factors involved in the redox regulation of ECs
Several key transcription factors in ECs, including forkhead box O (FoxO), nuclear factor erythroid-2-related factor 2 (Nrf2), and hypoxia-inducible factor 1 (HIF-1), are redox-sensitive. They act as distinct redox sensors and regulators. The redox state regulates the levels and activities of these transcription factors, which control EC fate and may inversely modulate redox homeostasis.
FoxO-mediated redox regulation in ECs
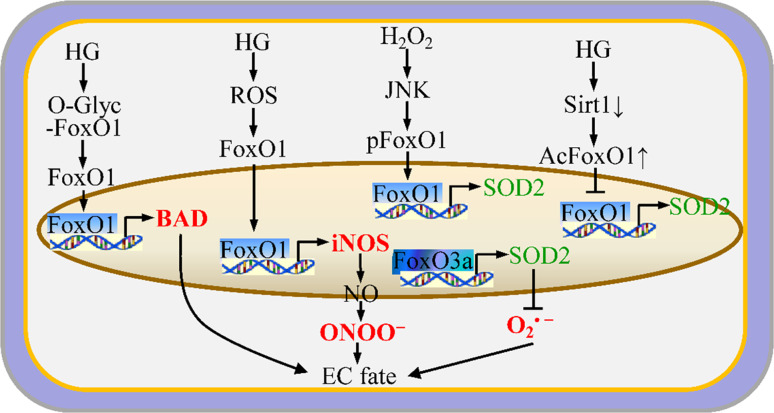
The FoxO family consists of many transcription factors, including FoxO1, FoxO3, FoxO4, and atypical FoxO6. The FoxO proteins have diverse physiological functions, including the regulation of EC fate. Among the FoxO family members, FoxO1 [89, 90], FoxO3a [91], and FoxO4 are implicated in EC biology. FoxO1 and FoxO3a are the most abundant FoxO isoforms in mature ECs [89]. EC redox regulates the activities of FoxOs through multiple posttranslational modifications, including phosphorylation, acetylation, and ubiquitination [92] (Fig. 3). For example, FoxO3a can be phosphorylated by the phosphoinositide 3-kinase (PI3K)/Akt kinase, leading to cytoplasmic retention and defective FoxO3a nuclear transcriptional activity [91]. FoxO1 has a similar profile in response to Akt activation [93]. FoxO1 is also a physiological substrate of protein kinase A-α (PKA-α) [94]. In contrast, c-Jun NH2-terminal kinase (JNK) phosphorylates FoxO1 at a distinct residue to promote nuclear import and transcriptional activation [90]. In addition, deacetylation of FoxO1 by Sirtuin 1 (SIRT1) promotes FoxO1-mediated transcription of target genes, such as SOD2 [95]. The redox state mutually regulates the biological functions of FoxOs. For example, H2O2 induces JNK-mediated nuclear translocation of FoxO1 in cultured ECs [90]. Deletion of FoxO1/FoxO3a/FoxO4 in ECs leads to the reduction of the NOX subunits, p22phox, p67phox, and NOX2, as well as decreased production of ROS, including O·−2 and H2O2 [25]. Moreover, FoxO3a functions as a direct transcriptional regulator of a group of antioxidant genes, including SOD2 [96]. However, activation of endothelial FoxO1 by hyperglycemia-mediated ROS promotes the expression of inducible NOS (iNOS), which mediates NO–ONOO− production [97]. FoxOs might regulate EC fate by maintaining redox homeostasis. Potente et al. [89] demonstrated that overexpression of constitutively active FoxO1 or FoxO3a significantly blocks EC migration and consequent tube formation in vitro. In addition, Tanaka et al. [97] found that activation of FoxO1 in response to high glucose results in LDL oxidation and eNOS dysfunction in ECs. Activation of FoxO1 [98] and FoxO3 [99] induces apoptosis in HUVECs. Thus, FoxO proteins play essential roles in the response to physiologic oxidative stress and thereby regulate EC survival. Loss of FoxO3 decreases the expression of SOD2 and CAT, leading to excessive amounts of ROS and EC dysfunction. These experiments suggest that FoxO-mediated repression of ROS determines EC fate.
Fig. 3.
FoxO-mediated redox system and EC fate. FoxOs regulate EC fate by affecting apoptosis, survival, proliferation, and migration due to the expression of FoxO target genes that are associated with redox balance
Nrf2-mediated redox regulation in the maintenance of EC fate
Nrf2 is a ubiquitously expressed transcription factor that can be induced by endogenous or exogenous stimulators in the cardiovascular system [100, 101]. Nrf2 activates antioxidant responsive element (ARE)-dependent gene expression to maintain cellular redox homeostasis [84]. Nrf2 is usually sequestered in the cytoplasm by binding with Kelch-like ECH-associated protein 1 (Keap1). Keap1 is a redox-sensitive substrate adaptor for the Cul3-dependent E3 ligase and a mediator of Nrf2 proteasomal degradation [102, 103]. H2O2 can enhance the formation of an intramolecular disulfide bridge between C226 and C613, which is associated with Keap1 inhibition [54]. H2S may decrease the C226-C613 disulfide in Keap1 to generate a free thiol and an S-sulfhydrated moiety [54]. Nrf2 plays important roles as an oxidant sensor and a regulator of redox homeostasis in ECs. Nrf2 combats detrimental levels of intracellular ROS by transcribing genes that encode several key antioxidant molecules, including Gpx, heme oxygenase-1 (HO-1) [104], and GSH. Expression of Nrf2 protects human aortic endothelial cells (HAECs) from H2O2-mediated cell death [104]. This finding suggests that Nrf2 might be a regulatory factor that controls EC fate by modulating redox homeostasis. Chen et al. further showed that overexpression of Nrf2 in HAECs suppresses TNF-α-induced expression of monocyte chemoattractant protein (MCP)-1 and vascular cell adhesion molecule-1 (VCAM-1), which are redox-sensitive inflammatory molecules. Moreover, expression of an upstream kinase for p38 MAP kinase, a constitutively active form of MKK6, partially reversed Nrf2-mediated inhibition of VCAM-1 expression. These results suggest that p38 MAP kinase mediates in part the anti-inflammatory actions of Nrf2 in ECs [104]. In addition, the shear stress in ECs that is generated by circulating blood increases intracellular ROS levels [105], which further stimulates nuclear translocation of Nrf2 and subsequent induction of HO-1 [106]. Nrf2 deletion inhibits both basal and inducible expression of antioxidant genes, increasing oxidative stress. Global deletion of Nrf2 inhibits EC sprouting and vascular density. This also occurred with EC-specific deletion of Nrf2 without affecting the surrounding non-vascular tissue [107]. Collectively, these reports suggest that Nrf2 may be a potent regulator of EC fate due in part to antioxidant activities.
Role of HIF-1 in the redox regulation of EC fate
Hypoxia-inducible factor-1 (HIF-1) is a heterodimeric transcription factor composed of the HIF-1α and HIF-1β subunits. Both subunits contain the basic helix-loop-helix and PAS domains [108], which mediate heterodimerization and binding to DNA [109]. The redox system controls the expression, stability, and activity of HIF-1 in ECs, which ultimately affects EC fate. S-nitrosylation by NO stabilizes the expression and activity of HIF-1 [110]. Hypoxia induces HIF-1 expression in ECs, resulting in reduced levels of the angiogenic factor interleukin-8 (IL-8). The downregulation of IL-8 by HIF-1 appears to be due to reduced expression of Nrf2 [111].
Redox modifications of cysteine residues in proteins in ECs
Post-translational protein modifications are dynamic, fine-tuned regulatory mechanisms that impact a wide variety of cellular processes. Recently, oxidative modification of cysteine thiols has been shown to be a central mechanism for dynamic post-translational regulation of several key proteins [112], such as AMPK, PTEN, HIF-1, eNOS, and VEGFR2. These modifications usually function as redox sensors and switches in ECs. There are five major redox modifications of protein cysteine in ECs (Fig. 2). The first redox modification is the reversible disulfide bond (RS-SR’) [113]. For example, H2O2 enhances disulfide formation between Cys124 in the active site of PTEN and a vicinal Cys71, leading to oxidative inactivation of PTEN. This modification contributes to the angiogenesis mediated by H2O2 [114]. The second redox modification is S-nitrosylation (also called S-nitrosation, R-SNO), which is generally mediated by NO [115]. For example, S-nitrosylation of eNOS at Cys99 is associated with monomerization and reduced enzymatic activity [116]. The third redox modification is S-glutathiolation (also known as S-glutathionylation, RS-SG). S-glutathiolation occurs through thiol-disulphide exchange with GSSG or through a reversible reaction between an oxidant-mediated protein thiyl radical and GSH during redox stress [59, 117–119]. For example, H2O2 induces S-glutathiolation of AMPKα at Cys304, which stimulates AMPK activation [120]. In addition, either vascular endothelial growth factor (VEGF) or H2O2 induce S-glutathiolation of sarco/endoplasmic-reticulum-Ca2+ ATPase-2b at Cys674, which mediates EC migration [121, 122]. Interestingly, oxidized low-density lipoproteins and ONOO− stimulate S-glutathiolation of p21Ras at Cys118, causing insulin resistance in ECs [60, 123]. Recently, Chen et al. demonstrated that GSSG induces S-glutathiolation of human eNOS at Cys689 and Cys908. This causes eNOS uncoupling and inhibition in association with reduction of NO and induction of O·−2 [124]. The fourth redox modification is sulfhydration (R-SSH) by H2S. The disulfide bond between Cys1045 and Cys1024 in VEGFR2 is disrupted by sulfhydration with H2S [40]. The fifth redox modification involves sulfenylation (R-SOH), sulfinic acid (R-SO2H), and sulfonic acid (R-SO3H). The general mechanism is that ROS react with H2O2 target proteins that contain redox-sensitive cysteine residues (R-SH) to produce the reversible sulfenic-(R-SOH) and the irreversible sulfinic-(R-SO2H) and sulfonic-(R-SO3H) acid derivatives [125]. The protein sulfenic acid derivative can further react with NO to generate nitrosothiol (R-SNO) or with another R-SH to yield a disulfide bond (R-SS-R) [126] (Fig. 2).
