Abstract
Humans with darkly-pigmented skin display superior permeability barrier function in comparison to humans with lightly-pigmented skin. The reduced pH of the stratum corneum (SC) of darkly-pigmented skin could account for enhanced function, because acidifying lightly-pigmented human SC resets barrier function to darkly-pigmented levels. In SKH1 (non-pigmented) vs. SKH2/J (pigmented) hairless mice, we evaluated how a pigment-dependent reduction in pH could influence epidermal barrier function. Permeability barrier homeostasis is enhanced in SKH2/J vs. SKH1 mice, correlating with a reduced pH in the lower SC that co-localizes with the extrusion of melanin granules. Darkly-pigmented human epidermis also shows substantial melanin extrusion in the outer epidermis. Both acute barrier disruption and topical basic pH challenges accelerate re-acidification of SKH2/J (but not SKH1) SC, while inducing melanin extrusion. SKH2/J mice also display enhanced expression of the SC acidifying enzyme, secretory phospholipase A2f (sPLA2f). Enhanced barrier function of SKH2/J mice could be attributed to enhanced activity of two acidic pH-dependent, ceramide-generating enzymes, β-glucocerebrosidase and acidic sphingomyelinase, leading to accelerated maturation of SC lamellar bilayers. Finally, organotypic cultures of darkly-pigmented-bearing human keratinocytes display enhanced barrier function in comparison to lightly-pigmented cultures. Together, these results suggest that the superior barrier function of pigmented epidermis can be largely attributed to the pH-lowering impact of melanin persistence/extrusion and enhanced sPLA2f expression.
Keywords: barrier function, melanin, melanocytes, melanosomes, pH, pigmentation, Crl:SKH1, SKH2/J hairless mice
INTRODUCTION
Because life in a terrestrial environment requires a highly-competent permeability barrier, we proposed recently that interfollicular pigmentation evolved as a strategy to enhance epidermal barrier function in hominids dwelling in the ultraviolet-B (UV-B)-enriched, arid milieu of Sub-Saharan Africa (Elias, et al., 2009, Elias, et al., 2010). While pigmentation provides additional functional advantages, such as thermal insulation, camouflage, and defense against photocarcinogenesis (Jablonski, 2006), the imperative to generate a competent permeability barrier is paramount. In support of this hypothesis, we showed that humans with darkly-pigmented skin (type IV/V – Fitzpatrick scale) display superior barrier function in comparison to age-, gender-, and occupationally-matched humans with lightly-pigmented skin (Type I/II), independent of ethnicity (Reed, et al., 1995, Gunathilake, et al., 2009). Moreover, in a large, homogenous Chinese population, epidermal barrier function was inferior in stable, non-inflamed, depigmented (vitiliginous) skin in comparison to adjacent, pigmented skin sites in the same individuals (Liu, et al., 2010).
The lower pH of darkly-pigmented human SC could account for enhanced barrier function (Gunathilake, et al., 2009), because acidification enhances epidermal structure and function by multiple mechanisms [e.g., (Mauro, et al., 1998, Hachem, et al., 2003)]. Accordingly, acidification of the SC of lightly-pigmented human subjects `reset' (improved) barrier function to levels found in darkly-pigmented humans (Gunathilake, et al., 2009, Hachem, et al., 2010). Yet, how pigmentation influences epidermal function remains uncertain. Melanocytes could regulate epidermal function (including acidification) by paracrine influences (Slominski and Paus, 1990, Slominski, et al., 1993), and/or by `juxtacrine' effects of melanocytes on neighboring keratinocytes. Melanosomes are acidic organelles that enclose several proteins in the eumelanin synthetic pathway (Moellmann, et al., 1988, Schallreuter, et al., 2008), whose activities require a reduced pH (Chen, et al., 2002). Although the internal pH of melanosomes increases immediately prior to secretion (Ancans, et al., 2001), our studies showed that putative melanosomes in dendrites of darkly-pigmented human melanocytes are more acidic than comparable vesicular structures in lightly-pigmented subjects (Gunathilake, et al., 2009). Yet, how the subsequent transfer of melanosomes impacts the pH of the epidermis in vivo remains unknown.
We explored this issue here in two closely-related mouse strains [SKH2/J-Hrrh/Hrrh (hairless pigmented inbred) and Crl:SKH1 (outbred hairless and non-pigmented)] that exhibit defined differences in both the extent and localization of pigmentation. Permeability barrier homeostasis is superior in SKH2/J mice, which correlates with a reduced pH in the lower SC in these mice. Not only the persistence of engulfed melanosomes, but also their subsequent delayed degradation and extrusion into the outer epidermis of SKH2/J mice (and darkly-pigmented human skin), correlates with acidification of these sites, as well as accelerated processing of secreted lamellar body-derived lipids into mature lamellar bilayers. Moreover, experimental maneuvers that increase the pH of the SC provoke more rapid re-acidification of the SC, and accelerate melanin granule extrusion, in SKH2/J mice. Finally, we showed that melanized keratinocytes display superior barrier function in comparison to lightly-pigmented keratinocytes in organotypic human keratinocytes (KC). Together, these results show that pigmentation enhances barrier function by a hitherto unrecognized, juxtacrine (acidifying) cellular mechanism.
