Abstract
Background
Despite recovery of hemodynamics by fluid resuscitation after hemorrhage, development of the systemic inflammatory response and multiple organ dysfunction syndromes can nonetheless lead to death. Minocycline and doxycycline are tetracycline derivatives that are protective in models of hypoxic, ischemic and oxidative stress. Our Aim was to determine whether minocycline and doxycycline protect liver and kidney and improve survival in a mouse model of hemorrhagic shock and resuscitation.
Methods
Mice were hemorrhaged to 30 mm Hg for 3 h and then resuscitated with shed blood followed by half the shed volume of lactated Ringer's solution containing tetracycline (10 mg/kg), minocycline (10 mg/kg), doxycycline (5 mg/kg) or vehicle. For pre-plus post-treatment, drugs were administered intraperitoneally prior to hemorrhage followed by second equal dose in Ringer's solution after blood resuscitation. Blood and tissue were harvested after 6 h.
Results
Serum alanine aminotransferase (ALT) increased to 1988 and 1878 U/L after post-treatment with vehicle and tetracycline, respectively, whereas minocycline and doxycycline post-treatment decreased ALT to 857 and 863 U/L. Pre-plus post-treatment with minocycline and doxycycline also decreased ALT to 849 and 834 U/L. After vehicle, blood creatinine increased to 279 μM, which minocycline and doxycycline post-treatment decreased to 118 and 112 μM. Minocycline and doxycycline pre- plus post-treatment decreased creatinine similarly. Minocycline and doxycycline also decreased necrosis and apoptosis in liver and apoptosis in both liver and kidney, the latter assessed by TUNEL and caspase-3 activation. Lastly after 4.5 h of hemorrhage followed by resuscitation, minocycline and doxycycline (but not tetracycline) post-treatment improved 1-week survival from 38%(vehicle) to 69% and 67%, respectively.
Conclusion
Minocycline and doxycycline were similarly protective when given before as after blood resuscitation and might therefore have clinical efficacy to mitigate liver and kidney injury after resuscitated hemorrhage.
Keywords: apoptosis, doxycycline, hemorrhagic shock, kidney, liver, minocycline, necrosis
Introduction
Despite recovery of hemodynamics by fluid resuscitation, survivors of hemorrhagic shock and resuscitation (HS/R) may develop a systemic inflammatory response syndrome (SIRS) leading to multiple organ damage and dysfunction (multiple organ dysfunction syndrome, MODS) and death (1-3). At the cellular level, HS/R injury leads to both necrosis and apoptosis as the apparent consequence of hypoxia/reoxygenation stress (4;5). Mitochondrial inner membrane permeabilization, called the mitochondrial permeability transition (MPT), is a major consequence of ischemia/reperfusion that leads to both necrotic and apoptotic cell death (6;7). The liver and kidneys are the most frequently affected organs after hemorrhage-induced hypotension in humans, whose protection during and after HS/R might prevent SIRS and MODS (8).
Minocycline and some other tetracycline derivatives, such as doxycycline, protect liver, kidney, brain and other organs in various models of hypoxic, ischemic and oxidative stress (4;9-13). Although the specific mechanisms of cytoprotection are still under study, minocycline likely acts by preserving mitochondrial function, and recent studies show that minocycline prevents MPT onset after ischemia/reperfusion to liver specifically by blocking the mitochondrial calcium uniporter, which catalyzes mitochondrial uptake of Ca2+ and Fe2+, two divalent cations that promote MPT onset (11;13). In a recent report, we also showed that minocycline treatment after blood resuscitation decreases liver injury and mitochondrial depolarization (a consequence of MPT onset) after HS/R to mice (4). Another recent report shows similar minocycline protection in a different model of HS/R (14). To our knowledge, no other studies have demonstrated that an agent given after HS/R is protective, the clinically relevant situation for most instances of resuscitated hemorrhage. Here, our goals were to determine in a mouse model of HS/R: 1) whether doxycycline also protects against HS/R injury; 2) whether minocycline and doxycycline protect against kidney as well as liver and injury after HS/R; 3) whether pre- plus post-treatment with minocycline and doxycycline is more protective than post-treatment alone and 4) whether minocycline and doxycycline improve long-term survival after HS/R.
Materials and Methods
Chemicals and Reagents
Minocycline, doxycycline, tetracycline and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
C57BL6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). All mice used were male, 8-10 weeks of age and 26-28 g in weight. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Hemorrhagic Shock and Resuscitation (HS/R)
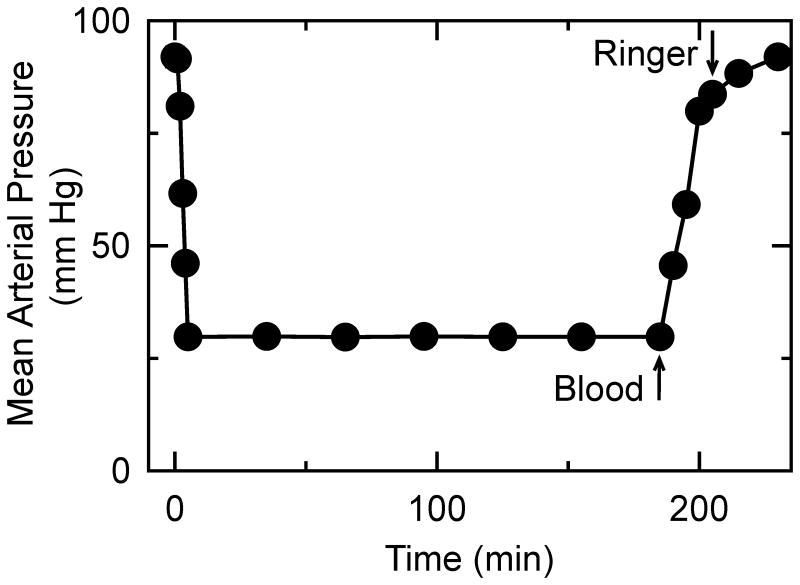
