Abstract
Increased vascular sensitivity to Ang II is a marker of a hypertensive human pregnancy. Recent evidence of interactions between the RAS and the endocannabinoid system (ECS) suggests that anandamide (AEA) and 2-arachidonoylglycerol (2-AG) may modulate Ang II contraction. We hypothesized that these interactions may contribute to the enhanced vascular responses in hypertensive pregnancy. We studied Ang II contraction in isolated uterine artery (UA) at early gestation in a rat model that mimics many features of preeclampsia, the transgenic hAGN×hREN (TgA), and control Sprague-Dawley (SD) rats. We determined the role of the cannabinoid receptor CB1 by blockade with SR171416A, and the contribution of AEA and 2-AG degradation to Ang II contraction by inhibiting their hydrolyzing enzymes FAAH (with URB597) or MAGL (with JZL184) respectively. TgA UA showed increased maximal contraction and sensitivity to Ang II that was inhibited by indomethacin. FAAH blockade decreased Ang IIMAX in SD UA, and decreased both Ang IIMAX and sensitivity in TgA UA. MAGL blockade had no effect on SD UA and decreased Ang IIMAX and sensitivity in TgA UA. Blockade of the CB1 receptor in TgA UA had no effect. Immunolocalization of FAAH and MAGL showed a similar pattern between groups; FAAH predominantly localized in endothelium and MAGL in smooth muscle cells. We demonstrated an increased Ang II contraction in TgA UA before initiation of the hypertensive phenotype. AEA and 2-AG reduced Ang II contraction in a CB1-independent manner. These RAS-ECS interactions may contribute to the enhanced vascular reactivity in early stages of hypertensive pregnancy.
Keywords: hypertensive pregnancy, endocannabinoids, Ang II, FAAH, MAGL
Introduction
Preeclampsia is a common disorder of pregnancy that manifests with hypertension and proteinuria. The renin angiotensin system (RAS) plays an important role in the normal and pathological regulation of the female reproductive system 1 and an increased response to activation of the RAS is characteristic of pregnancies at risk of developing preeclampsia. Thus, since the pioneering work of Gant et al, an increased sensitivity to angiotensin II (Ang II), one of the main agonists of the RAS, was early recognized as a marker for the development of a hypertensive pregnancy2-4.
The transgenic female rat containing the human angiotensinogen (hAGN) gene mated with the male transgenic containing human renin (hREN), the hAGTxhREN rat (TgA) mimics many features of human preeclampsia. This model shows increased blood pressure, proteinuria, and placenta alterations of edema and necrosis in the last half of gestation5. Mean blood pressure increases abruptly approximately 10 days before delivery reaching values of 160±10mmHg 5. Among the vascular effects observed in this model, a prostanoid-mediated endothelial dysfunction of the uterine artery has been described at late gestation 6, 7.
The endocannabinoid system (ECS) is composed of mediators such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), receptors (CB1, CB2 and non-CB1/CB2) and enzymes in charge of synthesis or hydrolysis of these mediators: i.e. fatty acid amide hydrolase (FAAH) for hydrolysis of AEA and monoacylglycerol lipase (MAGL) for 2-AG 8. Thus, AEA and 2-AG act as endogenous ligands for cannabinoid receptors. Endocannabinoids possess vasoactive, mitogenic, and differentiating properties, and are implicated in placentation 9 and in several pregnancy disorders including preeclampsia 10. Endocannabinoids participate in the regulation of angiogenesis during implantation and decidualization 11, control myometrial contractility 12, and regulate uterine and umbilical blood flow 13. All these events are determinants of a successful pregnancy and may have devastating consequences when altered 14.
Once generated, the actions of AEA and 2-AG are terminated by specific reuptake 15 and subsequent degradation by either FAAH 16 or MAGL 17, respectively. The ability to manipulate in vivo endocannabinoid levels by blocking their degradation makes these enzymes attractive therapeutic targets for several pathologies where AEA and 2-AG are involved 18-20, hence emphasizing the relevance of studying FAAH and MAGL blockers 21. Notably, the localization and expression pattern of FAAH in the blastocyst suggests a role for this enzyme in limiting AEA levels as a protection for a successful implantation 22. Data also suggest that these metabolizing enzymes regulate endocannabinoid levels in maternal tissues during pregnancy 23.
Recent evidence of interactions between the RAS and ECS suggests a regulatory role for endocannabinoids in modulating Ang II vascular responses. Thus, the contraction to Ang II in mice gracilis arteries is increased by blockade of the CB1 receptor suggesting a vasodilatory role of endocannabinoids modulating contraction to Ang II in arteries from the systemic circulation 24.