RESULTS
Distinctive Differences in Melanocyte and Melanin Localization in SKH1 and SKH2/J Epidermis
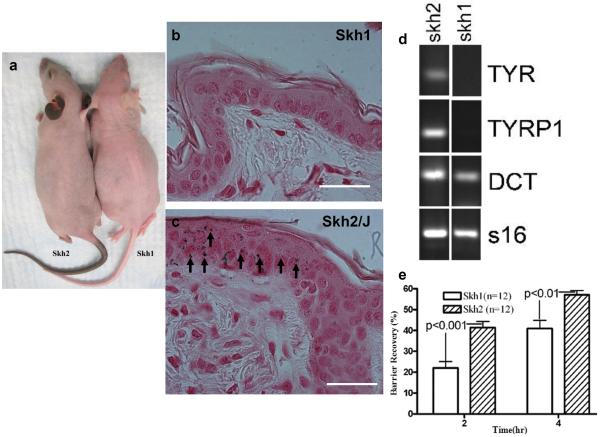
Adult SKH1 mouse skin appears non-pigmented (Fig. 1A), and lacks Fontana-Masson-positive melanin staining in epidermis (Fig. 1B), as well as an absence of the melanocyte marker, Mel-5 (not shown). Yet, neonatal SKH1 skin contains dendritic cells, identified as melanoblasts by Western blotting, and by immunohistochemical staining for tyrosinase-related protein (TYRP)2 and dopachrome tautomerase (DCT) (Fig. 1D), and melanocytes could be cultured from neonatal SKH1 skin (Suppl. Fig. 1). In contrast, both Mel-5-positive melanocytes and abundant melanin are present in SKH2/J mouse epidermis (Fig. 1C), where they localize solely to the interfollicular epidermis - neither melanocytes nor melanin could be detected below the follicular infundibulum of SKH2/J mice (Fig. 1C). Based upon this background information, we deployed these two closely-related hairless mouse models to assess the impact of epidermal pigmentation on a variety of cutaneous functions, as well as to address potential cellular and metabolic mechanisms that could account for the putative, pigmentation-induced enhancement of epidermal barrier function.
Figure 1. Localization of Melanocytes and Pigmentation Differs in SKH1 vs. SKH2/J Epidermis.
A: Appearance of SKH2/J and SKH1 mice. Note intense pigmentation of face, ears and tail, and moderate pigmentation of shoulders and flanks in SKH2/J mice. B&C: Note absence of Fontana-Masson staining for melanin in adult SKH1, with moderate staining for melanin in the interfollicular epidermis of SKH2/J mice (C, arrows). D. Adult SKH2/J mouse skin, but not SKH1 skin, expresses the melanocyte differentiation markers, tyrosinase (TYR) and tyrosinase-related protein 1 (TRYP1). Both mouse strains express the melanoblast differentiation marker, dopachrome tautomerase (DCT), also known as tyrosinase-related protein 2. Mag bars = 10 μm. E: After a comparable extent of acute barrier disruption by sequential tape stripping, recovery kinetics accelerate significantly in SKH2/J vs. SKH1 mice. All shown data reflect mean +/− SEM.
Pigmentation Positively Impacts Barrier Function By Acidifying the Outer Epidermis
We first assessed the role of pigmentation in regulating epidermal structure and function by quantitating differences in a suite of functions in the flank skin of SKH1 and SKH2/J mice. Although there were no significant differences in either basal barrier function or SC hydration (not shown), epidermal permeability barrier homeostasis, assessed as the kinetics of barrier recovery after acute perturbations (tape stripping), accelerated significantly in SKH2/J vs. SKH1 mice (Fig. 1E). These studies are consistent with our prior studies that demonstrated a comparable enhancement in permeability barrier homeostasis in pigmented human skin (Reed, et al., 1995, Gunathilake, et al., 2009).
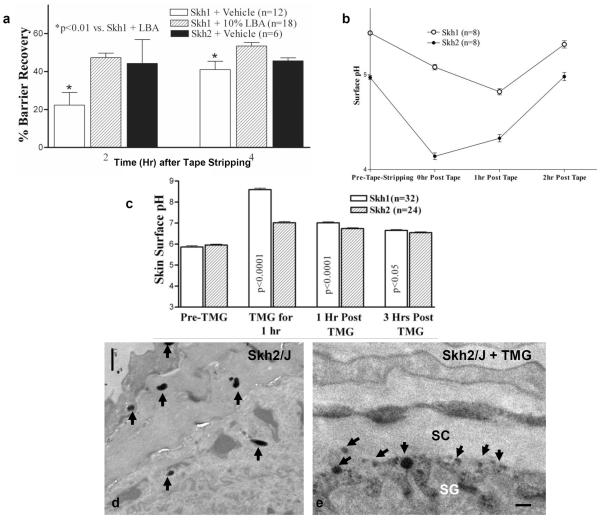
Our prior studies showed that darkly-pigmented humans display a significantly lower skin surface pH than do lightly-pigmented humans (Gunathilake, et al., 2009, Hachem, et al., 2010). Though the surface pH of SKH2/J mice appeared slightly reduced in comparison to SKH1 mice, this difference did not achieve statistical significance (not shown). Yet, using a more sensitive method (i.e., dual-photon confocal microscopy), we detected a lower pH (≈1/3 pH unit) within the lower SC and at the stratum granulosum-SC interface of SKH2/J mice (Figs. 2A&B). The importance of pH for enhanced function in SKH2/J mice could be demonstrated directly, because acidification of the SC of SKH1 mice with the `superacid,' lactobionic acid (LBA), accelerated barrier recovery kinetics to rates comparable to those in SKH2/J epidermis (Fig. 3A). Together, these experiments demonstrate that the presence of pigmentation correlates with a reduction in extracellular acidity in the lower SC and enhanced barrier function in SKH2/J mice.
Figure 2. Selective Acidification of the Lower SC and the Stratum Granulosum (SG)-SC Interface in SKH2/J Mice.