In addition to the direct redox modification of protein cysteines, several central molecules in ECs are also regulated by redox homeostasis via signaling pathways. For example, NO activates AMPK through a process mediated by the Ca2+/calmodulin-dependent protein kinase kinase [127]. In addition, berberine-elevated ONOO− stimulates AMPK activation in bovine aortic endothelial cells via phosphorylation of liver kinase B1 (LKB1) at Ser307 [128].
Regulation of EC fate by redox homeostasis
Redox systems, including ROS/RNS, regulate oxygen sensing, vascular tone, apoptosis, cell growth, proliferation, migration, senescence, and inflammatory responses in vascular ECs. As reviewed above, ECs can cope with redox stress to maintain redox homeostasis. These coping strategies involve unique regulatory mechanisms through which endogenous antioxidant defense systems are activated. In addition, a growing body of literature supports the notion that redox homeostasis is an important regulator of EC fate. However, most studies do not clarify the underlying molecular mechanisms. Here, we introduce several potential or well-documented redox-sensitive molecules that participate in the regulation of EC fate. For example, AMPK that has pleiotropic roles in vascular biology [129, 130], including regulation of EC fate, is the major redox sensor in ECs [64, 131].
Redox regulation of EC survival and apoptosis
Redox balance is required for the survival of ECs. In contrast, redox imbalance promotes apoptosis of ECs. We previously showed that ONOO− dose-dependently (10 μM) inhibits the activity of Akt [132], which is a pro-growth and pro-survival signal in ECs. ONOO− stimulated by high glucose significantly increases phosphorylation of Ser428 in the tumor suppressor LKB1, which phosphorylates PTEN at Ser380/Thr382/383 in vitro. Increased phosphorylation of PTEN increases PTEN activity. The enhanced lipid phosphatase activity of PTEN inhibits basal and insulin-stimulated phosphorylation of protein kinase B/Akt at Ser473. Thus, Akt activity is reduced, promoting EC apoptosis in response to high glucose in vitro and hyperglycemia in vivo [133]. The exposure of cultured HUVECs to a low concentration of ONOO− (5 μM) significantly increases the phosphorylation of protein kinase C ζ (PKCζ) at Thr410, which promotes nuclear import of PKCζ. Phosphorylated PKCζ directly phosphorylates Ser248 of LKB1 in vitro and in intact cells, leading to LKB1 nuclear export, PTEN activation, and consequent EC apoptosis [134]. These results suggest that PKCζ mediates LKB1-dependent inhibition of Akt in response to ONOO−, which leads to endothelial apoptosis. In contrast, a high concentration of ONOO− (>50 μM) oxidizes and inactivates PTEN in ECs (Song et al., unpublished data) or in a cell-free system [31]. Very recently, Ang II was demonstrated to induce EC apoptosis and dysfunction via NOX2 activation by kynureinine pathway [135].
AMPK activation by oxidative stress inhibits apoptosis in multiple types of cells, including ECs [131, 136]. AMPK activation is regulated by redox, such as that produced by H2O2 [137], and helps to mediate redox balance [131]. We showed that a low concentration (<10 μM) of ONOO− enhances the activity of AMPK and its substrate enzymes, including eNOS and acetyl-CoA carboxylase, in ECs [132, 138]. In addition, we demonstrated that ONOO−-dependent AMPK activation occurs subsequently to hypoxia–reoxygenation [139], and in ECs treated with metformin [138], simvastatin [140], or berberine [128]. Moreover, AMPK activation by ONOO− occurs independently of changes in the AMP/ATP ratio [138, 139]. Ido et al. [136] were the first to report that AMPK activation protects ECs from apoptosis induced by hyperglycemia-derived oxidative stress. The combination of hypoxia and glucose deprivation (OGD) profoundly activates AMPK within 30 min. AMPK activity reaches a maximum within 2 h of OGD in cultured ECs. However, EC apoptosis is clearly elevated after 6 h of OGD treatment. Furthermore, AMPK inhibition by Compound C or due to overexpression of a dominant-negative AMPK (AMPK-DN) aggravates EC apoptosis in response to OGD. In contrast, pharmacological AMPK activation by AICAR or genetic activation by overexpression of constitutively active AMPK mitigates EC apoptosis in response to OGD. This appears to be due to increased expression of survival factors. AMPK activation activates IкKα and NF-кB nuclear translocation, which enhances the expression of anti-apoptotic Bcl-2 and survivin. In addition, genetic deletion of AMPKα1 but not AMPKα2 blunts OGD-augmented activation of NFкB, reducing expression of Bcl-2 and survivin and increasing EC apoptosis [141]. In addition, knockdown of AMPKα1 in HUVECs downregulates antioxidant molecules, such as SOD2 and CAT, and increases production of ROS in response to low oxygen [142]. AMPKα activation by C-peptide, AICAR, or metformin prevents high glucose-stimulated ROS production, mitochondrial fission, destruction of the mitochondrial membrane potential, and EC apoptosis. Moreover, C-peptide replacement therapy blocks hyperglycemia-induced AMPKα inhibition, ROS generation, and mitochondrial deformation in the aorta of diabetic mice [143].
The FoxO transcription factor family plays important roles in the regulation of EC survival by controlling the expression of the apoptotic factor BAD. Hyperglycemia enhances O-glycosylation of FoxO1 in human aorta ECs and promotes nuclear localization of FoxO1. Nuclear FoxO1 increases the expression of BAD, which leads to EC apoptosis during hyperglycemia [144]. Moreover, FoxO1 expression is deregulated in the aorta of streptozotocin-induced diabetic mice and diabetic db/db mice. FoxO1 deregulation during hyperglycemia and effects on EC survival are reversed by endothelin-1 [144]. Furthermore, deletion of FoxO1/FoxO3a/FoxO4 in ECs inhibits apoptosis induced by TNFα, FFA, H2O2, 7-keto-cholesterol, and free cholesterol [25].
Redox and the cell cycle in ECs
Cellular redox status oscillates during the cell cycle [145, 146]. For example, entry into early G1 is linked to GSH recruitment and sequestration in the nucleus [55]. Redox regulation of the cell cycle involves modification of cell cycle regulatory proteins, such as transcription factors, cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors [146]. The GSH levels in cultured ECs are elevated during the initial exponential growth phase and decline as cells become confluent. Thus, inhibition of GSH synthesis and GSH deprivation in human microvascular ECs delay S-to-G2 transition [55]. Importantly, growth factor-stimulated ROS generation and redox regulation of p16, p27, and cyclin D1 drive differentiated cells into the cell cycle [147, 148].
Redox regulation of EC migration and angiogenesis
Angiogenesis is the formation of new blood vessels and occurs by EC sprouting from existing vessels. Angiogenesis is a critical event during embryonic development and multiple disease processes. Cell migration and adhesion are essential for the physiological and pathological functions of ECs. Angiogenesis is initiated by EC sprouting, which is driven and guided by a VEGF gradient. Increasing evidence indicates that the redox system tightly regulates EC migration and angiogenesis [149]. VEGF is a well-known mediator of EC migration. Interestingly, VEGF augments mitochondria-derived ROS and Rac-1 activation in HUVECs, which promotes EC migration [150]. However, activation of the thromboxane A2 receptor (TP) blocks VEGF-induced EC migration and angiogenesis by inhibiting Akt and eNOS phosphorylation [151], possibly by the RNS-dependent Rho/Rho-associated kinase/LKB1/PTEN pathway [152]. FoxO1 over-expression impairs cell migration and tube formation in primary ECs, whereas knockdown of FoxO1 by siRNA augments angiogenesis [89]. We recently demonstrated that AMPKα2 deletion or inhibition of AMPKα2 impairs expression of UCP2. There are also concomitant increases in ROS and ONOO−, which ultimately inhibits endothelial tube formation. Furthermore, ischemia significantly enhances UCP2 expression, eNOS phosphorylation at Ser 1177, and angiogenesis in ischemic adductor muscles from the thighs of wild-type mice but not in those from either AMPKα1 −/− or AMPKα2 −/− mice [153]. These results suggest that AMPK-dependent UCP2 expression in ECs enhances angiogenesis in vivo through redox balance. In contrast, AMPKα1 activation by transforming growth factor (TGF)-β-activated kinase 1 stimulates EC proliferation, migration, and subsequent VEGF-induced angiogenesis via SOD2 upregulation and O·−2:H2O2 balance [154].