In this study, we compared the Ang II-mediated contraction of isolated uterine artery from TgA and SD rats at early gestation and the effects of blocking AEA and 2-AG hydrolysis, by inhibiting FAAH or MAGL. We hypothesize that prostanoids have a greater impact in modulating Ang II contractions in preeclamptic rats and that blocking endocannabinoid degradation antagonizes Ang II contraction.
Material and Methods
Animals
Female hAGN transgenic rats, when mated with a hREN transgenic male (hAGN×hREN), develop hypertension and proteinuria in the second half of pregnancy 5. Seven days pregnant preeclamptic hAGN×hREN (TgA, n=9) and 7 days pregnant Sprague-Dawley (SD, n=6) rats were used. All experiments were performed in accordance with the guidelines of the Wake Forest School of Medicine Institutional Animal Care and Use Committee. (See online-only Supplemental Data for details).
Vascular Reactivity Experiments
Segments of the main uterine artery (UA), maximum of 2 mm in length, were mounted between an isometric force transducer (Kistler Morce DSC 6, Seattle, WA, USA) and a displacement device on a myograph (Multi Myograph, Model 620M Danish Myo Technologies, Aarhus, Denmark) using two stainless steel wires (diameter 40 μm), as described previously25. In a subgroup of arteries from the control and TgA rats the endothelium was destroyed by passing a human hair through the lumen 26. (See online-only Supplemental Data for details).
Response to Potassium Chloride (KCl)
After equilibration, in order to test the viability of the arterial preparations and determine the response to non-receptor mediated contraction, UA segments were exposed to 75 mmol/L KCl in KHB during 5 min, after washing the incubation was repeated twice. Contraction measured at the third incubation was recorded as maximal contraction to KCl (KMAX).
Response to Ang II
After washing and resting for 30 min, UA segments were exposed to a cumulative concentration-response curve of Ang II by exposing arteries to eleven (10-11–10-8M) increasing concentrations in fourth-log steps, with each subsequent dose being introduced only after a steady response had been reached (2 min). Since at higher concentrations of ligand the Ang II contraction decreased as a result of desensitization, only doses as high as 10 nmol/L were used. In parallel experiments, different arterial segments were denuded or pre-incubated for 15 min with the cyclooxygenase inhibitor indomethacin (10-5 mol/L) or the nitric oxide synthase inhibitor L-NAME (10-4 mol/L). Additional arterial segments were pre-incubated for 15 min with the FAAH blocker URB597 at 1x10-6 mol/L or the MAGL blocker JZL184 at 1x10-6 mol/L. Some TgA UA were pre-incubated with the CB1 receptor blocker SR141716A at 1x10-6 mol/L or SR141716A at 1x10-6 mol/L plus URB597 at 1x10-6 mol/L.
Immunohistochemistry
Expression of FAAH and MAGL in UA was detected by immunostaining using commercial antibodies. For details see online-only Suplemental Data. Images were acquired at 400 × magnifications using a Leica DM 4000B upright microscope (Leica Microsystems, Bannockburn, IL, USA). Illumination settings were held constant for image capture sessions (Retiga 1300R CCD Digital camera, QImaging, Surrey, BC, Canada; SimplePCI v6 software Cranberry Twp., PA). Regions of interest (ROI) were defined using the open source Fiji software (ImageJ, NIH) (http://fiji.sc/Fiji) covering smooth muscle and endothelial layers in each arterial segment. Intensity of the staining in five ROI's per segment was quantified following the reciprocal intensity method 27.
Data Analysis
All data analysis was performed using GraphPad Prism v5 statistical analysis package (GraphPad Software Inc., La Jolla, CA). See online-only Suplemental Data for details. Data are expressed as mean ± SEM. One-way ANOVA with Bonferroni's multiple comparisons was used to determine significant differences. A p< 0.05 was accepted as an indication of statistical significance.
Results
Blood pressure in TgA transgenic rats at early gestation
Mean blood pressure values were not different between SD (n=6) and TgA (n=7) animals at 7 days of gestational age (98.8±2 vs. 97±3 mmHg, p>0.05).
Contractile response to Ang II in UA at early gestation in control rats
Optimal diameters of the isolated UA segments used in these studies were not different between control and TgA (339±13 vs. 317±4 μm, p>0.05). Maximal response to KCl was increased in TgA compared to control UA (4.6±0.5 vs. 3.3±0.3 mN/mm, p<0.05). In control arteries from SD rats Ang II (10-11-10-8 mol/L) elicited a dose-dependent contraction that reached a plateau around 90% of KMAX. Arterial denudation increased maximal Ang II contraction and sensitivity (Figure 1A, Table 1, p<0.05) whereas pre incubation of intact arteries with indomethacin increased Ang II sensitivity (Figure 1A, Table 1, p<0.05). Blockade of NO production with L-NAME in intact arteries increased maximal contraction and sensitivity to Ang II (Figure 1A, Table 1, p<0.05).