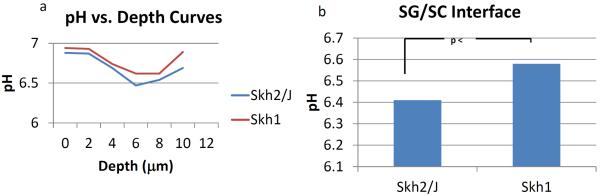
Although surface pH did not differ, assessment of pH at different levels of the SC by dual photon confocal microscopy show that the lower stratum corneum (SC) (A), and extracellular domains at the SG SC interface (A&B) are more acidic in SKH2/J mice than the same sites in SKH1 mice. Results shown represent average values (+/−SEM) for two regions of SKH1 and SKH2/J epidermis from a representative experiment.
Figure 3. Barrier Disruption and Alkaline Challenges Stimulate Rapid Re-acidification of SC of SKH2/J Mice, Paralleled By Pigment Granule Extrusion.
A: Acidification of SKH1 SC accelerates barrier recovery in SKH1 mice to rates similar to those in SKH2/J epidermis. B: Acute barrier disruption stimulates a more rapid return of an acidic pH in SKH2/J mice. C: A single application of the `superbase,' tetramethylguanidine (TMG), induces a rapid increase in SC pH in SKH1 and SKH2/J mice that normalizes more rapidly in SKH2/J mice. D&E, arrows: Note melanin granule persistence (C) and extrusion 1 hr after TMG applications to SKH2/J, but not SKH1 mouse skin. C&D, osmium tetroxide post fixation. Mag bars = 1 μm.
The Reduced pH of the Lower SC in Pigmented Skin Correlates with Persistence and Extrusion of Melanin Granules
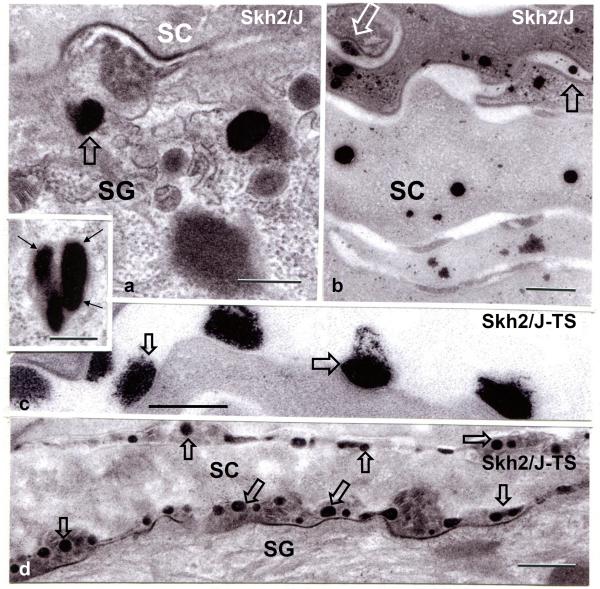
Melanosomes, with large, single melanin granules, persist within putative phagolysosomes into the outer epidermis of darkly-pigmented humans (Szabo, et al., 1969, Quevedo, et al., 1975, Boissy, 2003). Likewise, intact large granule-containing membrane-bound organelles also persist within the outer epidermis and corneocyte cytosol of SKH2/J mice, where they gradually become smaller in size (Figs. 3D, 4A&B). In addition to the persistence of melanin granules within the cytosol of SG and SC cells, some melanin granules are extruded at both the SG-SC interface (Fig. 4A, open arrows), as well as from corneocytes into the SC extracellular domains even under basal conditions (Fig. 4B, open arrows). To examine whether granule persistence and extrusion also correlate with the reduced pH of the SC in darkly- vs. lightly-pigmented humans, we next assessed human skin biopsies (n=4 each), archived during our prior studies (Gunathilake, et al., 2009). As in SKH2/J mouse epidermis, melanin granules persisted into the outer epidermis of darkly-pigmented humans, accompanied by occasional extrusion of these granules into the SC extracellular domains (Suppl. Fig. 2A&B, open arrows).
Figure 4. Pigment Granule Persistence, with Rapid Extrusion after Barrier Disruption in SKH2/J Mice.
A: Under basal conditions, large pigment granules persist into the SG and SC, where they largely disintegrate within the corneocyte cytosol (B). Under basal conditions, some granules appear to be extruded into the extracellular spaces (B, open arrows). A, insert: pigment granules appear enclosed within membrane bound organelles (likely phagolysosomes) in the outer nucleated cell layers of the SKH2/J epidermis. Shortly after acute barrier disruption by tape stripping (SKH2/J TS), pigment granules are extruded at the SG–SC interface, and also within the SC extracellular spaces in SKH2/J mice (C&D, open arrows). Osmium tetroxide post-fixation. Mag bars, A-C + insert: 0.25 μm, D: 0.5 μm.
Acute barrier disruption induces an abrupt increase in the pH of the SC that returns towards baseline over several hours (Mauro, et al., 1998). In order to ascertain whether the reduced pH of darkly-pigmented skin is linked to melanin granule extrusion, we next assessed changes in melanin granule extrusion in SKH2/J mice after acute barrier disruption. By one hour after tape stripping, almost all melanin granules had been extruded from the cytosol of the outermost granular (SG) cells into the SG-SC interface (Figs. 4C&D, open arrows). While this procedure results in an abrupt increase in pH, which returned towards baseline over three hours, the initial decline towards normal was much more robust in SKH2/J mice (Fig. 3B), correlating with pigment extrusion at that same time point (c.f., Figs. 4C&D).