After an overnight fast, each mouse was anesthetized with pentobarbital sodium (50 mg/kg body weight). Under spontaneous breathing, both femoral arteries were exposed and cannulated with polyethylene-10 catheters (SIMS Portex, Fort Myers, FL). The catheters were flushed with normal saline containing heparin (100 IU/L) before insertion. One catheter was connected via a transducer to a pressure analyzer (Micro-Med, Louisville, KY), and blood was withdrawn via the second catheter into a heparinized syringe (10 IU) over 5 min to a mean arterial pressure of 30 mm Hg. Hypotension at 30 mm Hg was maintained for 3 h by withdrawal or reinfusion of shed blood. Body temperature was monitored with a rectal thermometer and maintained at 37°C with a heating pad (ATC 1000, World Precision Instruments, Sarasota, FL). After 3 h of hemorrhagic shock, mice were resuscitated with shed blood plus a volume of lactated Ringer's solution corresponding to 50% of the shed blood volume containing tetracycline (10 mg/kg body weight), minocycline (10 mg/kg), doxycycline (5 mg/kg) or vehicle, which were infused via syringe pump over 30 minutes (post-treatment). Vehicle treatment was different from sham operation in that vehicle-treated mice underwent HS/R in the same fashion as mice treated with minocycline, doxycycline or tetracycline. The timing of post-treatment was intended to simulate a likely clinical scenario in which resuscitation with blood and fluids is initiated as soon as possible on an emergency basis, which is then followed by a potentially therapeutic drug treatment. Since injury after HS/R can depend on the age of the mice, mice were assigned to treatment groups in a randomized, prospective fashion. The extent of injury was within the range of previous findings in this model (4;15-19). For some experiments, mice were treated twice − 1 h before the hemorrhagic shock (i.p.) followed by a second equal dose after blood resuscitation (pre- plus post-treatment). Doses were the same as post-treatment except given twice. Adequacy of resuscitation was determined by restoration of blood pressure. As shown in Figure 1, mean arterial pressure recovered fully after resuscitation, and pre-hemorrhage and post-resuscitation blood pressures were not different. Catheters were then removed, the vessels were ligated, and the groin incisions were closed. Sham-operated animals underwent the same surgical procedures, but hemorrhage was not carried out. No mortality in any group occurred over the course of the surgery.
Figure 1. Mean arterial pressure during hemorrhage shock and resuscitation.
Mice were bled for ∼5 min to a mean arterial pressure of 30 mmHg. After 3 h, the mice were resuscitated with 100% of the shed blood followed by half the shed volume of lactated Ringer's solution, as described in MATERIALS AND METHODS. The data represents all groups combined, since there was no statistical difference in mean arterial pressure between groups. Ringer, lactated Ringer's solution.
For determination of HS/R-dependent liver and kidney injury, mice were re-anesthetized 6 h after resuscitation. For each mouse, blood was collected from the inferior vena cava, and the right kidney and two liver lobes (right and caudate) were cut and snap-frozen in liquid nitrogen. The remaining liver and left kidney was perfused with 4% buffered paraformaldehyde through the portal and renal vein for paraffin embedding.
Alanine aminotransferase, creatinine and urea
Serum alanine aminotransferase (ALT), creatinine and urea were measured using commercial kits (Pointe Scientific Inc., Canton, MI and Bio Assay Systems, Hayward, CA).
Histology
Paraffin sections (4-μm) were stained with hematoxylin and eosin (H&E). Ten random fields (10X magnification) were assessed for necrosis by standard morphologic criteria (e.g., loss of architecture, vacuolization, etc.), and the area percentage of necrosis was quantified (Image J, National Institutes of Health, Bethesda, MD).
Immunohistochemistry
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed on paraffin sections using an in situ cell death detection kit (Roche Diagnostics, Penzberg, Germany). TUNEL-positive parenchymal & non-parenchymal liver cells and kidney tubular cells were counted by light microscopy in 10 random high-power fields (HPF).
Caspase-3
The activity of caspase-3 in liver tissue was determined using a Caspase-3 Colorimetric Assay Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instruction. Activity was normalized to protein concentration of each sample and expressed as fold increase compared to sham operation.
Survival
The hemorrhagic shock period was lengthened from 3 h to 4.5 hours to assess survival, since 7-day survival in mice undergoing 3 h of hemorrhagic shock was 100%. Post-treatments with vehicle, minocycline, tetracycline and doxycycline were performed in a randomized, prospective fashion, and mice were followed 7 days after surgery.
Statistical Analysis
Data are presented as means ± SEM. Statistical analysis was performed by ANOVA plus Student-Newman-Keuls post-hoc analysis or Kaplan-Meier analysis, as appropriate, using p<0.05 as the criterion of significance. Error bars representing S.E.M. are shown in figures. If not shown, SEMs were smaller than the symbol size. Differences between means noted were statistically significant unless otherwise specified.
Results
Minocycline and doxycycline post-treatment decreases serum alanine aminotransferase after hemorrhagic shock/resuscitation
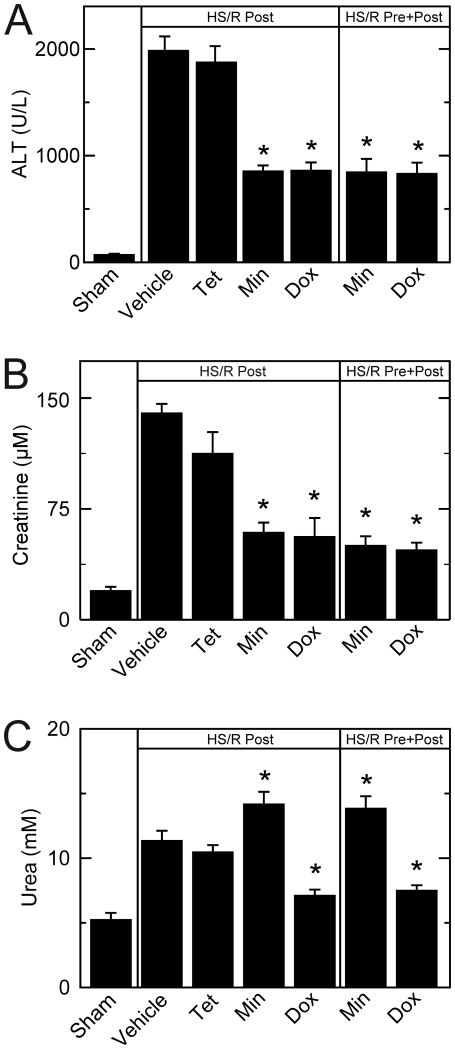
C57BL6 mice were hemorrhaged for 3 h and resuscitated with shed blood plus half the volume of lactated Ringer's solution containing tetracycline (10 mg/kg body weight), minocycline (10 mg/kg), doxycycline (5 mg/kg) or vehicle. At 6 h postoperatively, serum ALT of sham-operated mice averaged 75 U/L (Figure 2A). After hemorrhagic shock and resuscitation, ALT increased to 1988 U/L and 1878 U/L, respectively, after post-treatment with vehicle and tetracycline. Post-treatment minocycline and doxycycline decreased ALT to 857 and 863 U/L, respectively. Pre- plus post-treatment with minocycline and doxycycline also decreased ALT to 849 and 834 U/L. There was no significant difference between pre- plus post-treatment and post-treatment alone for either minocycline or doxycycline (Figure 2A). Thus, minocycline and doxycycline, but not tetracycline, protected against ALT release after HS/R.