Figure 1. Contraction to Ang II in UA from SD and TgA rats at early gestation.
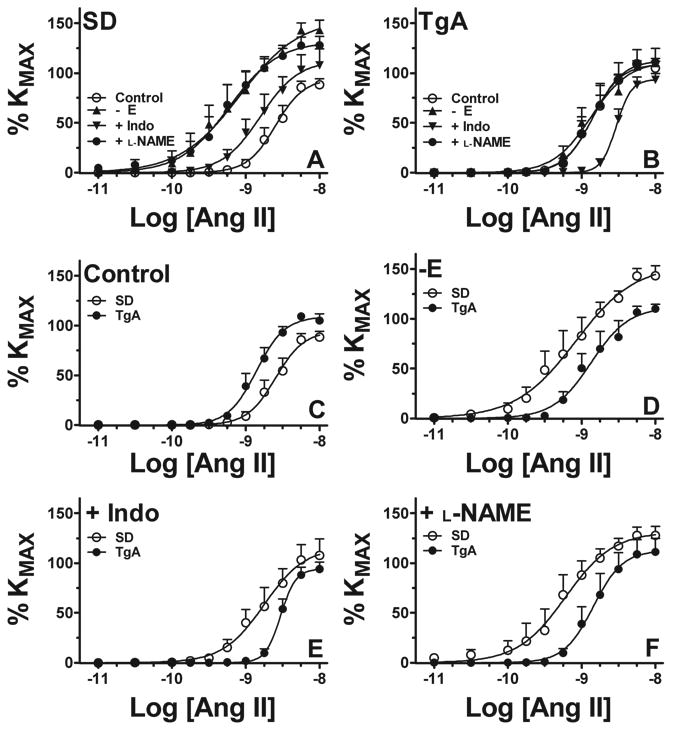
Contraction to Ang II in isolated UA from Sprague-Dawley (SD, A, n=6) and transgenic (TgA, B, n=9) animals. Parallel experiments in intact arteries (C), denuded arteries (-E, D), intact arteries pre-incubated with indomethacin 10-5M (+Indo, E) or L-NAME 10-4M (+L-NAME, F) are shown. See Table 1 for analysis.
Table 1. Contractile responses to angiotensin II (Ang II) in uterine arteries from SD and TgA rats at early gestation.
Maximal response to Ang II (Ang IIMAX) is expressed as % KMAX and sensitivity as pD2. Results are shown for intact, denuded arteries and arteries pre-incubated with indomethacin (10-5 mol/L) or L-NAME (10-4 mol/L) as indicated.
| Variables measured | Intact | Denuded | ||
|---|---|---|---|---|
| SD | TgA | SD | TgA | |
| Ang IIMAX, % KMAX | 91±5 | 109±4* | 150±17† | 108±4* |
| pD2 | 8.60±0.08 | 8.86±0.08* | 9.14±0.2† | 8.87±0.13 |
| + Indomethacin | + L-NAME | |||
| SD | TgA | SD | TgA | |
| Ang IIMAX, % KMAX | 111±15 | 92±7† | 128±8† | 110±13 |
| pD2 | 8.79±0.08† | 8.54±0.03†* | 9.28±0.17† | 8.82±0.01 |
p<0.05 vs SD;
p<0.05 vs intact arteries.
Contractile response to Ang II in TgA UA at early gestation
In uterine arteries from TgA rats the contraction to Ang II was increased compared to SD controls. Maximal response and sensitivity values were higher in TgA UA (Figure 1B, Table 1, p<0.05). In denuded arteries, contraction was similar to intact arteries whereas pre-incubation of intact arteries with indomethacin diminished maximal response and sensitivity as compared to TgA intact. Compared to intact arteries, sensitivity to Ang II in indomethacin-treated arteries was lower in TgA (Figure 1E, Table 1, p<0.05). Blockade of NO production with L-NAME in intact arteries did not alter Ang II contraction in TgA (Figure 1F, Table 1, p<0.05). Ang II contraction in SD and TgA UA was completely abolished by pre-incubation with losartan 10-6 mol/L (data not shown).
Effects of blocking endogenous production of AEA and 2-AG on Ang II contraction
Pre-incubation with the FAAH blocker URB597 10-6 mol/L reduced maximal response to Ang II in SD UA, whereas pre-incubation with the MAGL blocker JZL184 10-6 mol/L had no effect (Figure 2A, B and Table 2). In contrast, both FAAH and MAGL blockade inhibited maximal response and sensitivity to Ang II (Figure 3A, B and Table 2) in TgA UA. The inhibitory effect of blocking FAAH on Ang II in TgA UA was not modified by concomitant blockade of the CB1 receptor with SR141716A 10-6 mol/L. We observed no effect of blocking CB1 receptor alone on Ang II contraction (Figure 4, and Table 2).