As a further test of the hypothesis that melanin granule extrusion is linked to SC acidification, we next assessed changes in granule extrusion, as well as the kinetics of re-acidification in SKH1 and SKH2/J mice after an experimental increase in SC pH, induced by a single, topical application of the `super-base,' tetramethylguanidine (TMG). Our prior studies showed that such TMG applications raise the pH across the entire SC in a dose- and time-dependent fashion, without evidence of toxicity (Hachem, et al., 2003). This maneuver stimulated more rapid re-acidification of SC pH in SKH2/J than in SKH1 mice, which was again most evident at the one hour time point (Fig. 3C), correlating with the abrupt release of melanin granules into the SG-SC interface (Fig. 3E), comparable to the abrupt release of melanin after tape stripping (c.f., Figs. 4C&D). Together, these results show that melanin granule persistence, as well as inducible granule extrusion into the extracellular spaces of the outer epidermis, likely contributes to the maintenance and the rapid restoration of an optimal pH in pigmented skin.
Pigmentation-Associated Acidification Enhances Barrier Function By Accelerating Lipid Processing and Lamellar Bilayer Maturation
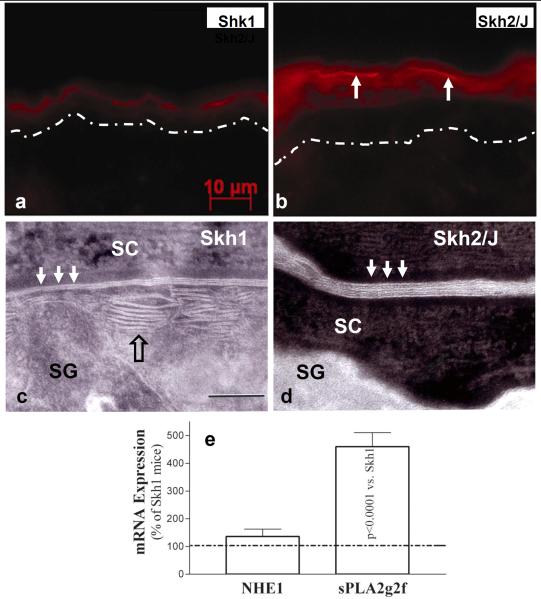
We next assessed which downstream mechanisms could account for the acidification-induced enhancement of barrier function in SKH2/J mice. The activities of the two, acidic pH-dependent, ceramide-generating enzymes, β-glucocerebrosidase (β-GlcCer'ase) and acidic sphingomyelinase (aSMase), were both greater in the outer epidermis of SKH2/J than in SKH1 mouse epidermis, as assessed by in situ zymography (Figs. 5B vs. A; aSMase not shown). Moreover, enhanced enzyme activity localized to the SG-SC junction and lower SC of SKH2/J epidermis (Fig. 5B, arrows), the same sites that are preferentially acidified in SKH2/J mice (c.f., Fig. 2). Moreover, the increased activities of these two enzymes in SKH2/J epidermis correlated with ultrastructural evidence of accelerated processing of newly-secreted lamellar body contents into mature lamellar bilayers (Figs. 5D vs. C).
Figure 5. Enhanced Activity of Ceramide-Generating Enzymes and Secretory Phospholipase A2f Correlates with Accelerated Post-Secretory Maturation of Lamellar Membranes in SKH2/J Mice.
Activities of β-glucocerebrosidase in SKH1 (A) and SKH2/J mouse epidermis (B), revealed by in situ zymography. Dotted line in A = basement membrane zone (B, note activity at SG SC interface). C: SKH1 epidermis reveals partial maturation of secreted lamellar material (open arrow) into mature lamellar bilayers (solid arrows), while SKH2/J epidermis reveals an accelerated metamorphosis of secreted lamellar material into mature lamellar bilayers (D, solid arrows). C&D: ruthenium tetroxide post fixation. E: Enhanced mRNA levels of two key endogenous acidifying mechanisms in SKH2/J epidermis. Mag bars – A&B: 10 μm; C+D: 0.5 μm.
Finally, we assessed whether other endogenous acidifying mechanisms could differ in SKH2/J vs. SKH1 epidermis. Our prior studies showed that the sodium-protein-exchanger, NHE1, and secretory phospholipase A2 account for over one pH unit of the skin's acidic surface (Man, et al., 1996, Behne, et al., 2001). mRNA levels of sPLA2f, the principal acidifying sPLA2 isoform in the outer epidermis (Ilic, et al., 2013), were almost five-fold higher in SKH2/J than in SKH1 epidermis, while NHE1 levels did not change (Fig. 5E). Together, these results show that melanin persistence/extrusion into the extracellular spaces, and enhanced expression of the acidifying enzyme, sPLA2f, likely contribute to the pH-dependent acceleration of lipid processing, lamellar bilayer maturation, and enhanced barrier function of pigmented epidermis.
Pigmented Keratinocytes Display Enhanced Barrier Function in Organotypic Cultures
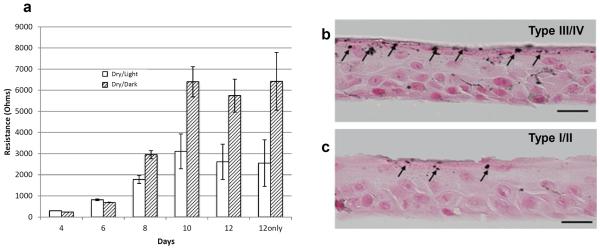
The experiments above, performed on whole skin, do not eliminate possible paracrine influences on epidermal function. To assess directly the impact of pigment within keratinocytes, we next compared the kinetics of barrier formation, assessed as changes in transepithelial electrical resistance (TER) in organotypic cultures of human keratinocytes, grown at an air-medium interface at a low relative humidity. Second-passage keratinocytes, generated from five darkly-pigmented (type III/IV Fitzpatrick scale) foreskins, displayed superior barrier function, measured as changes in TER over time, in comparison to keratinocytes derived from lightly-pigmented human foreskins (type I/II) (Fig. 6A). This finding correlated with the persistence of melanin granules in darkly-pigmented, but not lightly-pigmented, keratinocytes (Fig. 6B&C). Together, these results suggest that dark pigment within keratinocytes could enhance barrier function in a juxtacrine fashion, independent of possible paracrine influences from melanocytes.