Figure 2. Decreased serum alanine transaminase, creatinine and urea after hemorrhagic shock/resuscitation with minocycline and doxycycline.
Mice were subjected to HS/R, as described in MATERIALS AND METHODS. Serum ALT (A), creatinine (B) and urea (C) were measured at 6 h after HS/R. *p <0.05 vs. vehicle; n=6 per group. Dox, doxycycline; Min, minocycline; Post, post-treatment; Pre, pre- plus post-treatment; Tet, tetracycline.
Minocycline and doxycycline decrease serum creatinine after hemorrhagic shock/resuscitation
At 6 h postoperatively, serum creatinine averaged 19.7 μM in sham-operated mice (Figure 2B). After resuscitation with vehicle, serum creatinine increased to 134 μM. Minocycline and doxycycline post-treatment decreased blood creatinine to 59.2 μM and 56.3 μM, respectively. Minocycline and doxycycline pre- plus post-treatment also decreased blood creatinine to 50.3 and 47.2, respectively (Figure 2B). Again, pre- plus post-treatment with either minocycline or doxycycline was not significantly more efficacious than post-treatment alone. Tetracycline post-treatment was not different than vehicle (Figure 2B).
Doxycycline diminishes blood urea after hemorrhagic shock/resuscitation
At 6 h postoperatively, blood urea of sham-operated mice averaged 5.3 mM (Figure 2C). After HS/R with vehicle, blood urea more than doubled to 11.4 mM. Tetracycline post-treatment did not decrease blood urea after HS/R, whereas both post-treatment and pre- plus post-treatment with doxycycline did decrease blood urea to 7.1 and 7.5 mM, respectively (Figure 2C). Minocycline post-treatment and pre- plus post-treatment unexpectedly led to an increase of blood urea (p<0.05) compared to vehicle treatment.
Minocycline and doxycycline decrease liver necrosis after hemorrhagic shock/resuscitation
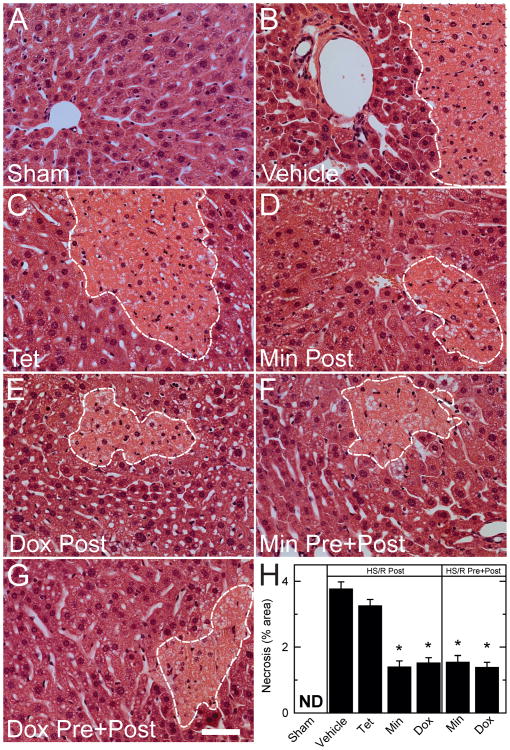
Liver injury was assessed histologically by H&E staining. At 6 h postoperatively, liver histology was normal in sham-operated mice (Figure 3). After HS/R, necrosis developed with a predominately pericentral distribution after post-treatment with vehicle (3.8% of area) and tetracycline (3.3%), whereas post-treatment with minocycline and doxycycline decreased necrosis to 1.4% and 1.5%, respectively (Figure 3). Pre- plus post-treatment with minocycline and doxycycline decreased liver necrosis to approximately the same extent as minocycline and doxycycline post-treatment only (Figure 3).
Figure 3. Protection against histological necrosis in liver by minocycline and doxycycline after hemorrhagic shock/resuscitation.
Representative fields of H&E-stained liver sections are shown for sham operation and for HS/R after various drug treatments. Bar is 50 μm. Lower right panel quantifies area percentage of liver necrosis after the various treatments.*p <0.05 vs vehicle; n=6 per group. Dox, doxycycline; Min, minocycline; ND, none detected; Post, post-treatment; Pre, pre- plus post-treatment; Tet, tetracycline. A – Sham; B – Vehicle; C – Tetracycline; D – Minocycline post-treatment; E – Doxycycline post-treatment; F – Minocycline pre- plus post-treatment; G – Doxycycline pre- plus post-treatment; H – Area of liver necrosis after various treatments (expressed as percentage). Dashed white lines outline areas of necrosis.