Ischemic retinopathy increases EC levels of ROS (H2O2 and O·−2), oxidative stress-mediated lipid oxidation, and subsequent activation of the ataxia telangiectasia mutated (ATM) protein due to phosphorylation of ATM at Ser1987 [155]. ATM activation induces pathological neoangiogenesis associated with EC proliferation and decreases apoptosis independent of VEGF signaling or the response to DNA damage [156]. In contrast, ATM deletion downregulates the activity of SOD2, CAT, and Gpx1, ultimately upregulating the amounts of cytosolic and mitochondrial ROS in ECs. In addition, ATM deletion inhibits neovascular tuft formation in response to ischemic retinopathy [31]. These results indicate that pathological neoangiogenesis, but not normal physiological neoangiogenesis, requires the ATM-mediated anti-oxidative response. Jansen et al. [157] demonstrated that the transfer of microRNA-126 (miR-126) via endothelial microparticles (MPs) released from apoptotic ECs stimulated EC migration and proliferation, which are both important steps for re-endothelialization. However, the miR-126 level in endothelial microparticles was significantly downregulated by high glucose, possibly due to the presence of ROS.
Thus, ROS act as “double-edged swords” in angiogenesis. Pathological angiogenesis appears to be stimulated by a certain amount of ROS. However, excessive amounts of ROS appear to suppress physiological or regenerative angiogenesis.
H2S is required for angiogenesis. For example, CSE-deleted mice show impaired wound healing probably due to disrupted angiogenesis [158].
Redox regulation of EC inflammation
Endothelial cell inflammatory activation is an early event that is tightly associated with insulin resistance and atherosclerosis. EC inflammation is regulated by redox signaling. AMPK activation in ECs is linked with anti-inflammatory actions. For example, AMPK activation inhibits fatty acid, palmitate, or cytokine TNF-α-induced NF-кB transactivation and VCAM-1 mRNA expression in cultured HUVECs [159]. Furthermore, AMPK activation by high-molecular-weight adiponectin enhances NO production and represses TNF-α-stimulated NF-кB activation [160]. Recently, Bess et al. [161] elucidated the molecular mechanisms by which AMPK mediates the anti-inflammatory actions of NO in EC. AMPKα2 directly phosphorylates IкB kinase at Ser177 and Ser181, leading to the inhibition of IкB kinase [162]. Inhibition of IкB kinase suppresses phosphorylation of IкB and p65 and inactivates NF-кB. Therefore, NO-mediated AMPK activation suppresses IL-1β and TNF-α-induced activation of NF-кB, E-selectin expression, and monocyte adhesion in human endothelial cells [161]. Deletion of FoxO1/FoxO3a/FoxO4 in ECs suppresses the expression of inflammatory cytokines, such as MCP-1, IL-1β, and IL-6, in response to lipopolysaccharide due to the inhibition of NF-κB transcriptional activity [25].
In addition, deletion of Hmox1 (encoding HO-1) in ECs increases ROS levels and expression of proinflammatory molecules, including VCAM-1, intercellular adhesion molecule-1 (ICAM-1), and E-selectin, as compared with that of wild-type ECs. In contrast, HO-1 downregulates RelA phosphorylation at Ser276 and subsequently eliminates the proinflammatory phenotype of activated ECs [163]. Very recently, Xiao et al. demonstrated for the first time that disrupted blood flow promotes the assembly and activation of the NLRP3 inflammasome, which is mediated by sterol regulatory element-binding protein 2 (SREBP2). Cleavage of pro-caspase-1 and pro-IL-1β is also increased in ECs via upregulation of NOX2. The activated NLRP3 inflammasome perturbs EC function and increases inflammation [164]. These results suggest that NOX-mediated redox signaling activates the NLRP3 inflammasome in ECs as in monocytes [165] and lung cells [166].
NaHS, an H2S donor, upregulates HO-1 and eliminates TNF-α-induced ROS production and NF-κB activation. Furthermore, NaHS inhibits TNF-α-induced expression of ICAM-1 and VCAM-1 mRNA and protein in a dose-dependent manner. NaHS also inhibits mRNA expression of P-selectin and E-selectin and blocks the adhesion of U937 monocytes to HUVECs. Overall, H2S attenuates TNF-α-induced inflammatory signaling in vascular ECs [167].
Redox and EC senescence
Chronic diseases, such as diabetes, accelerate endothelial dysfunction during aging [168], a process that is associated with cellular senescence. However, the mechanisms underlying this process are unclear. High glucose downregulates Sirt1 via ROS with consequent induction of acetylated FoxO1, which reduces SOD2 in association with EC senescence [95]. Deletion of FoxO1/FoxO3a/FoxO4 prevents EC senescence via eNOS-derived induction of NO [25]. Burger et al. sequentially passaged primary mouse ECs to induce senescence and discovered that cells maintain the phenotypic characteristics of ECs from passage 4 through passage 21. However, ECs from passage 21 have features of senescence, including increased staining for senescence-associated β-galactosidase, a greater percentage of cells in G1/G0 phase, and increased phosphorylation of p66Shc [169]. Furthermore, the number of MPs from passage 21 ECs is higher than that of passage 4 ECs (approximately 2.2-fold increase versus passage 4). The increased number of MPs is blocked by the Rho kinase inhibitor Fasudil. Exposure of passage 4 ECs to MPs shifts cells from a proliferating to a non-proliferating phenotype and increased staining for senescence-associated β-galactosidase. MPs increase the production of O·−2 and H2O2, which is blocked by apocynin, a NOX inhibitor, and rotenone, a mitochondrial oxidase inhibitor, but not by allopurinol, an XO inhibitor. In addition, MPs increase expression of p21cip1 and p16ink4a and stimulate phosphorylation of p66Shc in ECs. Pretreatment with the ROS scavenger sodium 4,5-dihydroxybenzene-1,3-disulfonate (tiron) abrogates the pro-senescent effects of MPs [169]. These results suggest that MPs promote EC senescence through NOX- and mitochondrial-derived ROS. In addition, ONOO− in ECs enhances EC senescence via post-translational oxidative modifications of intracellular regulatory proteins [43]. These redox-sensitive processes may be important in vascular aging.
Redox signaling and EC transdifferentiation
Endothelial cells are derived from several cell types, including embryonic stem cells [170, 171], induced pluripotent stem cells [172], fibroblasts [173, 174], mesenchymal stem cells [175], adipose-derived stem cells [176–178], and glioblastoma cells [179]. Furthermore, the local environment, such as fluid shear stress [180–182], affects EC differentiation of stem cells. In addition, ECs and blood cells are closely intermixed in the extra-embryonic blood islands of mammals and express the same molecular markers [183]. Thus, they may be derived from a common precursor called the hemangioblast. Myers et al. [184] found that bone morphogenetic protein (BMP) signaling mediates erythroid differentiation. Erythroid lineage and BMP signaling constrain EC fate in Xenopus blood islands. Our laboratory showed that AMPKα1 deletion increases the number of reticulocytes in blood [185] and impairs angiogenesis due to elevated oxidative stress [153]. However, the mechanisms by which AMPKα1 may control EC and blood-cell lineage through redox balance remain elusive.
ECs can also transdifferentiate into other cell types, including smooth muscle (SM)-like cells [186], mesenchymal cells [187], and blood cells. Arciniegas et al. [186] reported that adult bovine ECs can transdifferentiate into SM-like cells in vitro. They showed that TGF-β1 induced α-SM-actin expression in aortic ECs. Cooley et al. [188–190] demonstrated that, during the process of tubulogenesis in three-dimensional matrices of fibrin or type I collagen, HUVECs show increased expression of lymphatic vascular endothelial hyaluronan receptor, PROX1, and vascular endothelial growth factor-3, which are markers of lymphatic endothelial cells. Expression of blood vascular markers, such as VEGFC and CD44, are decreased in ECs during tubulogenesis. These results indicate that vascular ECs can switch to a lymphatic-like phenotype. The induction of this phenotype is reversible and is partially inhibited by co-culture of HUVECs with perivascular cells [191]. Medici et al. [192] reported that HUVECs that express constitutively active activin-like kinase-2 (mutant R206H) show an endothelial-to-mesenchymal transition (EndMT) and acquisition of a stem cell-like phenotype. Treatment with TGF-β2 or BMP4 promotes transdifferentiation of ECs into stem-like cells. These stem-like cells can be further differentiated into osteoblasts, chondrocytes, or adipocytes. More importantly, EndMT may contribute to cardiac fibrosis, leading to a progressive stiffening of the ventricular walls [193]. Later, Gupta et al. [194] demonstrated that capillary ECs within white and brown fat may transdifferentiate into preadipocytes. Thus, vascular ECs possess significant plasticity, which may be highly relevant to the function of ECs. However, the mechanisms by which redox regulates the EC-lineage switch remain largely unknown.
Redox regulation of EC fate in the pathogenesis of disease
In addition to the regulatory functions of the redox system during physiological conditions, excessive or sustained redox imbalance is implicated in the pathogenesis of various diseases, such as cardiovascular disease, cancer, obesity, and diabetes.
EC redox imbalance in cardiovascular diseases
An unbalanced redox status in ECs leads to endothelium dysfunction, which is strongly associated with cardiovascular diseases, such as atherosclerosis, hypertension, diabetic cardiovascular complications [195], and ischemia–reperfusion injury.
EC redox imbalance and atherogenesis
Atherosclerosis is a chronic inflammatory disease and is strongly associated with EC dysfunction [196], particularly during the early stages of atherosclerotic disease. Redox imbalance in ECs plays a pivotal role in the initiation, promotion, and progression of atherosclerosis [197, 198]. A number of key signaling pathways in the EC redox system are highly relevant to atherogenesis (Fig. 4).