Figure 2. Effects of blocking AEA and 2-AG hydrolysis on UA Ang II contraction in SD arteries at early gestation.
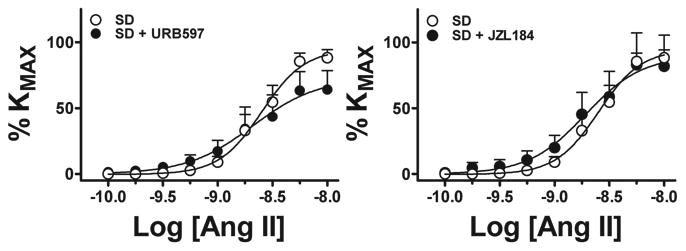
Contraction to Ang II in isolated UA from Sprague-Dawley rats (○, n=6) in the presence of FAAH (URB597 10-6M, •, n=9, A) or MAGL (JZL184 10-6M, •, n=9, B) blockade. See Table 2 for analysis.
Table 2. Effects of FAAH and MAGL blockade on contractile responses to angiotensin II (Ang II) in uterine arteries from SD and TgA rats at early gestation.
Maximal response to Ang II (Ang IIMAX) is expressed as % KMAX and sensitivity as pD2. Results are shown for non-treated arteries (control), FAAH blockade (URB597, 10-6 mol/L), MAGL blockade (JZL184, 10-6 mol/L) in SD UA. In TgA UA results are shown for FAAH blockade (URB597, 10-6 mol/L), MAGL blockade (JZL184, 10-6 mol/L), CB1 receptor blockade (+SR, SR141716A 10-6 mol/L), and concomitant FAAH and CB1 blockade (+URB595+SR).
| Variables measured | SD | ||
|---|---|---|---|
| Control | +URB595 | +JZL184 | |
| Ang IIMAX, % KMAX | 91±5 | 61±12* | 85±20 |
| pD2 | 8.60±0.08 | 8.68±0.14 | 8.68±0.15 |
| TgA | |||
| Control | +URB595 | +JZL184 | |
| Ang IIMAX, % KMAX | 109±4 | 86±8* | 78±10* |
| pD2 | 8.86±0.08 | 8.58±0.06* | 8.65±0.07* |
| +SR | +URB595+SR | ||
| Ang IIMAX, % KMAX | 98±4 | 76±7* | |
| pD2 | 9.01±0.09 | 8.45±0.13* | |
p<0.05 vs control arteries.
Figure 3. Effects of blocking AEA and 2-AG hydrolysis on UA Ang II contraction in transgenic TgA arteries at early gestation.
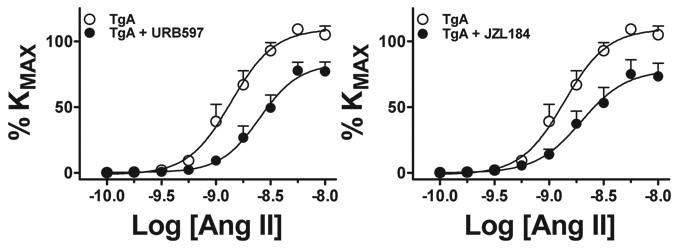
Contraction to Ang II in isolated UA from TgA rats (○, n= 9) in the presence of FAAH (URB597 10-6M, •, n=9, A) or MAGL (JZL184 10-6M, •, n=9, B) blockade. See Table 2 for analysis.
Figure 4. Effects of blocking the CB1 receptor and AEA hydrolysis on UA Ang II contraction in transgenic TgA arteries.
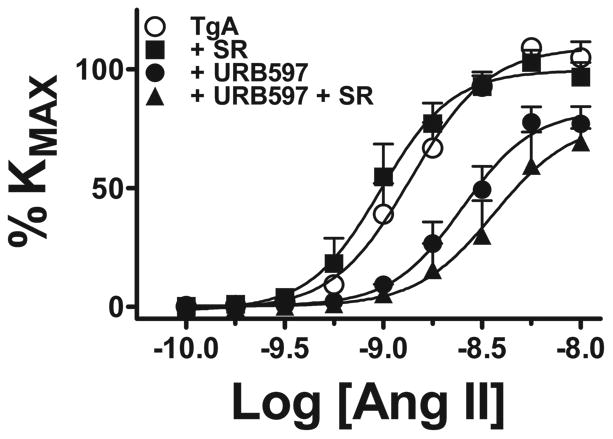
Contraction to Ang II in isolated UA from TgA rats (○, n=9) in the presence of FAAH blockade (URB597 10-6M, •, n=9), CB1 blockade (SR141716A 10-6M (▪, n= 5) or FAAH plus CB1 blockade (▲, n=3). See Table 2 for analysis.