Minocycline and doxycycline decrease liver apoptosis after hemorrhagic shock/resuscitation
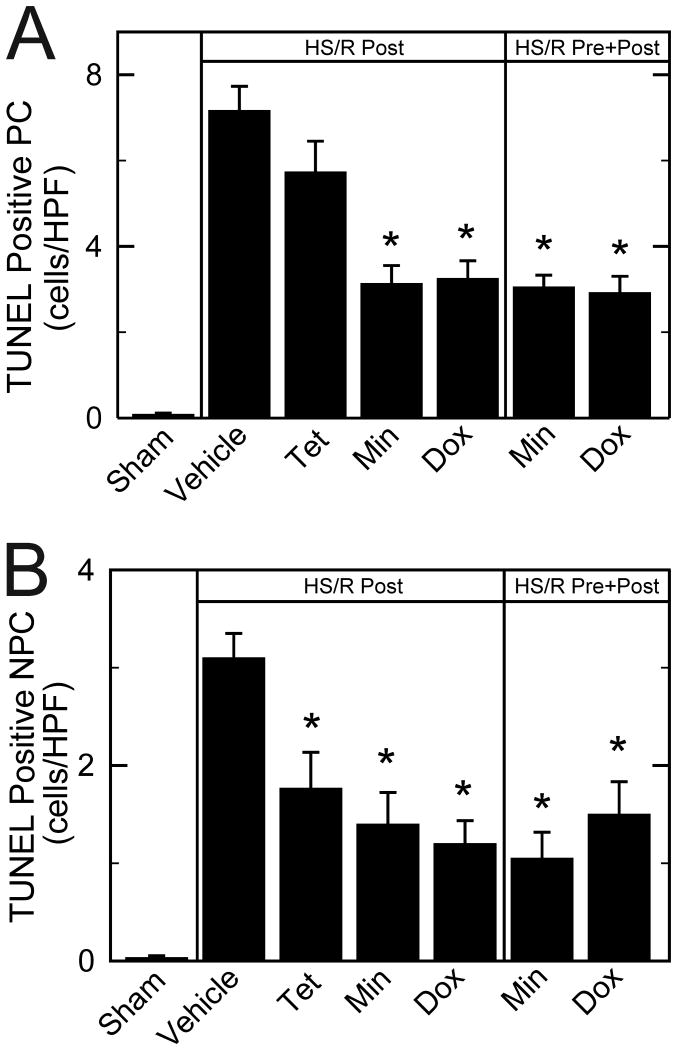
TUNEL was performed on paraffin sections to assess double-stranded DNA breaks that are characteristic of apoptosis. TUNEL-positive hepatic parenchymal and non-parenchymal cells were rare after sham operation, averaging less than 1 cell/HPF. In parenchymal cells (hepatocytes) at 6 h after HS/R with vehicle, TUNEL in non-necrotic areas increased to 7.2 cells/HPF (Figure 4A), corresponding to 1.2% of total parenchymal cell nuclei. Tetracycline failed to decrease parenchymal cell apoptosis. By contrast post-treatment with minocycline and doxycycline decreased parenchymal cell TUNEL to 3.1 and 3.2 cells/HPF, respectively. Pre- plus post-treatment with minocycline and doxycycline also decreased parenchymal cell TUNEL to a similar extent (3.0 and 2.9 cells/HPF, respectively). In non-parenchymal cells at 6 h after HS/R with vehicle treatment, TUNEL-averaged to 3.1 cells/HPF, corresponding to 1.3% of total non-parenchymal cell nuclei. Post-treatment with tetracycline, minocycline and doxycycline decreased TUNEL to 1.8, 1.4, and 1.2 cells/HPF, respectively (Figure 4B). Pre- plus post-treatment with minocycline and doxycycline also decreased TUNEL similarly to 1.0 and 1.5 cells/HPF, respectively.
Figure 4. Protection against TUNEL in liver by minocycline and doxycycline after hemorrhagic shock/resuscitation.
TUNEL positive parenchymal cells (PC, A) and non-parenchymal cells (NPC, B) were quantified after various treatments at 6 h postoperatively. *p <0.05 vs vehicle, n=6 per group. Dox, doxycycline; Min, minocycline; Post, post-treatment; Pre, pre- plus post-treatment; Tet, tetracycline.
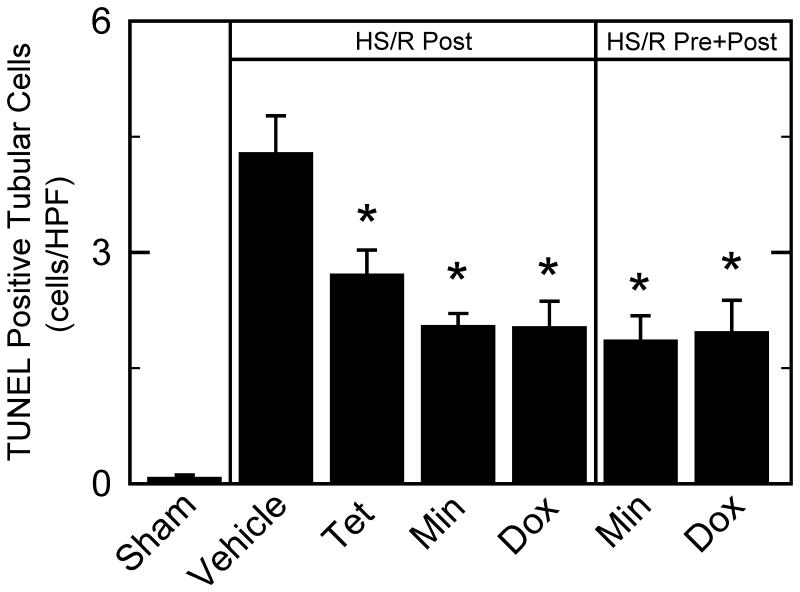
Minocycline, doxycycline, and tetracycline decrease kidney apoptosis after hemorrhagic shock/resuscitation
TUNEL-positive renal tubular cells were rare after sham operation. At 6 h after HS/R with vehicle, TUNEL of tubular cells increased to 4.3 cells/HPF, corresponding to 1.8% of tubular cell nuclei (Figure 5). Post-treatment with minocycline and doxycycline decreased TUNEL in tubular cells to 2.0 and 2.0 cells/HPF, respectively. Unexpectedly, tetracycline also decreased TUNEL significantly to 2.7 cells/HPF. Pre- plus post-treatment with minocycline and doxycycline similarly decreased TUNEL-positive parenchymal cells to 1.8 and 2.0 cells/HPF, respectively (Figure 5). TUNEL-positive glomerular cells at 6 h after HS/R were not observed after any treatment.
Figure 5. Protection against TUNEL in kidney by minocycline and doxycycline after hemorrhagic shock/resuscitation.