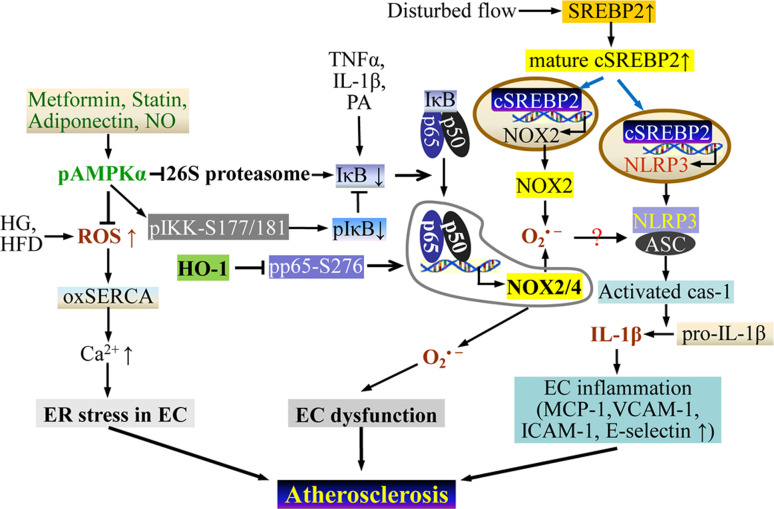
Fig. 4.
Redox-regulated EC fate in atherogenesis. AMPK activation in ECs inhibits NF-κB activation by suppressing 26S-proteasome activity or by directly phosphorylating IκK. This ameliorates the ER stress that is induced by ROS due to high glucose or a high-fat diet. Disturbed blood flow activates the NLRP3 inflammasome in ECs through SREBP2-mediated upregulation of NOX2. The activated NLRP3 inflammasome instigates EC inflammation, which accelerates atherogenesis. Cas-1 caspase-1; other abbreviations as indicated in the text
Atherogenesis preferentially affects branches and curvatures in the arterial tree. Notably, disturbances in blood flow at vascular branches and curvatures promote inflammation of ECs due to SREBP2-mediated NLRP3 inflammasome activation. This process accelerates atherosclerosis [164]. Furthermore, the SREBP2-NOX2-NLRP3 inflammasome was shown to be elevated and activated in the atherosclerosis-prone intima of the mouse aortic arch but not in the thoracic aorta. EC-SREBP2(N) overexpression in ApoE −/− mice augments atherosclerosis in the aortic arch and in thoracic aorta in response to a high-fat diet [164].
High glucose is the major source of ROS/RNS in the ECs of diabetic models [14]. For example, 3-nitrotyrosine (a biomarker of ONOO−) is significantly increased in the aortas of streptozotocin-induced diabetic mice [133]. AMPK activation protects ECs against redox imbalance, such as that which occurs during diabetes. Recently, we demonstrated that deletion of AMPKα2 upregulates NOX2/4 and O·−2 in ECs via NF-κB activation, leading to EC dysfunction and increased atherosclerosis in low-density-lipoprotein-receptor-knockout (LDLR −/−) mice [199]. Interestingly, reduction of AMPKα2 enhances the oxidation and inactivation of SERCA and increases ER stress in ECs, which further accelerates atherosclerosis in LDLR−/− mice [2]. Activation of AMPK mitigates ER stress due to oxidized LDL/ROS in ECs and attenuates atherosclerosis in ApoE −/− mice fed a high-fat diet [200]. Activation of the thromboxane receptor induces phosphorylation of PKCζ at Thr410 and promotes membrane translocation, activating NOX2 in response to elevated O·−2 and ONOO−. These activities cause eNOS to become uncoupled in ECs [201]. Thus, TP activation may be associated with atherothrombotic diseases. In addition, the development of atherosclerosis in the aorta, aortic arch, and descending aorta in response to a western-type diet is mitigated in LDLR −/− mice that have EC-specific deletions in FoxO1/FoxO3a/FoxO4 compared to that of LDLR −/− controls. Attenuated atherosclerosis may be attributed to reduced levels of ROS and increased levels of eNOS-derived NO, both of which inhibit inflammation [25]. A western-type diet can promote insulin resistance, which activates FoxO1/FoxO3 early during the process of atherogenesis. Activation of FoxO1/FoxO3 is believed to contribute to atherosclerosis [25]. These results strongly support therapeutic inhibition of FoxOs in ECs as a strategy for treating and/or preventing diabetes-associated cardiovascular diseases.
H2S is anti-atherosclerotic [202]. For example, in one study, treatment of ApoE −/− mice with exogenous H2S decreased the size of atherosclerotic lesions, whereas CSE inhibition with DL-propargylglycine (PAG) increased the size of these lesions [203]. In addition, although a high level of early mortality was observed in CBS −/− mice, a zinc-inducible human CBS transgene blocked this effect in CBS −/−/ApoE −/− mice. During pregnancy and weaning, zinc was provided in the drinking water, activating the transgene. Homocysteine levels were only slightly elevated, preventing early mortality. Zinc was removed from the drinking water after the mice were weaned, resulting in severe hyper-homocysteinemia and accelerated atherosclerosis. This study was consistent with prior observations in conventional CBS −/−/ApoE −/− mice. The size of the lesions and the increased levels of tumor necrosis factor, MCP-1, and Ly-6-Chi monocytes/macrophages were proportional to the homocysteine levels. However, insights from other studies suggested that the major factor was the decrease in H2S. In vitro studies showed that elevated plasma homocysteine inhibited CBS and impaired H2S production. However, H2S supplementation completely reversed the inflammation caused by hyper-homocysteinemia. Chemical inhibition of cortical thickness in ApoE −/− mice caused a marked increase in atherosclerosis. This excess lesion formation was decreased (by 38 %) with supplementation of sodium hydrosulfide (NaHS), an H2S donor. CSE −/− mice fed an atherogenic diet displayed increased lesional oxidative stress and elevated expression of ICAM-1 in aortae, resulting in the development of early fatty streak lesions in the aortic root [42]. CSE −/−/ApoE −/− mice developed more severe atherosclerosis. Treatment with NaHS, but not N-acetylcysteine, retarded the development of atherosclerosis in CSE −/− mice, suggesting that H2S employs an anti-atherosclerotic mechanism distinct from that which is activated by antioxidants.
EC redox imbalance and hypertension
Redox imbalance leads to EC dysfunction [8], which may contribute to impaired EC-dependent relaxation. eNOS uncoupling and dysfunction are implicated in hypertension, in part due to decreased production of NO and increased levels of O·−2. GTPCH1 is the rate-limiting enzyme for de novo synthesis of BH4, the essential cofactor for eNOS. We demonstrated that inhibition or knock down of endothelial GTPCH1 causes an imbalance of NO and O·−2, which increases systolic, diastolic, and mean blood pressures in C57BL6 mice [204]. In addition, ONOO−-mediated tyrosine nitration of PA700, a 26S proteasome regulatory subunit, activates the 26S proteasome and accelerates GTPCH1 degradation, BH4 deficiency, and endothelial dysfunction, which is highly associated with angiotensin II-induced hypertension [205]. Furthermore, NOX-derived O·−2 impairs vasodilation by inactivating NO [206].
Some drugs or agents that alter redox signaling also regulate EC-dependent relaxation. For example, eicosapentaenoic acid (EPA, 20:5 n-3), an ω-3 polyunsaturated fatty acid that is abundant in fish oils, has cardiovascular-protective effects [207]. We demonstrated that EPA activates AMPK and its substrate eNOS via UCP2 upregulation. Further, EPA improves high-fat/high-cholesterol-induced endothelium-dependent relaxation of the aortic rings. These effects of EPA appear to be dependent on AMPKα1 or eNOS [208]. Thus, fish oils may maintain normal blood pressure via improved redox balance due to NO release.
Moderate levels of the reductant H2S are required to maintain normal blood pressure. Two of the major enzymes in the trans-sulfuration pathway, CSE and CBS, metabolize homocysteine and serve as major sources of H2S. H2S is a physiologic vasorelaxant that opens ATP-sensitive K+ channels in VSMCs [209]. CSE and H2S levels are repressed in pulmonary-hypertensive rats [210]. Administration of the CSE inhibitor PAG leads to hypertension in rats [211] and pregnant mice [18]. Deficiency of either CSE or CBS markedly impairs cellular production of H2S. CSE-deleted mice have dramatically reduced levels of H2S in the serum, heart, and aorta. In addition, they show diminished endothelium-dependent vasorelaxation, which results in pronounced hypertension [19, 26]. However, H2S levels are higher in patients with coronary artery disease, peripheral arterial disease, or vascular diseases compared to patients who do not have vascular diseases [212].