Figure 6. Pigmented Keratinocytes in Organotypic Cultures Rapidly Establish a Superior Barrier.
A: Changes in barrier function over time was assessed by trans epithelial electrical resistance measurements. B&C: Melanin persists into the stratum corneum in pigmented keratinocytes in organotypic cultures. Fontana Masson stain (Bars=20 μm).
DISCUSSION
Because the epidermis of our furred, great ape ancestors was lightly pigmented, it is safe to assume that interfollicular pigmentation developed following the loss of most body hairs as hominids emerged from forest cover into open savannahs 2–3 million years ago (mya) (Jablonski, 2006, Westerhof, 2007). It is widely assumed that epidermal pigmentation developed to protect the epidermis from UV-B-induced carcinogenesis (Young and Sheehan, 2001, Goding, 2007, Lin and Fisher, 2007, Miyamura, et al., 2007, Brenner and Hearing, 2008, Schallreuter, et al., 2008). But epidermal pigmentation can provide camouflage (Mueller and Neuhauss, 2014); could protect against folic acid degradation (Jablonski, 1999); and likely is critical for thermal insulation in northern-dwelling mammals, such as polar bears (Elias and Williams, 2014). We proposed recently that pigmentation could have developed instead to protect the epidermis from UV-B-induced damage to the permeability barrier, as well as to defend against excessive water loss into the extremely arid environment of sub-Saharan Africa (Elias, et al., 2009, Elias, et al., 2010, Elias and Williams, 2013). Indeed, erythemogenic UV-B produces dose-dependent abnormalities in permeability barrier function (Haratake, et al., 1997, Holleran, et al., 1997), while sub-erythemogenic doses instead benefit barrier function (Hong, et al., 2008). In the highly arid, UV-B saturated environment of sub-Saharan Africa, a competent barrier would become the pre-eminent water conservation strategy, required for hominids to inhabit open savannahs, particularly because accelerated water loss from eccrine sweating in service to thermoregulation, would have placed still additional stress on the permeability barrier (Elias and Williams, 2013). Thus, the development of interfollicular pigmentation likely shifted the UV-B dose-response curve from the toxic to the beneficial (Elias and Williams, 2013).
In support of the pigment-barrier evolutionary hypothesis, darkly-pigmented humans display superior barrier function in comparison to light-pigmented humans, independent of ethnicity, gender, geographic location, or occupation (Reed, et al., 1995, Gunathilake, et al., 2009). Pertinently, the depigmented macules of stable, non-inflamed vitiligo display inferior barrier function in comparison to adjacent, pigmented skin sites of the same subjects (Liu, et al., 2010). In the current study, we compared barrier function in two closely-related, pigmented vs. non-pigmented hairless mouse strains, demonstrating again the functional superiority of pigmented skin. Moreover, as in lightly-pigmented humans (Gunathilake, et al., 2009, Hachem, et al., 2010), experimental reductions in the pH of the SC of SKH1 mice `reset' barrier function to levels comparable to those in SKH2/J mice. Using these two models, we then explored possible cellular and metabolic mechanisms that could illuminate how pigmentation enhances barrier function.
Because a lower pH is known to confer multiple advantages for barrier function (Mauro, et al., 1998, Fluhr and Elias, 2002, Hachem, et al., 2003), we also proposed previously that the functional superiority of darkly-pigmented epidermis could be due to its distinctly lower pH (Gunathilake, et al., 2009). Although the surface pH of the SC does not differ in SKH2/J vs. SKH1 hairless mice, the pH of extracellular compartments in the lower SC of SKH2/J mice is lower than is the pH of comparable sites in SKH1 mice, demonstrable by dual photon confocal microscopy. The question then becomes – how does pigmentation influence epidermal acidification? In prior studies, we provided evidence that the lower pH of darkly-pigmented human skin could be linked to the apparent transfer of more acidic melanosomes to keratinocytes (Gunathilake, et al., 2009). Moreover, after secretion and uptake into adjacent keratinocytes, the larger, single granule-containing phagolysosomes in darkly-pigmented epidermis persist much higher in the epidermis than do the `crumbly' melanin granules of lightly-pigmented humans (Szabo, et al., 1969, Pathak, 1995, Boissy, 2003). Accordingly, we showed here that following their transfer to keratinocytes, melanin granules persist into the outer epidermis of SKH2/J mice (and darkly-pigmented humans), slowly decomposing within the SC. It therefore seems safe to assume that the larger, heavily melanized granules of darkly-pigmented human skin are more resistant to intracellular hydrolysis than their smaller, multigranular equivalents in lightly-pigmented epidermis, likely because they present a smaller net surface area to endogenous, cytosolic proteases, and/or they are less susceptible to autophagy (Murase, et al., 2013).
Though pre-melanosomes and melanosomes possess highly-acidic contents (Moellmann, et al., 1988, Ancans, et al., 2001, Chen, et al., 2002, Schallreuter, et al., 2008), their contents approach neutrality immediately prior to secretion (Ancans, et al., 2001), an antecedent that is apparently necessary for pigment transfer (Seiberg, 2001, Boissy, 2003). Melanin granules then are secreted into the extracellular spaces before being phagocytized by neighboring keratinocytes (Ando, et al., 2012). These melanin granule-containing phagolysosomes express lysosomal markers, consistent with the known acidity of these organelles (Nyberg, et al., 1989, Hackam, et al., 1997). The present studies strongly suggest that not only the persistence, but also in part the subsequent extrusion of these acidic, melanin-containing granules from the outer epidermis contribute to the reduced pH of the lower SC of pigmented mouse and human skin, likely through the release of protons from the acidic milieu of phagolysosomes.