TUNEL positive tubular cells were quantified after various treatments at 6 h postoperatively. *p <0.05 vs vehicle, n=6 per group. Dox, doxycycline; Min, minocycline; Post, post-treatment; Pre, pre-treatment; Tet, tetracycline.
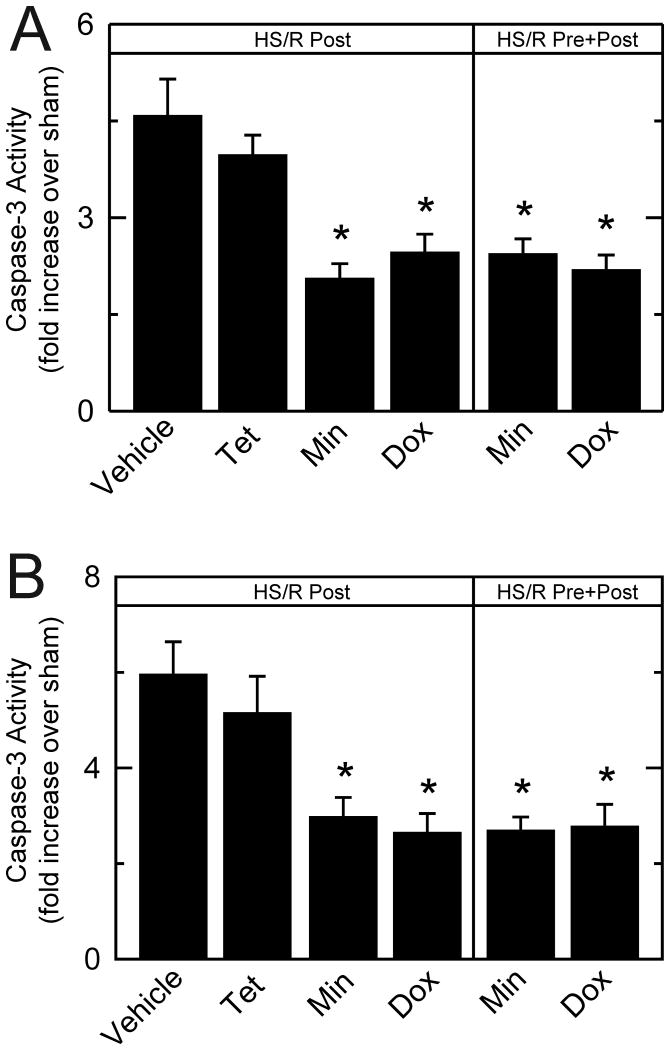
Minocycline and doxycycline decrease caspase-3 activity after hemorrhagic shock/resuscitation
In liver homogenates, caspase-3 activity after HS/R with vehicle treatment increased 4.6–fold over sham (Figure 6A). Tetracycline failed to decrease caspase-3 activity significantly, whereas post-treatment with minocycline and doxycycline decreased caspase-3 activity to 2.1– and 2.5–fold, respectively. Minocycline and doxycycline pre- plus post-treatment also decreased caspase-3 activity to 2.4– and 2.2–fold, respectively (Figure 6A).
Figure 6. Protection of liver and kidney against caspase-3 activation in liver (A) and kidney (B).
Caspase-3 activity was measured in liver and kidney homogenates at 6 h after HS/R. *p <0.05 vs vehicle, n=6 per group. Dox, doxycycline; Min, minocycline; Post, post-treatment; Pre, pre-treatment; Tet, tetracycline.
In kidney homogenates, caspase-3 activity after HS/R with vehicle treatment increased 6.0–fold over sham (Figure 6B). Tetracycline did not significantly decrease caspase-3 activity (5.1–fold over sham), whereas post-treatment with minocycline and doxycycline decreased caspase-3 activity to 3.0– and 2.6–fold, respectively (Figure 6B). Pre- plus post-treatment with minocycline and doxycycline decreased caspase-3 activity similarly (2.7 and 2.8–fold, respectively, Figure 6B).
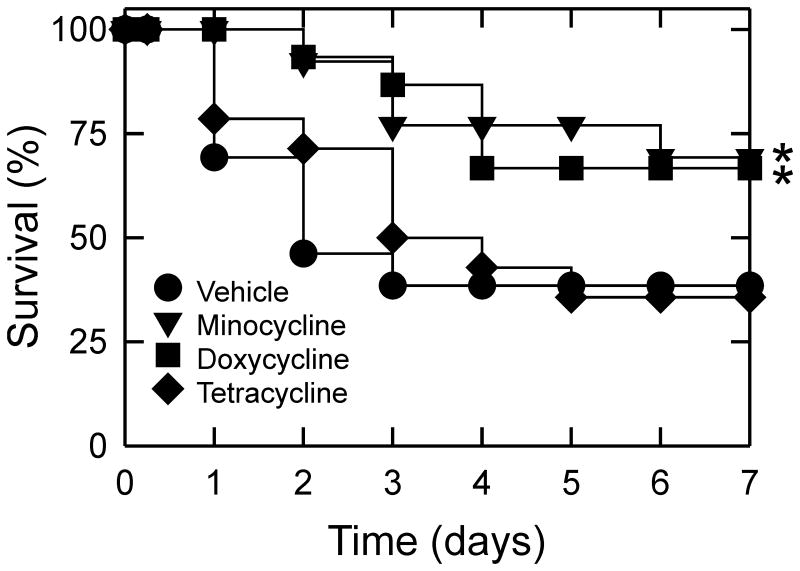
Survival after hemorrhagic shock/resuscitation
The duration of hemorrhagic shock leading to organ failure and mortality after resuscitation is species specific. For mice after 3 h of hemorrhage followed by resuscitation, long-term survival was 100%, although organ damage nonetheless occurred. To assess whether minocycline and doxycycline would improve survival after HS/R, we lengthened the time of hemorrhage to 4.5 h. Restoration of blood pressure by blood and fluid resuscitation was successful after 4.5 h of hemorrhage, and 6 h survival was 100% in all groups. By contrast, 7-day survival after HS/R with vehicle treatment was 38%, which did not improve after post-treatment with tetracycline (Figure 7). Most deaths occurred between days 1 and 3 postoperatively. Post-treatment with minocycline and doxycycline increased 7-day survival to 69% and 67%, respectively, which was significant by Kaplan-Meier analysis.