Redox regulation of EC fate and cancer development
Tumor angiogenesis is essential for tumor development [213]. EC fate, including migration, proliferation, apoptosis, and the formation of tight junctions, is important for tumor angiogenesis and metastasis [214]. Redox systems, including ROS/RNS, regulate angiogenesis during cancer development [215, 216]. Elevated production of ROS mediated by NOX4 and the mitochondrial electron chain increases the expression of HIF-1 and VEGF in ovarian and prostate cancer cells. Increased levels of endogenous ROS are required for angiogenesis and tumor growth [215]. Increased production of ROS enhances tumor angiogenesis and tumor growth of ovarian cancer cells via the recruitment and proliferation of ECs. B16 melanoma cell subcutaneous implants display increased amounts of intracellular H2O2 and activated ATM kinase in ECs. In addition, tumor angiogenesis is enhanced in this in vivo model compared with that of normal subcutaneous tissue. However, endothelial-specific deletion of ATM significantly blocks pathological neoangiogenesis in association with increased apoptosis and reduced proliferation due to elevated production of ROS [156].
Interestingly, several oxidized products are increased in cancer tissue. For example, the end products of lipid oxidation by oxidative stress, ω-(2-carboxyethyl)pyrrole (CEP) and other related pyrroles, accumulate at high levels in highly vascularized tumors, such as murine and human melanomas [217]. Elevated CEP is recognized by Toll-like receptor 2, but not Toll-like receptor 4, and scavenger receptors on ECs, leading to Rac1 activation, EC migration, and tumor angiogenesis independent of VEGF. Moreover, neutralization of endogenous CEP diminishes the vascularization of melanomas [217].
In addition, H2S enhances angiogenesis. The H2S-generating enzyme CBS is selectively upregulated in human colon cancers, resulting in a high rate of H2S production. CBS knockdown by shRNA or pharmacological inhibition with aminooxyacetic acid inhibits EC migration in colon cancers or co-cultures. These results indicate that endogenous H2S promotes tumor angiogenesis in human colon cancers [20].
Redox regulation of EC fate in obesity and diabetes
Obesity is characterized by the hypertrophy and hyperplasia of adipocytes in white adipose tissue (WAT). Angiogenesis plays a critical role in the modulation of adipogenesis and the development of obesity [7, 218, 219]. Angiogenic vessels provide nutrients and oxygen to fat tissue, enrich the concentrations of growth factors and cytokines in plasma [220], and increase the numbers of circulating stem cells, including bone marrow-derived stem cells [221, 222]. Angiogenesis also increases the delivery of EC-derived growth factors and cytokines to adipocytes, increasing the growth and expansion of fat tissue in a paracrine manner [223]. ECs from different fat depots have distinct characteristics. Visceral adipose tissue (VAT) has a higher vascular density and number of ECs than that of the corresponding subcutaneous adipose tissue (SAT) from obese human subjects. ECs from VAT display remarkable levels of angiogenesis and inflammation with reduced expression of metabolism-associated genes, including endothelial lipase and PPARγ. VAT-derived ECs express increased levels of cellular senescence markers, such as IGFBP3 and γH2AX, and express lower levels of SIRT1 [224]. In contrast, human SAT embedded in Matrigel has a stronger angiogenic capacity than VAT. Moreover, the angiogenic capacity of SAT, but not VAT, is inversely associated with insulin sensitivity [225]. Modulation of EC fate is highly associated with angiogenesis. However, because EC fate is tightly regulated by redox, the development of adipose tissue may be affected by the redox state. The growth of adipose tissue in mice was inhibited by angiogenesis inhibitors, such as TNP-470 [226], angiostatin (a plasminogen fragment containing kringle domains 1–4), and endostatin (a C-terminal fragment of collagen XVIII). The mechanisms implicate decreased proliferation and increased apoptosis of ECs [227]. Tam et al. [228] further demonstrated that formation of new vessels in fat tissues during diet-induced obesity largely depends on angiogenesis rather than de novo vasculogenesis. Anti-angiogenic treatment with monoclonal antibodies that block VEGFR2, but not VEGFR1, may limit the expansion of adipose tissue. Notably, brown adipose tissue (BAT) is probably the most highly vascularized tissue in the body, as each adipocyte is encircled by capillaries [229, 230]. Thus, EC fate may tightly regulate BAT development and function, as well as beige adipocytes differentiation in WAT [231].
Diabetes is well known to alter EC fate and function, since ECs are primary target of hyperglycemia-mediated oxidative damage [101]. For example, gestational diabetes mellitus weakens Nrf2 antioxidant signaling, and consequently induces oxidative stress in fetal ECs in utero [232], which may result in the increased risk of type 2 diabetes in offspring [233]. Additionally, EC fate also affects diabetes progression. Emerging evidence suggests that vascular ECs regulate the activities of insulin associated with metabolism [234, 235]. For example, adipose tissues from eNOS-transgenic mice that were provided a high-fat diet displayed higher levels of PPAR-α and PPAR-γ, increased expression of mitochondrial proteins, higher metabolic rates, and decreased hypertrophy of white adipocytes. Consistent with these alterations, eNOS transgenic mice were resistant to diet-induced obesity and hyper-insulinemia [236]. In addition, NO bioavailability associated with EC function directly stimulates EC insulin transport [237]. These results indicate that adipose tissue mass and diabetes may be regulated by vascular EC fate.
Obesity and most obesity-associated disorders, including diabetic complications, cardiovascular disorders, and malignancies [238], are associated with pathological angiogenesis [223, 239]. Thus, the modulation of EC fate-associated angiogenesis is a promising therapeutic approach for anti-obesity and insulin sensitivity. In particular, the stimulation of angiogenesis in metabolically active BAT or during beige adipocytes induction may facilitate energy expenditure [240, 241]. However, the mechanisms by which redox regulation of EC fate mediates the BAT/WAT switch [242] remain largely unknown.
Therapeutic strategies for redox regulation of EC fate
Endothelial cell fate is a critical determinant of overall health and the development of diseases, such as cardiovascular disease, cancer, obesity, and diabetes. Thus, the normalization of EC function is a potential approach for preventing and treating many human diseases.
Strategies for maintaining redox homeostasis in ECs
Exogenous and direct exposure to oxidants or reductants is an important strategy for maintaining endogenous redox balance in ECs. Nitro-fatty acids (NO2-FAs) are nitrated derivatives of unsaturated fatty acids that form in human blood and tissues [243]. NO2-FAs release NO and possess antioxidant functions [244]. Therefore, NO2-FAs may be promising pharmacological tools as NO donors to prevent inflammatory diseases associated with redox imbalance [245]. In addition, NO2-FAs may function independently of NO. For example, nitrated oleic acid upregulates HO-1 and HIF1-α and activates Ca2+/calmodulin-dependent protein kinase kinase-β in bovine aortic endothelial cells. This leads to the activation of AMPK and eNOS, which are tightly associated with EC function [246].
H2S is a chemical reductant with anti-oxidant function. Because H2S has vasculoprotective functions, including vasorelaxation, prevention of inflammation, anti-proliferation, and anti-thrombotic effects, it is a potential alternative approach for treating hypertension [247]. This is supported by the physiologic role of H2S as a regulator of blood pressure. Several H2S donors demonstrate preventive and therapeutic effects. For example, morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate (GYY4137) is a water-soluble, slow-release formulation of H2S. Chronic administration of GYY4137 during a 14-day period elicited antihypertensive effects in a rat model of spontaneous hypertension [32]. Sodium hydrosulfide (NaHS), an H2S donor, increased collateral vessel growth and capillary density in a rat model of hind-limb ischemia via upregulation of VEGF in skeletal muscles and phosphorylated VEGFR2 in neighboring vascular ECs [42]. NaHS administration blocked atherogenesis [19]. Recently, H2S-donating agents in combination with aspirin, the so-called HS-ASA hybrids, have been shown to have anti-cancer potential [248]. Importantly, a number of human intervention trials currently involve various H2S donors. For example, NaHS controls EC redox homeostasis and has been used in clinical trials [249]. Interestingly, naturally occurring polysulfides in garlic yield biologically meaningful amounts of H2S by interacting with GSH in red blood cells [250]. These insights may help explain the cardiovascular protective outcomes of several randomized, controlled trials that utilized garlic extracts [251, 252].
Regulation of redox-sensitive signaling molecules
Activation or inhibition of endogenous oxidants or reductants would help create a balanced redox system and may be a promising strategy for preventing or curing diseases associated with altered EC fate. For example, several inhibitors of NOX have been developed to specifically target dysfunctional NOX [253]. The polyphenolic derivative S17834, benzo(b)pyran-4-one, inhibits NOX activity in EC membranes and in atherogenesis [254]. The NOX inhibitor also decreases Sirt-1-mediated acetylation of p53 on lysine-382 and ultimately reduces signaling pathways that promote apoptosis of ECs [255]. Redox sensors and modulators normalize the redox state and functions of ECs in vivo. For example, AMPK activation occurs in response to metformin, which is widely prescribed for type-2 diabetes [78]. In addition, gene therapy and peptide/protein therapy are potential strategies for modulating redox signaling. Gene therapy targets include enzymes involved in redox homeostasis and those that control EC fate. The delivery of genes that encode antioxidant-defense enzymes, such as SOD, CAT, Gpx, or eNOS, ameliorates ROS production and atherogenesis in animal models [256, 257]. Delivery or knockdown of genes that encode redox-sensitive transcriptional factors, such as FoxOs, NF-κB, and Nrf-2, has also been successfully employed in animal models [258]. However, the delivery method is often a limiting factor for gene therapy. Nanoparticles targeted to ECs of the desired tissue or organ may provide a promising therapeutic intervention with increased precision and efficacy [259–261]. The administration of nanoparticles that delivered antioxidants, such as CAT and SOD, to ECs was recently shown to elicit favorable outcomes [262].