Expression of MAGL and FAAH in control and TgA UA at early gestation
Immunolocalization of FAAH and MAGL revealed the presence of both enzymes in UA. FAAH was localized predominantly in the endothelium (Figure 4B and E), whereas MAGL was detected predominantly in smooth muscle cells (Figure 4C and F). A similar intensity of FAAH and MAGL signals was observed in both control and TgA arteries with stronger staining for MAGL than FAAH in both groups. (Supplemental Figure S1).
Discussion
For the first time we report that the transgenic hAGTxhREN rat, a rodent model that mimics many features of preeclampsia, displayed increased contraction to Ang II in the uterine artery at early gestation, before the onset of blood pressure rise. Herein, we also present additional novel findings of functional interactions between RAS and ECS in the uterine vasculature, with an increased role for the endocannabinoids AEA and 2-AG in reducing Ang II contraction in this preeclamptic model.
Increased vascular sensitivity to Ang II was reported in human pregnancies with an increased risk of developing hypertension2-4. We observed this effect at early gestation in the TgA rat before the hypertensive phenotype is established. Since increased sensitivity to Ang II constitutes a hallmark for the development of a hypertensive pregnancy our results contribute to the characterization of the hAGTxhREN rat as a suitable model of preeclampsia. The presence of agonistic autoantibodies to the AT1 receptor (AT1-AA) is another characteristic of human preeclampsia that this model replicates 28. AT1-AA have been shown to modulate vascular responses in systemic and placental vessels 29, 30, and increase Ang II sensitivity in pregnant rats 31. The effects of AT1-AA on blood pressure and Ang II sensitivity in isolated vessels of late pregnant rats required the presence of both AT1-AA and Ang II32, and these vascular responses are blocked by the epitope peptide AFHYESQ32. Since AT1-AA appear to be secondary to a hypoxic/ischemic vascular disorder 33, it is less probable that AT1-AA are playing a role in the increased Ang II sensitivity in early gestation TgA UA before the pathogenic syndrome is established.
The effects of endothelium denudation or inhibition of NO production on Ang II contraction were lower in TgA than controls suggesting an endothelium-derived dysfunction in TgA UA, consistent with a previous report in this model at late gestation 6. Ang II contraction was dependent on prostaglandin generation. Indomethacin pre incubation shifted Ang II contraction to the left in arteries from control animals and to the right in arteries from TgA animals. This suggests a change in the type of prostanoids being generated in UA; from vasodilatory prostanoids in control UA to vasoconstrictor prostanoids in TgA arteries. This observation is consistent with the reported imbalance in the production of prostanoids described in preeclampsia: endothelial production of prostacyclin PGI2 is decreased, whereas thromboxane TXA2 levels are increased 34, 35, an effect proposed to be mediated by increased ROS generation in preeclamptic pregnancies. Interestingly, PGI2 levels decreased months before the clinical onset of preeclampsia 36. TgA rats appear to reproduce this imbalance before the installation of the hypertensive phenotype. Recent reports also support the contribution of CYP epoxygenase to the effects on blood pressure, albuminuria, and vascular function observed in the TgA rat at late gestation 7.
The actions of endocannabinoids in the vasculature are complex and not explained by a single mechanism or target tissue 37. An endothelium-dependent vasodilatory action involving a CB1 receptor-dependent pathway, as well as NO generation and K+ channels activation has been demonstrated for endocannabinoids 38. Although the development of highly specific blockers of FAAH and MAGL has allowed the study of the mechanisms mediated by AEA and 2-AG 39, 40, one limitation of our study is that our approach to examine endocannabinoid -mediated responses relies exclusively on pharmacological blockade. Using this approach however, an efficient catabolism of AEA and 2-AG by their corresponding hydrolyzing enzymes was demonstrated in vascular tissues 38, 41. Thus, in the mesenteric artery AEA and 2-AG are able to relax pre-constricted arteries, an effect that is potentiated by blockade of the hydrolases FAAH and MAGL 38. We used the enzyme blockers, URB597 and JZL184, at concentrations previously shown effective in vascular studies; i.e. URB597 10-6 M abolished AEA vascular actions in rat gracilis arteries 42. Our observations of a reduced Ang II contraction in TgA UA upon blockade of FAAH or MAGL suggest the induction of a vasodilatory mechanism or the attenuation of vasoconstrictor mediators. Studies on coronary arteries showed that the vasodilatory effects of endocannabinoids, particularly AEA, are mediated by their catabolism to arachidonic acid and subsequent conversion to vasodilatory eicosanoids such as prostacyclin or epoxyeicosatrienoic acids 43. Interestingly, these effects in coronary arteries are not mediated by the CB1 receptor 43, in agreement with the absence of effects of CB1 receptor antagonism on the reduction of Ang II contraction induced by FAAH blockade in TgA UA. Vasodilatory effects of AEA in rat aorta 44, as well as AEA-mediated nitric oxide production in endothelial cells 45, have both been described as being independent of CB1 or CB2 receptors, making it possible to explain the involvement of non-CB1/CB2-mediated vasodilatation in the reduction of Ang II contraction that we observed after blockade of endocannabinoid degradation.