Figure 7. Seven-day survival after hemorrhagic shock and resuscitation.
Seven-day survival was assessed in mice that underwent 4.5 h of hemorrhage followed by resuscitation after post-treatments with tetracycline derivatives or vehicle. *p <0.05 vs vehicle, n≥13 per group.
Discussion
Despite recovery of hemodynamics by fluid resuscitation after hemorrhage, major organ systems may nonetheless progressively dysfunction and fail. In patients that initially survive HS/R, SIRS and MODS frequently develop when unresuscitated hemorrhage is longer than 1 h (1;2). MODS has a mortality of ∼30% despite aggressive treatment and supportive care (3). Thus, effective therapy for the adverse sequelae of HS/R is an important unmet medical need.
Some tetracyclines have therapeutic properties beyond their antimicrobial action. For example, minocycline mitigates ischemia/reperfusion injury in various organs (9-11;13;20). Recently, we showed that minocycline administered only during the last phase of resuscitation also protects against liver injury after HS/R in mice (4). Doxycycline is another protective tetracycline derivative that decreases injury after myocardial, cerebral and hepatic hypoxia/ischemia (11;21;22). Here, we extend our earlier study to show that 1) doxycycline protects against liver and kidney injury after HS/R as effectively as minocycline, 2) pretreatment with minocycline and doxycycline does not increase protection by post-treatment alone, and 3) minocycline and doxycycline improve long-term survival after HS/R. Dosing for minocycline and doxycycline was based on prior studies and the observation in in vitro models that doxycycline is approximately twice as potent a cytoprotectant as minocycline (11). However, future studies of the pharmacokinetics and dosing regimens for maximal therapeutic effect will be needed in preparation for possible human trials.
In a mouse model of 3 h of hemorrhage to 30 mm Hg followed by sequential resuscitation with shed blood and half the shed volume of lactated Ringer's solution containing vehicle or drug, minocycline and doxycycline each substantially decreased liver and kidney injury assessed at 6 h after resuscitation, whereas tetracycline did not. Specifically, hepatic necrosis and apoptosis, blood creatinine, and renal apoptosis were all significantly decreased by minocycline and doxycycline treatment (Figures 2-6). Blood creatinine reflects glomerular filtration rate, considered the best single index of overall renal function (23;24).
To investigate renal function further, blood urea was also measured. After HS/R, blood urea increased more than 2-fold (Figure 2C). Doxycycline but not tetracycline or minocycline decreased blood urea after HS/R. Lack of protection against elevated blood urea by minocycline after HS/R was unexpected, but previous studies show that tetracycline and minocycline increase blood urea, an effect attributed to decreased renal excretion and a catabolic effect by these tetracyclines (25;26). We also compared pre- plus post-treatment to post-treatment alone. Remarkably, pre- plus post-treatment with minocycline and doxycycline was no more protective than post-treatment alone during the final phase of resuscitation (Figures 2-6).
Since long-term survival after 3 h of hemorrhage followed by resuscitation was 100%, we increased the time of hemorrhage to 4.5 h. After the longer time of hemorrhagic shock, 7-day survival decreased to 38%, which minocycline and doxycycline but not tetracycline treatment significantly improved to 69% and 67%, respectively (Figure 7). Thus, minocycline and doxycycline but not tetracycline protected against liver and kidney injury and significantly improved long-term survival even when administered at the end of resuscitation. Although our mouse model required a longer time of hemorrhage to produce mortality than for humans or rats, restoration of blood pressure by resuscitation was successful after 4.5 h of hemorrhage to mice, and 6-h survival was 100% in all groups. Rather, mortality did not occur until 1-3 days after resuscitation. Early recovery of hemodynamics followed by late mortality is characteristic of human MODS after HS/R, and for this reason our mouse model seems relevant to the human disease.
After HS/R to mice, both necrotic and apoptotic cell death occurred. In the liver, minocycline and doxycycline protected against both modes of cell death and decreased histological necrosis and ALT release by ∼60% at 6 h after HS/R compared to vehicle and tetracycline treatment (Figures 2 and 3). Hepatic parenchymal and nonparenchymal apoptosis assessed by TUNEL and caspase-3 activity also decreased by ∼60% with minocycline and doxycycline treatment (Figures 4 and 6). Overall, hepatic necrosis predominated over apoptosis. Necrosis involved 3.8%of the liver after HS/R with vehicle treatment, whereas TUNEL representing apoptosis in non-necrotic areas occurred in 1.2 and 1.3% of parenchymal and non-parenchymal cell nuclei, respectively (Figure 4).
In kidneys, apoptotic cell death occurred in tubular cells but not glomeruli. Necrosis by histology was not evident, at least at 6 h after HS/R (data not shown). Compared to vehicle, minocycline and doxycycline decreased TUNEL in kidneys after HS/R by an average of 53%(Figure 5). Unexpectedly, tetracycline also significantly decreased renal TUNEL by 37% after HS/R, although tetracycline did not decrease caspase-3 activity or serum creatinine after HS/R compared to vehicle treatment. In hepatic nonparenchymal cells, tetracycline also modestly decreased TUNEL (Figure 3B). These results suggest that tetracycline exerts a weak protective effect that is only evident by TUNEL in some cell types, perhaps because these cell types are experiencing the mildest injury. Future studies will be needed to determine the basis for tetracycline protection against TUNEL in certain cell types.