Conclusions and perspectives
Redox molecules are created during normal EC metabolism. Redox systems play pivotal roles in the modulation of EC fate. Thus, it is important to understand the molecular mechanisms that regulate EC fate. Although great progress has been made in the last two decades, the redox mechanisms that regulate EC fate remain unclear. Future research into redox regulation of EC fate should focus on the following.
An emerging feature of redox systems is the spatial and temporal compartmentalization [83]. Recent research shows that different redox-signaling and redox-buffering systems are spatially segregated and have unique compartmentalized functions. Furthermore, oxidative signals selectively oxidize target molecules. The maintenance of compartmentalized redox homeostasis is pivotal for EC fate and function. Thus, determining how this compartmentalized nature of cellular redox systems is linked to EC fate will be critical for fully understanding how EC fate promotes disease development and progression.
Intriguingly, many of the molecules that are highly associated with redox regulation of EC fate appear to be modified at redox-sensitive cysteine residues. Such molecules include FoxOs, Nrf2, HIF-1, AMPK, and PTEN. Thus, these molecules may function as ‘redox sensors.’ Understanding the mechanisms by which these molecules act as ‘redox sensors’ and regulators of EC fate is an important area of research.
The potential physiological and clinical significance of H2S has received considerable attention in recent years. However, the molecular targets and molecular mechanisms of H2S action in ECs remain unknown. Novel pharmacological agents that are developed based on the principles of H2S donation may be able to prevent disease onset by balancing the redox system.
Further investigations must be performed to understand the molecular mechanisms by which the redox system regulates EC fate during the development and switching of white adipocytes and beige adipocytes.
Additional techniques should be developed to target or deliver genes that encode redox sensors and modulators to ECs.
Acknowledgments
The authors sincerely apologize to those colleagues whose original work was not cited due to limited space. The authors also thank all current and former members of Dr. Zou’s laboratory for the work described in this review. This study was supported by funding from the following agencies: National Institutes of Health RO1 (HL110488, HL105157, HL096032, HL089920, HL080499, HL079584, and HL074399), the Warren Chair in Diabetes Research of the University of Oklahoma Health Sciences Center (all to Dr. Zou), Scientist Development Grant (11SDG5560036) from National Center of American Heart Association, and Oklahoma Center for the Advancement of Science and Technology (HR12-061) (both to Dr. Song). Dr. Zou is a recipient of the National Established Investigator Award of the American Heart Association.
Conflict of interest
The authors declare that there are no conflicts of interest.
Abbreviations
- 3-MST
3-Mercaptopyruvate sulfurtransferase
- AICAR
5-Amino-4-imidazole carboxamide riboside
- AMPK
Adenosine monophosphate-activated protein kinase
- BAT
Brown adipose tissue
- BH4
Tetrahydrobiopterin
- BMP
Bone morphogenetic protein
- CAT
Catalase
- CBS
Cystathionine β-synthase
- CEP
ω-(2-Carboxyethyl)pyrrole
- CSE
Cystathionine γ-lyase
- EPA
Eicosapentaenoic acid
- ER
Endoplasmic reticulum
- ETC
Mitochondrial electron-transport chain
- FoxO
Forkhead homeobox type O
- Gpx
Glutathione peroxidase
- GR
Glutathione reductase
- Grx
Glutaredoxin
- GTPCH I
GTP-cyclohydrolase I
- H2O2
Hydrogen peroxide
- HIF-1
Hypoxia-inducible factor 1
- HUVEC
Human umbilical vein endothelial cells
- ICAM-1
Intercellular adhesion molecule-1
- IKK
IкB kinase
- JNK
c-Jun N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- LDLR
Low-density lipoprotein receptor
- LKB1
Liver kinase B1
- MCP
Monocyte chemoattractant protein
- NaHS
Sodium hydrosulfide
- NO
Nitric oxide
- NO2-FAs
Nitro-fatty acids
- NOS
Nitric oxide synthase
- NOX
NADPH oxidase
- Nrf2
Nuclear factor erythroid-2-related factor 2
- O·−2
Superoxide anion
- OGD
Hypoxia and glucose deprivation
- PAG
DL-propargylglycine
- PI3K
Phosphoinositide 3-kinase
- Prx
Peroxiredoxins
- PTEN
Phosphatase and tensin homolog
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- RSH
Thiol
- RSO2H
Sulfinic acids
- RSO3H
Sulfonic acids
- RSOH
Sulfenic acids
- RS-SG
S-glutathionylation
- RS-SR, RS-SR’
Disulfide bonds
- SIRT1
Sirtuin 1
- SOD
Superoxide dismutase
- SM
Smooth muscle
- SREBP2
Sterol regulatory element binding protein 2
- TP
Thromboxane receptor
- Trx
Thioredoxin
- VEGF
Vascular endothelial growth factor
- VEGFR
VEGF receptor
- VCAM-1
Vascular cell adhesion molecule-1
- WAT
White adipose tissue
Contributor Information
Ping Song, Phone: +1-405-271-8001, FAX: +1-405-271-3973, Email: ping-song@ouhsc.edu.
Ming-Hui Zou, Phone: +1-405-271-3974, FAX: +1-405-271-3973, Email: ming-hui-zou@ouhsc.edu.
References
- 1.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Dong Y, Zhang M, Liang B, et al. Reduction of AMP-activated protein kinase {alpha}2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P. Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transpl. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 6.Pasula S, Cai X, Dong Y, et al. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. J Clin Invest. 2012;122:4424–4438. doi: 10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18:478–489. doi: 10.1016/j.cmet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 11.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 13.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- 15.Nie H, Wu JL, Zhang M, Xu J, Zou MH. Endothelial nitric oxide synthase-dependent tyrosine nitration of prostacyclin synthase in diabetes in vivo. Diabetes. 2006;55:3133–3141. doi: 10.2337/db06-0505. [DOI] [PubMed] [Google Scholar]
- 16.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Ahmad S, Cai M, et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127:2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 19.Mani S, Li H, Untereiner A, et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 20.Szabo C, Coletta C, Chao C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 23.Yadav PK, Yamada K, Chiku T, Koutmos M, Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Li X, Du J. Hydrogen sulfide as a new endogenous gaseous transmitter in the cardiovascular system. Curr Vasc Pharmacol. 2006;4:17–22. doi: 10.2174/157016106775203144. [DOI] [PubMed] [Google Scholar]
- 26.Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 28.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltowski J, Jamroz-Wisniewska A. Modulation of h(2)s metabolism by statins: a new aspect of cardiovascular pharmacology. Antioxid Redox Signal. 2012;17:81–94. doi: 10.1089/ars.2011.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greiner R, Palinkas Z, Basell K, et al. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Whiteman M, Guan YY, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 33.Keefe AD, Miller SL, McDonald G, Bada J. Investigation of the prebiotic synthesis of amino acids and RNA bases from CO2 using FeS/H2S as a reducing agent. Proc Natl Acad Sci USA. 1995;92:11904–11906. doi: 10.1073/pnas.92.25.11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyagi N, Moshal KS, Sen U, et al. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal. 2014;20:783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collman JP, Ghosh S, Dey A, Decreau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci USA. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo G, Veres G, Radovits T, et al. Cardioprotective effects of hydrogen sulfide. Nitric Oxide. 2011;25:201–210. doi: 10.1016/j.niox.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen YD, Wang H, Kho SH, et al. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS ONE. 2013;8:e53147. doi: 10.1371/journal.pone.0053147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Tao BB, Liu SY, Zhang CC, et al. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys 1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2013;19:448–464. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coletta C, Papapetropoulos A, Erdelyi K, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 43.Polhemus DJ, Kondo K, Bhushan S, et al. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013;6:1077–1086. doi: 10.1161/CIRCHEARTFAILURE.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mustafa AK, Sikka G, Gazi SK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang G, Yang G, Jiang B, Ju Y, Wu L, Wang R. H2S is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal. 2013;19:1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 46.Guan Q, Zhang Y, Yu C, Liu Y, Gao L, Zhao J. Hydrogen sulfide protects against high-glucose-induced apoptosis in endothelial cells. J Cardiovasc Pharmacol. 2012;59:188–193. doi: 10.1097/FJC.0b013e31823b4915. [DOI] [PubMed] [Google Scholar]
- 47.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 48.Perna AF, Sepe I, Lanza D, et al. Hydrogen sulfide reduces cell adhesion and relevant inflammatory triggering by preventing ADAM17-dependent TNF-alpha activation. J Cell Biochem. 2013;114:1536–1548. doi: 10.1002/jcb.24495. [DOI] [PubMed] [Google Scholar]
- 49.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiteman M, Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King AL, Polhemus DJ, Bhushan S, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 53.Mustafa AK, Gadalla MM, Sen N, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 55.Li W, Busu C, Circu ML, Aw TY. Glutathione in cerebral microvascular endothelial biology and pathobiology: implications for brain homeostasis. Int J Cell Biol. 2012;2012:434971. doi: 10.1155/2012/434971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okouchi M, Okayama N, Aw TY. Preservation of cellular glutathione status and mitochondrial membrane potential by N-acetylcysteine and insulin sensitizers prevent carbonyl stress-induced human brain endothelial cell apoptosis. Curr Neurovasc Res. 2009;6:267–278. doi: 10.2174/156720209789630348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langston W, Chidlow JH, Jr, Booth BA, et al. Regulation of endothelial glutathione by ICAM-1 governs VEGF-A-mediated eNOS activity and angiogenesis. Free Radic Biol Med. 2007;42:720–729. doi: 10.1016/j.freeradbiomed.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kugiyama K, Ohgushi M, Motoyama T, et al. Intracoronary infusion of reduced glutathione improves endothelial vasomotor response to acetylcholine in human coronary circulation. Circulation. 1998;97:2299–2301. doi: 10.1161/01.cir.97.23.2299. [DOI] [PubMed] [Google Scholar]