It has also been described that vasodilatory prostanoids released by endocannabinoid hydrolysis would cause vasorelaxation in part via opening K+ channels 46. Given the important effect of blockade of prostaglandin generation we observed in TgA, it is conceivable that similar mechanisms may be operating in UA. Thus, increased endocannabinoid levels, via inhibition of their degradation, would increase vasodilatory prostanoids in UA that in turn would limit Ang II contraction.
Our immunohistochemistry data indicate differences in tissue localization for FAAH and MAGL in UA. FAAH was mainly localized to the cells lining the arterial lumen consistent with endothelial location and sparsely located in smooth muscle cells in SD and TgA arteries. MAGL was observed along the whole arterial wall spanning both endothelial and smooth muscle cells. Previous evidence indicated the presence of FAAH in bovine coronary arteries, kidney endothelial cells and human umbilical vein endothelial cells43, 47-49. Recently FAAH expression has also been reported in arterial smooth muscle cells42. The localization we observed for FAAH agrees with the endothelium-dependent effects described for the FAAH blocker URB597 42. As a key mediator of 2-AG degradation 50, MAGL is ubiquitously expressed 51. In the vasculature, 2-AG relaxation of mesenteric artery has been shown to be endothelium-independent 52, in agreement with the localization in smooth muscle cells we observed in UA. In both SD and TgA UA it appears that the staining for MAGL was higher than what we observed for FAAH, and this may be related to the reported greater levels of 2-AG compared to AEA observed in the rodent uterus53. Thus, a higher expression of MAGL would contribute to modulate 2-AG levels. Similar intensity of immunohistochemistry signals between arterial segments from control and TgA animals suggests that the observed differences in vascular effects are not related to different levels of expression of FAAH or MAGL in arteries from preeclamptic animals.
In terms of the possible prostanoid compounds involved in the vascular responses to endocannabinoid hydrolysis, AEA and 2-AG are also substrates of the enzyme cyclooxygenase-2 (COX-2)54. Thus, if endocannabinoids are not able to be metabolized by FAAH or MAGL due to their blockade, more substrate would be available for COX-2. AEA and 2-AG are oxidized by COX-2 to prostaglandins-ethanolamides (prostamides) (PG-EA) and prostaglandins-glyceryl esters (PG-G) respectively 54. These compounds do not interact with cannabinoid or prostaglandin receptors suggesting additional pathways involved in their cellular function55. Interestingly, the effects of URB597 on myogenic tone of mouse gracilis arteries are inhibited by indomethacin42, suggesting the involvement of PG-EA and/or PG-G on endocannabinoid-derived vascular responses. In the mesenteric artery the vasodilatory responses to endocannabinoids are endothelium-dependent 38, however we did not test the influence of endothelium on the responses to FAAH and MAGL blockers. Thus the role of the prostanoids families of compounds in the regulation of the vascular actions of Ang II by endocannabinoids warrants further investigation.
Perspectives
We observed a functional interaction between RAS and ECS in the uterine circulation. As in the clinical manifestation of preeclampsia, an increased role of prostanoids in the vasculature may help explain the enhanced effects of endocannabinoids we observed on Ang II contraction. The vascular response to Ang II is a clinical target for antihypertensive therapies, and since FAAH inhibitors have no hemodynamic effects under normotensive conditions56, our results also point to the use of endocannabinoid degradation blockers as effective pharmacotherapies for hypertension.
Supplementary Material
Figure 5. Immunolocalization of FAAH and MAGL in UA from control and TgA rats at early gestation.
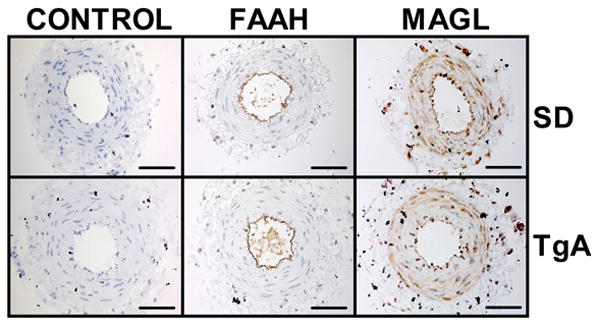
UA segments from control (SD, A-C, n=5) or TgA rats (D-F, n=5) were incubated with anti-FAAH (B and E) or anti-MAGL (C and F) antibodies. Representative pictures from each group are shown. Signals were developed by incubation with 3, 3′-diaminobenzidine (brown) and counterstained with hematoxylin (nuclei, blue). Control incubations for SD (A) and TgA UA (D) did not contain primary antibodies. Scale bar = 200 μm.