We describe several further experiments that provide support for a granule-associated mechanism for SC acidification (Suppl. Fig. 3). First, with dual photon confocal microscopy, we showed that the sites of melanin granule persistence and extrusion, at and just above the SG-SC interface of SKH2/J mice, co-localize with a reduced pH of extracellular domains in the same location. Second, acute barrier disruption, which induces a temporary increase in pH (Mauro, et al., 1998), stimulates a more rapid reduction in the surface pH of SKH2/J than in SKH1 mouse SC, paralleled by more rapid extrusion of granules at the SG-SC interface. Third, we showed that melanin granule release into extracellular domains, and an accelerated normalization of pH, occur in SKH2/J mice in response to experimental elevations of SC pH, achieved with a topical `superbase.' These changes in pigment extrusion and localized acidification likely account, at least in part, for enhanced permeability barrier function in SKH2/J mice. Notably, this previously-unreported, melanin-extruding phenomenon was also readily evident in multiple archival samples of darkly-pigmented (but not lightly-pigmented) human skin, and it therefore likely also accounts, at least in part, for the superior barrier function of darkly-pigmented human SC (Reed, et al., 1995, Gunathilake, et al., 2009). Yet, whether melanin granule extrusion is a regulated secretory process, akin to lamellar body secretion at the SG-SC interface (Menon, et al., 1992, Man, et al., 1995, Elias, et al., 1998), remains to be determined. Finally, we performed studies in organotypic cultures, derived from keratinocytes with and without the presence of pigment. These studies show that pigment-bearing keratinocytes generate a more competent barrier than the barrier formed by keratinocytes contains a paucity of pigment. Yet, keratinocytes derived from dark and light skins differ in numerous pathways and their non-identical genetic composition could be the reason for the barrier-differential results, regardless of the pigmentary contribution. The definite study would be to create cultures from same keratinocyte donor(s), with and without the addition of melanocytes or melanosomes. Nonetheless, while melanocytes likely also influence epidermal function by paracrine mechanisms (see below), these studies show that pigmentation of keratinocytes alone enhances epidermal function.
Together, these observations provide strong support for the concept that melanin granule persistence and extrusion comprise yet another endogenous acidifying mechanism in addition to phospholipid hydrolysis into free fatty acids (Man, et al., 1995), the sodium-protein exchange mechanism (NHE1) (Behne, et al., 2001), and likely the deimination of filaggrin-derived amino acids into polycarboxylic acids (Krien and Kermici, 2000, Fluhr, et al., 2010, Gruber, et al., 2011). Pertinently, at least one of these acidifying mechanisms; i.e., the sPLA2 isoform, sPLA2f, previously shown to be regulated by barrier requirements (Ilic, et al., 2013), is amplified in SKH2/J epidermis, likely providing a further, feed-forward mechanism that contributes to the lower pH of pigmented SC (Suppl. Fig. 3). The resultant downstream reduction in pH due not only to melanin persistence and extrusion, as well as increased sPLA2f expression likely contribute to enhanced barrier function through stimulation of the activities of two key, ceramide-generating enzymes, β-GlcCer'ase and aSMase (Gunathilake, et al., 2009), which both display acidic pH optima (Takagi, et al., 1999). We show here a similar increase in the activities of these enzymes in SKH2/J vs. SKH1 mouse epidermis, which correlated further with ultrastructural evidence of accelerated maturation of extracellular lamellar bilayers. Together, these studies identify both a `juxtacrine' mechanism (melanin pigment persistence and extrusion), as well as a likely paracrine acidifying mechanism; i.e., enhanced sPLA2f expression, that contribute to the reduced pH and superior barrier function of pigmented epidermis.
Though juxtacrine mechanisms clearly contribute to enhanced barrier function in pigmented skin, it is likely that pigmented melanocytes exert additional paracrine influences on barrier function (Slominski, et al., 1993). As noted above, the increase in sPLA2f expression, described here, cannot be attributed to juxtacrine processes. Moreover, in preliminary studies (Man, et al., 2013), we found enhanced epidermal differentiation, lipid content, and antimicrobial peptide expression in SKH2/J mouse epidermis (Man, et al., 2013). These findings would explain the noticeable differences in epidermal thickness in SKH2/J mice (c.f., Fig. 1). However, which secreted factors, or intermediates in the melanin synthetic pathway, stimulate epidermal function in a paracrine fashion remains unknown.
MATERIALS AND METHODS
Animals and Materials
Male and female hairless, non-pigmented mice (Crl:SKH1-Hrrh/Hrrh), hereafter and above-referred to as SKH1 mice, obtained from Charles River Laboratories, Wilmington, MA), and pigmented (SKH2/J-Hrrh/Hrrh) hairless mice from Jackson Laboratory (Bar Harbor, ME), were used in all these studies. All mice were fed a standard mouse diet (Ralston-Purina Co., St Louis, MO, USA), tap water ad libitum, and studied at 6–8 weeks of age. Mice were maintained in a temperature- and humidity-controlled, pathogen free (SPF) facility. Affinity-purified, rabbit anti-mouse antibodies to filaggrin, involucrin, and loricrin were purchased from Covance/BabCo (Richmond, CA). HMB-45 antibody was from Dako (Carpinteria, CA), MEL-5 antibody was from Covance (Princeton, NJ). Secondary biotinylated, goat anti-rabbit IgG and ABC-peroxidase kit were purchased from Vector laboratories (Burlingame, CA, USA). Biotinylated anti-PCNA antibody was from CalTag Laboratories (Burlingame, CA, USA). ABC-peroxidase kit was purchased from Vector Laboratories (Burlingame, CA, USA). For melanin localization, we utilized a Fontana-Masson staining kit from American Master Tech (Lodi, CA).