- 59.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 61.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 62.Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci USA. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012;52:1607–1619. doi: 10.1016/j.freeradbiomed.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thum T, Fraccarollo D, Schultheiss M, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 69.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Coronary artery endothelial transcriptome in vivo: identification of endoplasmic reticulum stress and enhanced reactive oxygen species by gene connectivity network analysis. Circ Cardiovasc Genet. 2011;4:243–252. doi: 10.1161/CIRCGENETICS.110.958926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 71.Landmesser U, Spiekermann S, Preuss C, et al. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol. 2007;27:943–948. doi: 10.1161/01.ATV.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 72.Kou B, Ni J, Vatish M, Singer DR. Xanthine oxidase interaction with vascular endothelial growth factor in human endothelial cell angiogenesis. Microcirculation. 2008;15:251–267. doi: 10.1080/10739680701651495. [DOI] [PubMed] [Google Scholar]
- 73.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal. 2013;19:1469–1480. doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 76.Therade-Matharan S, Laemmel E, Carpentier S, et al. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1756–R1762. doi: 10.1152/ajpregu.00480.2004. [DOI] [PubMed] [Google Scholar]
- 77.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5’-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Wang S, Zhang M, Wang Q, Asfa S, Zou MH. Tyrosine nitration of PA700 links proteasome activation to endothelial dysfunction in mouse models with cardiovascular risk factors. PLoS ONE. 2012;7:e29649. doi: 10.1371/journal.pone.0029649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Zhao Y, Wu J, Zhu H, Song P, Zou MH. Peroxynitrite-dependent zinc release and inactivation of guanosine 5’-triphosphate cyclohydrolase 1 instigate its ubiquitination in diabetes. Diabetes. 2013;62:4247–4256. doi: 10.2337/db13-0751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stangherlin A, Reddy AB. Regulation of circadian clocks by redox homeostasis. J Biol Chem. 2013;288:26505–26511. doi: 10.1074/jbc.R113.457564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oelze M, Kroller-Schon S, Steven S, et al. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 87.Whiteman M, Armstrong JS, Chu SH, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 88.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am J Physiol Heart Circ Physiol. 2010;298:H1048–H1054. doi: 10.1152/ajpheart.00826.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skurk C, Maatz H, Kim HS, et al. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 92.Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsuchiya K, Tanaka J, Shuiqing Y, et al. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JW, Chen H, Pullikotil P, Quon MJ. Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA. J Biol Chem. 2011;286:6423–6432. doi: 10.1074/jbc.M110.180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olmos Y, Valle I, Borniquel S, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanaka J, Qiang L, Banks AS, et al. Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes. 2009;58:2344–2354. doi: 10.2337/db09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daly C, Wong V, Burova E, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HS, Skurk C, Maatz H, et al. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. FASEB J. 2005;19:1042–1044. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- 100.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 101.Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14:469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen XL, Dodd G, Thomas S, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 105.Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 106.Hsieh CY, Hsiao HY, Wu WY, et al. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J Biomed Sci. 2009;16:12. doi: 10.1186/1423-0127-16-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Y, Gong J, Thimmulappa RK, Kosmider B, Biswal S, Duh EJ. Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc Natl Acad Sci USA. 2013;110:E3910–E3918. doi: 10.1073/pnas.1309276110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 110.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58:1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 111.Loboda A, Stachurska A, Florczyk U, et al. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxid Redox Signal. 2009;11:1501–1517. doi: 10.1089/ars.2008.2211. [DOI] [PubMed] [Google Scholar]
- 112.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res. 2013;112:382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013;288:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Connor KM, Subbaram S, Regan KJ, et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 115.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hill BG, Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59:21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- 118.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evangelista AM, Thompson MD, Weisbrod RM, et al. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxid Redox Signal. 2012;17:1099–1108. doi: 10.1089/ars.2011.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Evangelista AM, Thompson MD, Bolotina VM, Tong X, Cohen RA. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med. 2012;53:2327–2334. doi: 10.1016/j.freeradbiomed.2012.10.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clavreul N, Bachschmid MM, Hou X, et al. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 124.Chen CA, Wang TY, Varadharaj S, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Yang J, Yi J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid Redox Signal. 2012;16:649–657. doi: 10.1089/ars.2011.4313. [DOI] [PubMed] [Google Scholar]
- 126.Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013;4:e537. doi: 10.1038/cddis.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J, Xie Z, Dong Y, Wang S, Liu C, Zou MH. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283:27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.Han Y, Wang Q, Song P, Zhu Y, Zou MH. Redox regulation of the AMP-activated protein kinase. PLoS ONE. 2010;5:e15420. doi: 10.1371/journal.pone.0015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song P, Wang S, He C, Liang B, Viollet B, Zou MH. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Song P, Zhou Y, Coughlan KA, et al. Adenosine monophosphate-activated protein kinase-alpha2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation. Arterioscler Thromb Vasc Biol. 2013;33:2800–2809. doi: 10.1161/ATVBAHA.113.301869. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Wang S, Song P, Zou MH. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond) 2012;122:555–573. doi: 10.1042/CS20110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 133.Song P, Wu Y, Xu J, et al. Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation. 2007;116:1585–1595. doi: 10.1161/CIRCULATIONAHA.107.716498. [DOI] [PubMed] [Google Scholar]
- 134.Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem. 2008;283:12446–12455. doi: 10.1074/jbc.M708208200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Wang Q, Zhang M, Ding Y, et al. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res. 2014;114:480–492. doi: 10.1161/CIRCRESAHA.114.302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 137.Zhang M, Dong Y, Xu J, et al. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 139.Zou MH, Hou XY, Shi CM, et al. Activation of 5’-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 140.Choi HC, Song P, Xie Z, et al. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283:20186–20197. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Liu C, Liang B, Wang Q, Wu J, Zou MH. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem. 2010;285:15346–15355. doi: 10.1074/jbc.M110.102491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 143.Bhatt MP, Lim YC, Kim YM, Ha KS. C-peptide activates AMPKalpha and prevents ROS-mediated mitochondrial fission and endothelial apoptosis in diabetes. Diabetes. 2013;62:3851–3862. doi: 10.2337/db13-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cifarelli V, Lee S, Kim DH, et al. FOXO1 mediates the autocrine effect of endothelin-1 on endothelial cell survival. Mol Endocrinol. 2012;26:1213–1224. doi: 10.1210/me.2011-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Burhans WC, Heintz NH. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med. 2009;47:1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 146.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 148.Latella L, Sacco A, Pajalunga D, et al. Reconstitution of cyclin D1-associated kinase activity drives terminally differentiated cells into the cell cycle. Mol Cell Biol. 2001;21:5631–5643. doi: 10.1128/MCB.21.16.5631-5643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Zang QS, Liu Z, et al. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Physiol Cell Physiol. 2011;301:C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- 152.Song P, Zhang M, Wang S, Xu J, Choi HC, Zou MH. Thromboxane A2 receptor activates a rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem. 2009;284:17120–17128. doi: 10.1074/jbc.M109.012583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Xu MJ, Song P, Shirwany N, et al. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase alpha subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zippel N, Malik RA, Fromel T, et al. Transforming growth factor-beta-activated kinase 1 regulates angiogenesis via AMP-activated protein kinase-alpha1 and redox balance in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:2792–2799. doi: 10.1161/ATVBAHA.113.301848. [DOI] [PubMed] [Google Scholar]
- 155.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 156.Okuno Y, Nakamura-Ishizu A, Otsu K, Suda T, Kubota Y. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med. 2012;18:1208–1216. doi: 10.1038/nm.2846. [DOI] [PubMed] [Google Scholar]
- 157.Jansen F, Yang X, Hoelscher M, et al. Endothelial microparticle-mediated transfer of MicroRna-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 158.Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 160.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 161.Bess E, Fisslthaler B, Fromel T, Fleming I. Nitric oxide-induced activation of the AMP-activated protein kinase alpha2 subunit attenuates IkappaB kinase activity and inflammatory responses in endothelial cells. PLoS ONE. 2011;6:e20848. doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 163.Seldon MP, Silva G, Pejanovic N, et al. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J Immunol. 2007;179:7840–7851. doi: 10.4049/jimmunol.179.11.7840. [DOI] [PubMed] [Google Scholar]