Based on in vitro studies, several mechanisms have been proposed to explain cytoprotection by minocycline and doxycycline, including calcium and iron chelation (27;28), mitochondrial depolarization preventing formation of reactive oxygen species (ROS) and MPT onset (27;29;30), inhibition of matrix metalloproteinases (9;10;31;32), inhibition of mitochondrial release of cytochrome c and caspase-3 activation (33;34), and upregulation of antiapoptotic proteins (35). Recently, we showed that minocycline and doxycycline specifically inhibit the electrogenic mitochondrial Ca2+ uniporter (MCU), which also mediates uptake of Fe2+ into mitochondria (11;13). In particular, uptake of Fe2+ into mitochondria during hypoxia/ischemia promotes ROS formation after reoxygenation/reperfusion with consequent onset of the MPT (36). The MPT in turn leads to abrupt mitochondrial depolarization, uncoupling of oxidative phosphorylation, mitochondrial swelling with cytochrome c release and both ATP depletion-dependent necrosis and cytochrome c-activated apoptosis (6;7). In support of this hypothesis, we showed previously by intravital multiphoton microscopy that hepatocellular mitochondria depolarize after HS/R prior to loss of cell viability and that minocycline, but not tetracycline, diminishes this mitochondrial dysfunction (4). Other work also indicates that minocycline and doxycycline are not protective by virtue of causing mitochondrial depolarization, chelating divalent cations or inhibiting MMPs, at least in cultured hepatocytes subjected to hypoxia and ischemia/reperfusion (11).
Importantly, minocycline and doxycycline administered after blood resuscitation were as efficacious against organ damage and mortality as the drugs administered both before and after hemorrhage. One implication of this finding is that irreversible liver and kidney damage does not occur until after fluid resuscitation of hemorrhagic shock. However, future studies will be needed to determine how long after resuscitation minocycline/doxycycline can be administered with beneficial effect. Additionally, hemorrhage is often associated with trauma, and the combination of trauma with another stress such as hemorrhage, burn injury or sepsis augments onset of SIRS and MODS, leading to increased mortality in a fashion further modulated by gender and genetic background (2;10;37-39). Again, future studies will be needed to characterize protection by tetracycline derivatives when hemorrhagic shock is combined with other stresses and as a function of gender and animal strain. Lastly, future work is needed to determine the relationship of injury to liver, kidney and possibly other organs (e.g., heart, brain, lung, intestine) to MODS and mortality after HS/R. In particular, the liver with its very high content of macrophages may be a key organ promoting systemic inflammation, MODS and mortality (40;41).
Minocycline and doxycycline are both safe and effective drugs approved for human use by the FDA. Rarely, hepatotoxicity with minocycline occurs that is associated with prolonged use (42). Hepatotoxicity after single dosing of minocycline may occur only if a dosage of 50 mg/kg or higher is used (25). By contrast, doxycycline users appear not to have an increased risk of developing hepatotoxicity (43). Thus in terms of potential clinical application, minocycline and doxycycline should have a high degree of safety in a rescue treatment regimen.
In conclusion, our results here suggest that minocycline and doxycycline might be used as rescue therapy for resuscitated hemorrhage. Doxycycline, which does not adversely affect blood urea, might be preferable over minocycline for such rescue therapy. Minocycline and doxycycline are both safe and widely used FDA-approved drugs that therefore could be adapted for clinical use in both civilian and military medicine to prevent mortality from SIRS and MODS in hemorrhagic shock patients.
Supplementary Material
Abbreviations
- ALT
alanine aminotransferase
- HS/R
hemorrhagic shock and resuscitation
- H&E
hematoxylin and eosin
- HPF
high power field
- I/R
ischemia/reperfusion
- MODS
multiple organ dysfunction syndrome
- MPT
mitochondrial permeability transition
- SIRS
systemic inflammatory response syndrome
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
Footnotes
This work was supported, in part, by Grant DoD W81XWH-09-1-0484 from the Department of Defense and Grants DK073336 and DK37034 from the National Institutes of Health. Imaging facilities were supported, in part, by P30 CA138313 and 5 P20 GM103542-03 with animal facility support from Grant C06 RR015455.
References
- 1.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches. Int J Mol Med. 1999;4(6):575–83. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 2.McGhan LJ, Jaroszewski DE. The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury. 2012;43(2):129–36. doi: 10.1016/j.injury.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Visser T, Pillay J, Koenderman L, Leenen LP. Postinjury immune monitoring: can multiple organ failure be predicted? Curr Opin Crit Care. 2008;14(6):666–72. doi: 10.1097/MCC.0b013e3283196522. [DOI] [PubMed] [Google Scholar]
- 4.Czerny C, Kholmukhamedov A, Theruvath TP, Maldonado EN, Ramshesh VK, Lehnert M, Marzi I, Zhong Z, Lemasters JJ. Minocycline decreases liver injury after hemorrhagic shock and resuscitation in mice. HPB Surg. 2012;2012:259512. doi: 10.1155/2012/259512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paxian M, Bauer I, Rensing H, Jaeschke H, Mautes AE, Kolb SA, Wolf B, Stockhausen A, Jeblick S, Bauer M. Recovery of hepatocellular ATP and “pericentral apoptosis” after hemorrhage and resuscitation. FASEB J. 2003;17(9):993–1002. doi: 10.1096/fj.02-0624com. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3(6):527–35. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- 7.Lemasters JJ. V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999;276(1 Pt 1):G1–G6. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- 8.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45(3):545–9. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Burggraf D, Trinkl A, Dichgans M, Hamann GF. Doxycycline inhibits MMPs via modulation of plasminogen activators in focal cerebral ischemia. Neurobiol Dis. 2007;25(3):506–13. doi: 10.1016/j.nbd.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Roach DM, Fitridge RA, Laws PE, Millard SH, Varelias A, Cowled PA. Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur J Vasc Endovasc Surg. 2002;23(3):260–9. doi: 10.1053/ejvs.2002.1598. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz J, Holmuhamedov E, Zhang X, Lovelace GL, Smith CD, Lemasters JJ. Minocycline and doxycycline, but not other tetracycline-derived compounds, protect liver cells from chemical hypoxia and ischemia/reperfusion injury by inhibition of the mitochondrial calcium uniporter. Toxicol Appl Pharmacol. 2013;273(1):172–9. doi: 10.1016/j.taap.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JR, Gabler WL. Effects of doxycycline in two rat models of ischemia/reperfusion injury. Proc West Pharmacol Soc. 1994;37:3–4. [PubMed] [Google Scholar]