Experimental protocols and functional studies
All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. Baseline transepidermal water loss (TEWL), stratum corneum hydration and skin surface pH were measured with respective probes (TM300, Corneometer CM825 and pH905), attached to a Courage-Khazaka MPA5 system under controlled atmospheric conditions (25° C; 60% relative humidity). For barrier recovery studies, TEWL was measured with an electrolytic water analyzer (Meeco, Warrington, PA) before and immediately after (0 time), then 2 and 4 hours after sequential D-squame tape stripping, until TEWL increased ≥10-fold. Percent barrier recovery rates were calculated as described previously (Hachem, et al., 2003). In some experiments, both flanks of 6–8 week old SKH1 or SKH2/J mice were treated topically with 60 μl of either 1:100 1,8-diazabicyclo [5,4,0] undec-7-ene (DBU) or 0.065%, 1,1,3,3-tetramethylguanidine (TMG) in propylene glycol:ethanol (7:3, v/v). One hour after DBU or TMG applications, the residual DBU or TMG was removed from the skin source, and skin surface pH was measured with a MPA5 skin physiology monitor (Courage & Khazaka, Cologne, Germany), immediately before treatments, 1, 3, and 4 hrs after DBU or TMC applications. Data were expressed as % changes over pretreatment.
In Situ Zymography
Skin biopsies from both SKH1 and SKH2/J mice were snap frozen, and then frozen sectioned (5 μm) prior to washing with a solution containing 0.025% Tween 20 three times followed by incubation with a fluorescent substrate solution containing 5 mM D-glucopyranoside for 2 hrs at 37°C to visualize and localize changes in β-glucocerebrosidase activity, as described previously (Hachem, et al., 2006). Sections were examined with a Zeiss microscope (Jena, Germany), and digital images were captured with AxioVision 2.05 software (Carl Zeiss Vision, Munich, Germany).
Electron Microscopy
Skin biopsies were obtained from SKH1 and SKH2/J mice, before and at various time points after tape stripping, fixed in Karnovsky's fixative overnight, followed by post-fixation in either 0.25% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide (Hou, et al., 1991). Ultrathin sections were examined using an electron microscope (Zeiss 10A, Carl Zeiss, Thornwood, NY) operated at 60 kV. Changes in lamellar body density and secretion, as well as in lamellar membrane maturation and melanin granule extrusion were assessed visually in coded micrographs obtained from randomly photographed sites in multiple samples (≥3 each).
Statistical Analyses
Data are expressed as the means ± SEM. Unpaired two-tailed student t test with Welch's correction was used to determine significant differences when two groups were compared.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AG028492 and AR019098, administered by the Northern California Institute for Research and Education, with additional resources provided by the Veterans Affairs Medical Center, San Francisco, CA. This content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or the Department of Veterans Affairs.
Abbreviations
- aSMase
acidic sphingomyelinase
- β-GlcCer'ase
β-glucocerebrosidase
- DAPI
4,6-diamidino-2-phenylindole
- DCT
dopachrome tautomerase
- Hrrh
hairless mice gene
- KC
keratinocyte
- LBA
lactobionic acid
- LSC
lower stratum corneum
- MYA
million years ago
- OSC
outer stratum corneum
- SC
stratum corneum
- SG
stratum granulosum
- sPLA2f
secretory phospholipase A2f
- TER
transepithelial electrical resistance
- TEWL
transepidermal water loss
- TMG
tetramethylguanidine
- TYR
tyrosinase
- TYRP
tyrosinase related protein
- UV-B
ultraviolet-B.
Footnotes
Conflict of Interest Statement: None of the authors have conflict of interest with regard to the contents or subject of this paper.
Please see Supplemental Materials for Quantification of mRNA levels, Primary melanocyte cultures, Cell and organotypic culture, TER Measurements, Quantitation and location of acidic microdomains.