- 164.Xiao H, Lu M, Lin TY, et al. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 166.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pan LL, Liu XH, Gong QH, Wu D, Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 168.van der Loo B, Schildknecht S, Zee R, Bachschmid MM. Signalling processes in endothelial ageing in relation to chronic oxidative stress and their potential therapeutic implications in humans. Exp Physiol. 2009;94:305–310. doi: 10.1113/expphysiol.2008.043315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burger D, Kwart DG, Montezano AC, et al. Microparticles induce cell cycle arrest through redox-sensitive processes in endothelial cells: implications in vascular senescence. J Am Heart Assoc. 2012;1:e001842. doi: 10.1161/JAHA.112.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 172.Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Margariti A, Winkler B, Karamariti E, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci USA. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Junker JP, Lonnqvist S, Rakar J, Karlsson LK, Grenegard M, Kratz G. Differentiation of human dermal fibroblasts towards endothelial cells. Differentiation. 2013;85:67–77. doi: 10.1016/j.diff.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 175.Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 176.Zhang P, Moudgill N, Hager E, et al. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev. 2011;20:977–988. doi: 10.1089/scd.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fischer LJ, McIlhenny S, Tulenko T, et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 179.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeng L, Xiao Q, Margariti A, et al. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174:1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamamoto K, Sokabe T, Watabe T, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 182.Zhang C, Zeng L, Emanueli C, Xu Q. Blood flow and stem cells in vascular disease. Cardiovasc Res. 2013;99:251–259. doi: 10.1093/cvr/cvt061. [DOI] [PubMed] [Google Scholar]
- 183.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Myers CT, Krieg PA. BMP-mediated specification of the erythroid lineage suppresses endothelial development in blood island precursors. Blood. 2013;122:3929–3939. doi: 10.1182/blood-2013-03-490045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 187.DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- 188.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 190.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 191.Cooley LS, Handsley MM, Zhou Z, et al. Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro. J Cell Sci. 2010;123:3808–3816. doi: 10.1242/jcs.064279. [DOI] [PubMed] [Google Scholar]
- 192.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 194.Gupta RK, Mepani RJ, Kleiner S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sena CM, Pereira AM, Seica R. Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 196.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 197.Sukumar P, Viswambharan H, Imrie H, et al. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes. 2013;62:2130–2134. doi: 10.2337/db12-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 199.Wang S, Zhang M, Liang B, et al. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dong Y, Zhang M, Wang S, et al. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang M, Song P, Xu J, Zou MH. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31:125–132. doi: 10.1161/ATVBAHA.110.207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mani S, Untereiner A, Wu L, Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid Redox Signal. 2014;20:805–817. doi: 10.1089/ars.2013.5324. [DOI] [PubMed] [Google Scholar]
- 203.Wang Y, Zhao X, Jin H, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 204.Wang S, Xu J, Song P, et al. Acute inhibition of guanosine triphosphate cyclohydrolase 1 uncouples endothelial nitric oxide synthase and elevates blood pressure. Hypertension. 2008;52:484–490. doi: 10.1161/HYPERTENSIONAHA.108.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu J, Wang S, Wu Y, Song P, Zou MH. Tyrosine nitration of PA700 activates the 26S proteasome to induce endothelial dysfunction in mice with angiotensin II-induced hypertension. Hypertension. 2009;54:625–632. doi: 10.1161/HYPERTENSIONAHA.109.133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 207.Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 208.Wu Y, Zhang C, Dong Y, et al. Activation of the AMP-activated protein kinase by eicosapentaenoic acid (EPA, 20:5 n-3) improves endothelial function in vivo. PLoS ONE. 2012;7:e35508. doi: 10.1371/journal.pone.0035508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yanfei W, Lin S, Junbao D, Chaoshu T. Impact of l-arginine on hydrogen sulfide/cystathionine-gamma-lyase pathway in rats with high blood flow-induced pulmonary hypertension. Biochem Biophys Res Commun. 2006;345:851–857. doi: 10.1016/j.bbrc.2006.04.162. [DOI] [PubMed] [Google Scholar]
- 211.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 212.Peter EA, Shen X, Shah SH, et al. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc. 2013;2:e000387. doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9:498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 214.Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13:858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 215.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 216.Parri M, Chiarugi P. Redox molecular machines involved in tumor progression. Antioxid Redox Signal. 2013;19:1828–1845. doi: 10.1089/ars.2012.5040. [DOI] [PubMed] [Google Scholar]
- 217.West XZ, Malinin NL, Merkulova AA, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Daquinag AC, Zhang Y, Kolonin MG. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharmacol Sci. 2011;32:300–307. doi: 10.1016/j.tips.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 219.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 220.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 221.Kim SJ, Moon GJ, Cho YH, et al. Circulating mesenchymal stem cells microparticles in patients with cerebrovascular disease. PLoS ONE. 2012;7:e37036. doi: 10.1371/journal.pone.0037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–597. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 223.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Villaret A, Galitzky J, Decaunes P, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gealekman O, Guseva N, Hartigan C, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brakenhielm E, Cao R, Gao B, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 227.Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLoS ONE. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lim S, Honek J, Xue Y, et al. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat Protoc. 2012;7:606–615. doi: 10.1038/nprot.2012.013. [DOI] [PubMed] [Google Scholar]
- 230.Xue Y, Lim S, Brakenhielm E, Cao Y. Adipose angiogenesis: quantitative methods to study microvessel growth, regression and remodeling in vivo. Nat Protoc. 2010;5:912–920. doi: 10.1038/nprot.2010.46. [DOI] [PubMed] [Google Scholar]
- 231.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 232.Cheng X, Chapple SJ, Patel B, et al. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013;62:4088–4097. doi: 10.2337/db13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sobngwi E, Boudou P, Mauvais-Jarvis F, et al. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361:1861–1865. doi: 10.1016/S0140-6736(03)13505-2. [DOI] [PubMed] [Google Scholar]
- 234.Duplain H, Burcelin R, Sartori C, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 235.Le Gouill E, Jimenez M, Binnert C, et al. Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56:2690–2696. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- 236.Sansbury BE, Cummins TD, Tang Y, et al. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ Res. 2012;111:1176–1189. doi: 10.1161/CIRCRESAHA.112.266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62:4030–4042. doi: 10.2337/db13-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 240.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 241.Xue Y, Petrovic N, Cao R, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 242.Wang J, Liu R, Wang F, et al. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nat Cell Biol. 2013;15:1455–1463. doi: 10.1038/ncb2867. [DOI] [PubMed] [Google Scholar]
- 243.Baker PR, Lin Y, Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lima ES, Bonini MG, Augusto O, Barbeiro HV, Souza HP, Abdalla DS. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic Biol Med. 2005;39:532–539. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 245.Trostchansky A, Bonilla L, Gonzalez-Perilli L, Rubbo H. Nitro-fatty acids: formation, redox signaling, and therapeutic potential. Antioxid Redox Signal. 2013;19:1257–1265. doi: 10.1089/ars.2012.5023. [DOI] [PubMed] [Google Scholar]
- 246.Wu Y, Dong Y, Song P, Zou MH. Activation of the AMP-activated protein kinase (AMPK) by nitrated lipids in endothelial cells. PLoS ONE. 2012;7:e31056. doi: 10.1371/journal.pone.0031056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 247.Streeter E, Ng HH, Hart JL. Hydrogen sulfide as a vasculoprotective factor. Med Gas Res. 2013;3:9. doi: 10.1186/2045-9912-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kashfi K. Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxid Redox Signal. 2014;20:831–846. doi: 10.1089/ars.2013.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Predmore BL, Lefer DJ. Development of hydrogen sulfide-based therapeutics for cardiovascular disease. J Cardiovasc Transl Res. 2010;3:487–498. doi: 10.1007/s12265-010-9201-y. [DOI] [PubMed] [Google Scholar]
- 250.Benavides GA, Squadrito GL, Mills RW, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ried K, Frank OR, Stocks NP. Aged garlic extract reduces blood pressure in hypertensives: a dose-response trial. Eur J Clin Nutr. 2013;67:64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Huang YT, Yao CH, Way CL, et al. Diallyl trisulfide and diallyl disulfide ameliorate cardiac dysfunction by suppressing apoptotic and enhancing survival pathways in experimental diabetic rats. J Appl Physiol. 2013;114:402–410. doi: 10.1152/japplphysiol.00672.2012. [DOI] [PubMed] [Google Scholar]
- 253.Schramm A, Matusik P, Osmenda G, Guzik TJ. Targeting NADPH oxidases in vascular pharmacology. Vasc Pharmacol. 2012;56:216–231. doi: 10.1016/j.vph.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cayatte AJ, Rupin A, Oliver-Krasinski J, et al. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- 255.Xu S, Jiang B, Hou X, et al. High-fat diet increases and the polyphenol, S17834, decreases acetylation of the sirtuin-1-dependent lysine-382 on p53 and apoptotic signaling in atherosclerotic lesion-prone aortic endothelium of normal mice. J Cardiovasc Pharmacol. 2011;58:263–271. doi: 10.1097/FJC.0b013e3182239eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yang H, Roberts LJ, Shi MJ, et al. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 257.Van Assche T, Huygelen V, Crabtree MJ. Targeting vascular redox biology through antioxidant gene delivery: a historical view and current perspectives. Recent Pat Cardiovasc Drug Discovery. 2011;6:89–102. doi: 10.2174/157489011795933873. [DOI] [PubMed] [Google Scholar]
- 258.Levonen AL, Inkala M, Heikura T, et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol. 2007;27:741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 259.Shuvaev VV, Ilies MA, Simone E, et al. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5:6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zern BJ, Chacko AM, Liu J, et al. Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano. 2013;7:2461–2469. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pan H, Myerson JW, Hu L, et al. Programmable nanoparticle functionalization for in vivo targeting. FASEB J. 2013;27:255–264. doi: 10.1096/fj.12-218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hood E, Simone E, Wattamwar P, Dziubla T, Muzykantov V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine. 2011;6:1257–1272. doi: 10.2217/nnm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]