Novelty and Significance.
What Is New?
Increased contraction to Ang II in the UA at early gestation in a rat model of preeclampsia
The endogenous cannabinoids anandamide and 2-arachidonoylglycerol reduced contraction of the UA to Ang II
What Is Relevant?
By increasing levels of endogenous cannabinoids, the inhibition of the enzymes FAAH and MAGL would counteract enhanced contractile responses to Ang II
These effects are enhanced in a model of preeclampsia at early gestation, before the hypertensive phenotype initiates.
Summary
A functional interaction between the RAS and the ECS was observed in the uterine circulation.
Acknowledgments
The authors gratefully acknowledge grant support in part provided by Unifi, Inc. Greensboro, NC and Farley-Hudson Foundation, Jacksonville, NC.
Source of Funding: Funded by NHLBI P01-HL51952.
Footnotes
Conflicts of Interest/Disclosure Statement: The authors have no conflict of interest to declare.
References
- 1.Herr D, Bekes I, Wulff C. Local renin angiotensin system in the reproductive system: a review of literature. Front Endocrinol. 2013;4:150. doi: 10.3389/fendo.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gant NF, Whalley PJ, Everett RB, Worley RJ, MacDonald PC. Control of vascular reactivity in pregnancy. Am J Kidney Dis. 1987;9:303–307. doi: 10.1016/s0272-6386(87)80126-9. [DOI] [PubMed] [Google Scholar]
- 4.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II Is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55:1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohlender J, Ganten D, Luft FC. Rats transgenic for human renin and human angiotensinogen as a model for gestational hypertension. J Am Soc Nephrol. 2000;11:2056–2061. doi: 10.1681/ASN.V11112056. [DOI] [PubMed] [Google Scholar]
- 6.Verlohren S, Niehoff M, Hering L, Geusens N, Herse F, Tintu AN, Plagemann A, LeNoble F, Pijnenborg R, Muller DN, Luft FC, Dudenhausen JW, Gollasch M, Dechend R. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension. 2008;51:547–553. doi: 10.1161/HYPERTENSIONAHA.107.103176. [DOI] [PubMed] [Google Scholar]
- 7.Herse F, LaMarca B, Hubel CA, Kaartokallio T, Lokki AI, Ekholm E, Laivuori H, Gauster M, Huppertz B, Sugulle M, Ryan MJ, Novotny S, Brewer J, Park JK, Kacik M, Hoyer J, Verlohren S, Wallukat G, Rothe M, Luft FC, Muller DN, Schunck WH, Staff AC, Dechend R. Cytochrome P450 subfamily 2J polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia. Circulation. 2012;126:2990–2999. doi: 10.1161/CIRCULATIONAHA.112.127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Xie H, Yang J, Wang H, Bradshaw HB, Dey SK. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc Natl Acad Sci U S A. 2010;107:16887–16892. doi: 10.1073/pnas.1010892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abán C, Leguizamón GF, Cella M, Damiano A, Franchi AM, Farina MG. Differential expression of endocannabinoid system in normal and preeclamptic placentas: effects on nitric oxide synthesis. Placenta. 2013;34:67–74. doi: 10.1016/j.placenta.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca BM, Correia-da-Silva G, Almada M, Costa MA, Teixeira NA. The endocannabinoid system in the postimplantation period: a role during decidualization and placentation. Int J Endocrinol. 2013;2013:11. doi: 10.1155/2013/510540. Article ID 510540. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennedy MC, Friel AM, Houlihan DD, Broderick VM, Smith T, Morrison JJ. Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol. 2004;190:2–9. doi: 10.1016/j.ajog.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Cotterill RW, Penney LL, Vaughn DL, Reimann BE, Rauls DO. Acute cardiovascular effects of delta-9-tetrahydrocannabinol in pregnant anesthetized sheep. Biol Res Pregnancy Perinatol. 1984;5:1–5. [PubMed] [Google Scholar]
- 14.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci U S A. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 17.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–246. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends Mol Med. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Feledziak M, Lambert DM, Marchand-Brynaert J, Muccioli GG. Inhibitors of the endocannabinoid-degrading enzymes, or how to increase endocannabinoid's activity by preventing their hydrolysis. Recent Pat CNS Drug Discov. 2012;7:49–70. doi: 10.2174/157488912798842223. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Xie H, Guo Y, Zhang H, Takahashi T, Kingsley PJ, Marnett LJ, Das SK, Cravatt BF, Dey SK. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006;116:2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca BM, Correia-da-Silva G, Taylor AH, Lam PMW, Marczylo TH, Bell SC, Konje JC, Teixeira NA. The endocannabinoid 2-arachidonoylglycerol (2-AG) and metabolizing enzymes during rat fetoplacental development: A role in uterine remodelling. Int J Biochem Cell Biol. 2010;42:1884–1892. doi: 10.1016/j.biocel.