References
- Ancans J, Tobin DJ, Hoogduijn MJ, et al. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001;268:26–35. doi: 10.1006/excr.2001.5251. [DOI] [PubMed] [Google Scholar]
- Ando H, Niki Y, Ito M, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012;132:1222–9. doi: 10.1038/jid.2011.413. [DOI] [PubMed] [Google Scholar]
- Behne M, Elias PM, Mauro T. The antiporter NHE1 influences the function of the SC pH gradient. J Skin Barrier Res. 2001;3:3–10. [Google Scholar]
- Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol. 2003;12(Suppl 2):5–12. doi: 10.1034/j.1600-0625.12.s2.1.x. [DOI] [PubMed] [Google Scholar]
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–49. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13:1953–64. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Cullander C, Mauro T, et al. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Invest Dermatol Symp Proc. 1998;3:87–100. doi: 10.1038/jidsymp.1998.20. [DOI] [PubMed] [Google Scholar]
- Elias PM, Menon G, Wetzel BK, et al. Evidence that stress to the epidermal barrier influenced the development of pigmentation in humans. Pigment Cell Melanoma Res. 2009;22:420–34. doi: 10.1111/j.1755-148X.2009.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Menon G, Wetzel BK, et al. Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. Am J Hum Biol. 2010;22:526–37. doi: 10.1002/ajhb.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Williams ML. Re-appraisal of current theories for the development and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687–92. doi: 10.1016/j.jhevol.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Williams ML. Encylopedia of Human Biology. Elsevier; 2014. Evolution: Skin Color. In Press. [Google Scholar]
- Fluhr JW, Elias PM. Stratum corneum pH: Formation and function of the `acid mantle'. Exog Dermatol. 2002;1:163–175. [Google Scholar]
- Fluhr JW, Elias PM, Man MQ, et al. Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification? J Invest Dermatol. 2010;130:2141–4. doi: 10.1038/jid.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding CR. Melanocytes: the new Black. Int J Biochem Cell Biol. 2007;39:275–9. doi: 10.1016/j.biocel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Gruber R, Elias PM, Crumrine D, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252–63. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunathilake R, Schurer NY, Shoo BA, et al. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol. 2009;129:1719–29. doi: 10.1038/jid.2008.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–53. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Roelandt T, Schurer N, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J Invest Dermatol. 2010;130:500–10. doi: 10.1038/jid.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Wagberg F, Schmuth M, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609–21. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Zhang WJ, et al. Regulation of phagosomal acidification. Differential targeting of Na+/H+ exchangers, Na+/K+-ATPases, and vacuolar-type H+-atpases. J Biol Chem. 1997;272:29810–20. doi: 10.1074/jbc.272.47.29810. [DOI] [PubMed] [Google Scholar]
- Haratake A, Uchida Y, Schmuth M, et al. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997;108:769–75. doi: 10.1111/1523-1747.ep12292163. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Uchida Y, Halkier-Sorensen L, et al. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 1997;13:117–28. doi: 10.1111/j.1600-0781.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Hong SP, Kim MJ, Jung MY, et al. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880–7. doi: 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- Hou SY, Mitra AK, White SH, et al. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991;96:215–23. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Ilic D, Bollinger JM, Gelb M, et al. sPLA2 and the epidermal barrier. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581–2. doi: 10.1054/mehy.1997.0697. [DOI] [PubMed] [Google Scholar]
- Jablonski NG. Skin: a natural history. 1. University of California Press; 2006. p. 281. [Google Scholar]
- Krien P, Kermici M. Evidence for the existence of a self-regulated enzymatic process within human stratum corneum-an unexpected role for urocanic acid. J Invest Dermatol. 2000;115:414–420. doi: 10.1046/j.1523-1747.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Liu J, Man WY, Lv CZ, et al. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol Physiol. 2010;23:193–200. doi: 10.1159/000288166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MQ, Feingold KR, Jain M, et al. Extracellular processing of phospholipids is required for permeability barrier homeostasis. J Lipid Res. 1995;36:1925–35. [PubMed] [Google Scholar]
- Man MQ, Jain M, Feingold KR, et al. Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J Invest Dermatol. 1996;106:57–63. doi: 10.1111/1523-1747.ep12327246. [DOI] [PubMed] [Google Scholar]
- Man MQ, Lin T, Santiago J, et al. IID 2013. Edinburgh; Scotland: 2013. Basis for pigmentation-enhanced barrier function. [Google Scholar]
- Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290:215–22. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992;98:279–89. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Moellmann G, Slominski A, Kuklinska E, et al. Regulation of melanogenesis in melanocytes. Pigment Cell Res. 1988;1:79–87. doi: 10.1111/j.1600-0749.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Mueller KP, Neuhauss SCF. Sunscreen for fish: co-option of UV light protection for camouflage. PLoS ONE. 2014;9:e87372. doi: 10.1371/journal.pone.0087372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase D, Hachiya A, Takano K, et al. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J Invest Dermatol. 2013;133:2416–24. doi: 10.1038/jid.2013.165. [DOI] [PubMed] [Google Scholar]
- Nyberg K, Johansson U, Rundquist I, et al. Estimation of pH in individual alveolar macrophage phagolysosomes. Exp Lung Res. 1989;15:499–510. doi: 10.3109/01902148909069614. [DOI] [PubMed] [Google Scholar]
- Pathak M. Functions of melanin and protection by melanin. In: Zeise L, Chedekel M, Fitzpatrick T, editors. Melanin: its role in human photoprotection. Valdenmar; Washington, DC: 1995. pp. 125–134. [Google Scholar]
- Quevedo WC, Fitzpatrick TB, Pathak MA, et al. Role of light in human skin color variation. Am J Phys Anthropol. 1975;43:393–408. doi: 10.1002/ajpa.1330430321. [DOI] [PubMed] [Google Scholar]
- Reed JT, Ghadially R, Elias PM. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch Dermatol. 1995;131:1134–8. [PubMed] [Google Scholar]
- Schallreuter KU, Kothari S, Chavan B, et al. Regulation of melanogenesis--controversies and new concepts. Exp Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001;14:236–42. doi: 10.1034/j.1600-0749.2001.140402.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Are l-tyrosine and l-dopa hormone-like bioregulators? J Theor Biol. 1990;143:123–138. doi: 10.1016/s0022-5193(05)80292-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–20. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- Szabo G, Gerald AB, Pathak MA, et al. Racial differences in the fate of melanosomes in human epidermis. Nature. 1969;222:1081–2. doi: 10.1038/2221081a0. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kriehuber E, Imokawa G, et al. Beta-glucocerebrosidase activity in mammalian stratum corneum. J Lipid Res. 1999;40:861–9. [PubMed] [Google Scholar]
- Westerhof W. Evolutionary, biologic, and social aspects of skin color. Dermatol Clin. 2007;25:293–302. vii. doi: 10.1016/j.det.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Young A, Sheehan J. UV-induced pigmentation in human skin. In: Giacomoni P, editor. Sun Protection in Man. Elsevier Science; Amsterdam: 2001. pp. 357–375. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.