- 13.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47(1):236–46. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CH, Tsai PS, Huang CJ. Minocycline ameliorates lung and liver dysfunction in a rodent model of hemorrhagic shock/resuscitation plus abdominal compartment syndrome. J Surg Res. 2013;180(2):301–9. doi: 10.1016/j.jss.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Chima RS, Maltese G, Lamontagne T, Piraino G, Denenberg A, O'Connor M, Zingarelli B. C-peptide ameliorates kidney injury following hemorrhagic shock. Shock. 2011;35(5):524–9. doi: 10.1097/SHK.0b013e31820b2e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotroneo TM, Nemzek-Hamlin JA, Bayliss J, Su GL. Lipopolysaccharide binding protein inhibitory peptide alters hepatic inflammatory response post-hemorrhagic shock. Innate Immun. 2012;18(6):866–75. doi: 10.1177/1753425912444641. [DOI] [PubMed] [Google Scholar]
- 17.Lehnert M, Arteel GE, Smutney OM, Conzelmann LO, Zhong Z, Thurman RG, Lemasters JJ. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19(4):345–51. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lehnert M, Uehara T, Bradford BU, Lind H, Zhong Z, Brenner DA, Marzi I, Lemasters JJ. Lipopolysaccharide-binding protein modulates hepatic damage and the inflammatory response after hemorrhagic shock and resuscitation. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G456–G463. doi: 10.1152/ajpgi.00480.2005. [DOI] [PubMed] [Google Scholar]
- 19.Lehnert M, Lind H, Zhong Z, Schoonhoven R, Marzi I, Lemasters JJ. Polyphenols of Camellia sinenesis decrease mortality, hepatic injury and generation of cytokines and reactive oxygen and nitrogen species after hemorrhage/resuscitation in rats. BMC Complement Altern Med. 2010;10:46. doi: 10.1186/1472-6882-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JR, Gabler WL. Doxycycline suppression of ischemia-reperfusion-induced hepatic injury. Inflammation. 1994;18(2):193–201. doi: 10.1007/BF01534560. [DOI] [PubMed] [Google Scholar]
- 21.Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64(6):551–60. doi: 10.1016/j.phrs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301(1):H87–H97. doi: 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: Part II. Glomerular filtration rate, proteinuria, and other markers. Am Fam Physician. 2004;70(6):1091–7. [PubMed] [Google Scholar]
- 24.Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: Part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70(5):869–76. [PubMed] [Google Scholar]
- 25.Bocker R, Estler CJ, Ludewig-Sandig D. Evaluation of the hepatotoxic potential of minocycline. Antimicrob Agents Chemother. 1991;35(7):1434–6. doi: 10.1128/aac.35.7.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wibell L, Norlen K, Hedstrand U. The antianabolic effect of tetracyclines in a consecutive clinical series. Scand J Infect Dis Suppl. 1976;9:109–13. [PubMed] [Google Scholar]
- 27.Antonenko YN, Rokitskaya TI, Cooper AJ, Krasnikov BF. Minocycline chelates Ca2+, binds to membranes, and depolarizes mitochondria by formation of Ca2+-dependent ion channels. J Bioenerg Biomembr. 2010;42(2):151–63. doi: 10.1007/s10863-010-9271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun. 2009;386(2):322–6. doi: 10.1016/j.bbrc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogueira CR, Damasceno FM, de Aquino-Neto MR, de Andrade GM, Fontenele JB, de Medeiros TA, Viana GS. Doxycycline protects against pilocarpine-induced convulsions in rats, through its antioxidant effect and modulation of brain amino acids. Pharmacol Biochem Behav. 2011;98(4):525–32. doi: 10.1016/j.pbb.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Relja B, Tottel E, Breig L, Henrich D, Schneider H, Marzi I, Lehnert M. Plant polyphenols attenuate hepatic injury after hemorrhage/resuscitation by inhibition of apoptosis, oxidative stress, and inflammation via NF-kappaB in rats. Eur J Nutr. 2012;51(3):311–21. doi: 10.1007/s00394-011-0216-1. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Yosef Y, Miller A, Shapiro S, Lahat N. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death. Am J Physiol Cell Physiol. 2005;289(5):C1321–C1331. doi: 10.1152/ajpcell.00079.2005. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich R, Gerhauser I, Seeliger F, Baumgartner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the developing mouse brain and spinal cord: a reverse transcription quantitative polymerase chain reaction study. Dev Neurosci. 2005;27(6):408–18. doi: 10.1159/000088455. [DOI] [PubMed] [Google Scholar]
- 33.Chu HC, Lin YL, Sytwu HK, Lin SH, Liao CL, Chao YC. Effects of minocycline on Fas-mediated fulminant hepatitis in mice. Br J Pharmacol. 2005;144(2):275–82. doi: 10.1038/sj.bjp.0706079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417(6884):74–8. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–54. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic Biol Med. 2013;63:243–53. doi: 10.1016/j.freeradbiomed.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14(2):81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 38.Cai C, Gill R, Eum HA, Cao Z, Loughran PA, Darwiche S, Edmonds RD, Menzel CL, Billiar TR. Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1175–R1182. doi: 10.1152/ajpregu.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czerny C, Theruvath TP, Maldonado EN, Lehnert M, Marzi I, Zhong Z, Lemasters JJ. C-Jun N-Terminal Kinase 2 Promotes Liver Injury via the Mitochondrial Permeability Transition after Hemorrhage and Resuscitation. HPB Surg. 2012;2012:641982. doi: 10.1155/2012/641982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt JP, Hunter CT, Brownstein MR, Ku J, Roberts L, Currin RT, Lemasters JJ, Baker CC. Alteration in Kupffer cell function after mild hemorrhagic shock. Shock. 2001;15(5):403–7. doi: 10.1097/00024382-200115050-00012. [DOI] [PubMed] [Google Scholar]
- 41.Huynh T, Lemasters JJ, Bracey LW, Baker CC. Proinflammatory kupffer cell alterations after femur fracture trauma and sepsis in rats. Shock. 2000;14(5):555–60. doi: 10.1097/00024382-200014050-00010. [DOI] [PubMed] [Google Scholar]
- 42.Lawrenson RA, Seaman HE, Sundstrom A, Williams TJ, Farmer RD. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23(4):333–49. doi: 10.2165/00002018-200023040-00006. [DOI] [PubMed] [Google Scholar]
- 43.Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case-control study. J Clin Pharm Ther. 2007;32(5):483–7. doi: 10.1111/j.1365-2710.2007.00853.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.