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Szekeres M, Nádasy GL, Turu G, Soltész-Katona E, Tóth ZE, Balla A, Catt KJ, Hunyady L. Angiotensin II induces vascular endocannabinoid release, which attenuates its vasoconstrictor effect via CB1 cannabinoid receptors. J Biol Chem. 2012;287:31540–31550. doi: 10.1074/jbc.M112.346296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulgar VM, Yamashiro H, Rose JC, Moore LG. Role of the AT2 receptor in modulating the angiotensin II contractile response of the uterine artery at mid-gestation. J Renin Angiotensin Aldosterone Syst. 2011;12:176–183. doi: 10.1177/1470320310397406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulgar VM, Figueroa JP. Antenatal betamethasone administration has a dual effect on adult sheep vascular reactivity. Pediatr Res. 2006;60:705–710. doi: 10.1203/01.pdr.0000246481.05231.17. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen DH, Zhou T, Shu J, Mao JH. Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes. 2013:2:e. [Google Scholar]
- 28.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brãsen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Müller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Wang F, Chang H, Zhang S, Yang L, Wang X, Cheng X, Zhang M, Ma XL, iu H. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens. 2008;26:1629–1635. doi: 10.1097/HJH.0b013e328304dbff. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Zheng R, Yang L, Zhang X, Zuo L, Yang X, Bai K, Song L, Tian J, Yang J, Liu H. Angiotensin type 1 receptor autoantibody from preeclamptic patients induces human fetoplacental vasoconstriction. J Cell Physiol. 2013;228:142–148. doi: 10.1002/jcp.24113. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II Type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension. 2011;58:77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN, LaMarca B. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62:886–892. doi: 10.1161/HYPERTENSIONAHA.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herse F, Staff A, Hering L, M++ller D, Luft F, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med. 2008;86:697–703. doi: 10.1007/s00109-008-0332-4. [DOI] [PubMed] [Google Scholar]
- 34.Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2004;70:223–232. doi: 10.1016/j.plefa.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–340. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 36.Mills JL, DerSimonian R, Raymond E. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: A multicenter prospective study. JAMA. 1999;282:356–362. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- 37.Hillard CJ. Endocannabinoids and vascular function. J Pharmacol Exp Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- 38.Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillard CJ, Ho WS, Thompson J, Gauthier KM, Wheelock CE, Huang H, Hammock BD. Inhibition of 2-arachidonoylglycerol catabolism modulates vasoconstriction of rat middle cerebral artery by the thromboxane mimetic, U-46619. Br J Pharmacol. 2007;152:691–698. doi: 10.1038/sj.bjp.0707468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czikora Á, Lizanecz E, Boczán J, Daragó A, Papp Z, Édes I, Tóth A. Vascular metabolism of anandamide to arachidonic acid affects myogenic constriction in response to intraluminal pressure elevation. Life Sciences. 2012;90:407–415. doi: 10.1016/j.lfs.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol Heart Circ Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- 45.McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor-independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321:930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- 46.Romano MR, Lograno MD. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: evidences for CB1 receptors, nitric oxide and potassium channels. Br J Pharmacol. 2006;147:917–925. doi: 10.1038/sj.bjp.0706687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maccarrone M, Bari M, Lorenzon T, Bisogno T, Di Marzo V, Finazzi-Agró A. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. J Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- 49.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Ann Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 50.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase: evolutionary relathionship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 52.Kagota S, Yamaguchi Y, Nakamura K, Sugiura T, Waku K, Kunitomo M. 2-Arachidonoylglycerol, a candidate of endothelium-derived hyperpolarizing factor. Eur J Pharmacol. 2001;415:233–238. doi: 10.1016/s0014-2999(01)00833-0. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Xie H, Sun X, Kingsley PJ, Marnett LJ, Cravatt BF, Dey SK. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins Other Lipid Mediat. 2007;83:62–74. doi: 10.1016/j.prostaglandins.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 55.Woodward DF, Carling RWC, Cornell CL, Fliri HG, Martos JL, Pettit SN, Liang Y, Wang JW. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol Ther. 2008;120:71–80. doi: 10.1016/j.pharmthera.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


