Abstract
Progress in HIV treatments has led to HIV-infected patients living into their 60s and older. Since HIV-associated Neurocognitive Disorder (HAND) in older age is associated with more executive dysfunction, cognitive screening instruments tapping this domain may be optimal. We examined the Montreal Cognitive Assessment (MoCA) to identify HAND in 67 HIV-infected patients over age 60, of which 40% were diagnosed with HAND. Receiver operator characteristics (ROC) curve identified an optimized cut-off ≤25/30 identified HAND with a sensitivity of 72% and specificity of 67%. We conclude that the MoCA has only moderate performance characteristics for cognitive screening of HIV-infected elders.
Keywords: Cognition disorders, HIV dementia, AIDS
Introduction
Infection with HIV has become a manageable chronic illness with the advent of combination antiretroviral therapy (cART). Consequently, the number of people living with HIV over age 60 has increased substantially, and some estimate that more than one-half of all HIV/AIDS cases in the U.S. will be over 50 years old by 2015.1 Considering the emergence of safer antiretroviral medications and the availability of cART worldwide, aging with HIV has become an internationally important issue.2
Despite the widespread use of cART and these treatment successes, HIV-associated neurocognitive disorders (HAND) are common, with a prevalence of up to 50% among community-dwelling patients with access to cART who are seen at academic medical centers in the U.S.3 In the current era, HAND is defined by the 2007 “Frascati” guidelines as one of three conditions: Asymptomatic Neurocognitive Impairment (ANI), Mild Neurocognitive Disorder (MND), or HIV-associated Dementia (HAD).4 Although the severity of cognitive impairment is attenuated in the cART era, milder forms of HAND persist and continue to impact everyday functioning.3,5,6
Both age and HIV are risk factors for cognitive decline, and older age is associated with greater risk for HAND.7,8 Older patients often have higher rates of comorbid illness, including cardiovascular disease, which further increases risk for impaired cognition and may impact the patterns of impairment on neuropsychological testing.9 This may explain why executive dysfunction is more frequently seen in older compared to younger HIV-infected patients.10
The Mini Mental State Examination (MMSE) continues to be a commonly employed cognitive screening test in clinical practice, but has been criticized for use in HIV due to ceiling effects.11 The Montreal Cognitive Assessment (MoCA) more broadly evaluates domains known to be affected by HIV and has sensitivity for the detection of Mild Cognitive Impairment (MCI) and milder Alzheimer's disease in the general population.12 The few studies that have examined the utility of the MoCA in an HIV population show promise; but, to date, none have examined performance in a sample of elder people with HIV.13-15 In this study, we analyze the performance characteristics of the MoCA in HIV-infected patients over age 60 to determine if it can be a clinically useful tool.
Methods
Subjects
We selected all subjects who completed the MoCA (n=67) among those who were enrolled in a larger cohort study of cognition in HIV-infected individuals aged 60 and above (UCSF HIV Over 60 Cohort). The parent study excluded subjects who had factors that would substantially affect cognition besides HIV infection or neurodegenerative disorder, as previously described.16 We also excluded one patient from this analysis who was not able to complete the MoCA due to advanced dementia. Participants were recruited through community fliers and physician referrals as previously described, with no exclusion based on cognitive symptoms.16
Cognitive and functional assessments
Participants completed a neuropsychological testing battery that assessed multiple cognitive domains important to diagnosis using 2007 HAND criteria, including: memory, executive function, psychomotor speed, visuospatial and motor abilities, and attention. Raw neuropsychological test results were interpreted by neurobehavioral neurologists and neuropsychologists aided by means and standard deviations from controls (n>2,000) available within the Alzheimer's Disease Research Center (ADRC) for tests included in the ADRC Uniform Data Set, from published normative data (California Auditory Verbal Learning (CVLT) and Finger tapping), or from local developed normative data (grooved pegboard test).17-19 A proxy informant interview was conducted using the Clinical Dementia Rating scale (CDR) to provide a global rating of functional compromise. Although not employed in HIV, the CDR is a widely validated formal interview assessing multiple domains of cognitive functioning which provides a global rating of functional compromise associated with dementia of the Alzheimer's type.20 Additionally, each subject underwent a standardized and comprehensive symptoms checklist conducted by a physician who also assessed motor and behavioral components of HAND. All subjects completed the Geriatric Depression Scale (GDS) questionnaire to evaluate mood. Symptoms of cognitive difficulty endorsed by either the subject or proxy were considered sufficient to diagnose symptomatic impairment in cases with neuropsychological deficits meeting HAND criteria. Final diagnoses were determined at consensus conference, attended by a nurse, neuropsychologist, and at least one behavioral neurologist. We assigned HAND diagnoses using clinical acumen informed by the Frascati criteria (2007).4 In general, subjects with mild and moderately impaired performance (1 to 2 SD below the mean, after adjustment for age and education) were diagnosed as MND, whereas those demonstrating severe impairment (typically worse than -2 SD) were diagnosed as HAD. Those with neuropsychological testing impairment but without documented symptoms were diagnosed with ANI regardless of disease severity. When possible, we measured CD4 T-lymphocyte counts and plasma HIV RNA if current labs (+/- 3 months) were not available and otherwise used self-reported most recent results for CD4 (n=12) and plasma HIV RNA (n=7).
Statistical Analysis
We used SPSS Version 13.0 software package (SPSS Inc., Chicago, IL) for statistical analysis. Descriptive statistics were calculated in standard fashion for quantitative variables. A non-parametric receiver operating characteristic (ROC) curve analysis was performed to examine the ability of the MoCA to detect cognitive impairment using the presence of HAND as the outcome.21 The area under the curve (AUC) and the associated standard error were calculated, with 1 and 0.50 respectively, indicating perfect and chance prediction. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were then computed for five different MoCA cut points.
Results
Most participants were Caucasian (94%, n=63) men who have sex with men (MSM, 81%, n=54) with high self-reported level of education (mean (SD) = 16.3 (2.32), Table 1). All were on cART and 79% had undetectable plasma HIV RNA (<75 copies/ml). Eight (12%) met the criteria for ANI, 19 (28%) for MND, and none met criteria for HAD. In all, 27 subjects (40%) were diagnosed with HAND.
Table 1. Demographics and clinical characteristics of the sample.
| Sample size, n | 67 |
|---|---|
| Age, years (median, range) | 64 (60-84) |
| Gender (% male) | 96% |
| Education, mean years (SD) | 16.3 (2.32) |
| Ethnicity, Caucasian (%) | 94% |
| Risk for HIV, MSM only (%) | 81% |
| CD4 count, mean (SD) | 549 (246) |
| Nadir CD4, mean (SD) | 240 (162) |
| HIV duration, (median, range) | 23 (1-32) |
| Undetectable viral load* (%) | 79% |
| on cART (%) | 100% |
| HAND diagnoses | |
| Cognitively normal (%) | 60% |
| ANI (%) | 12% |
| MND (%) | 28% |
less than 75 copies/ml
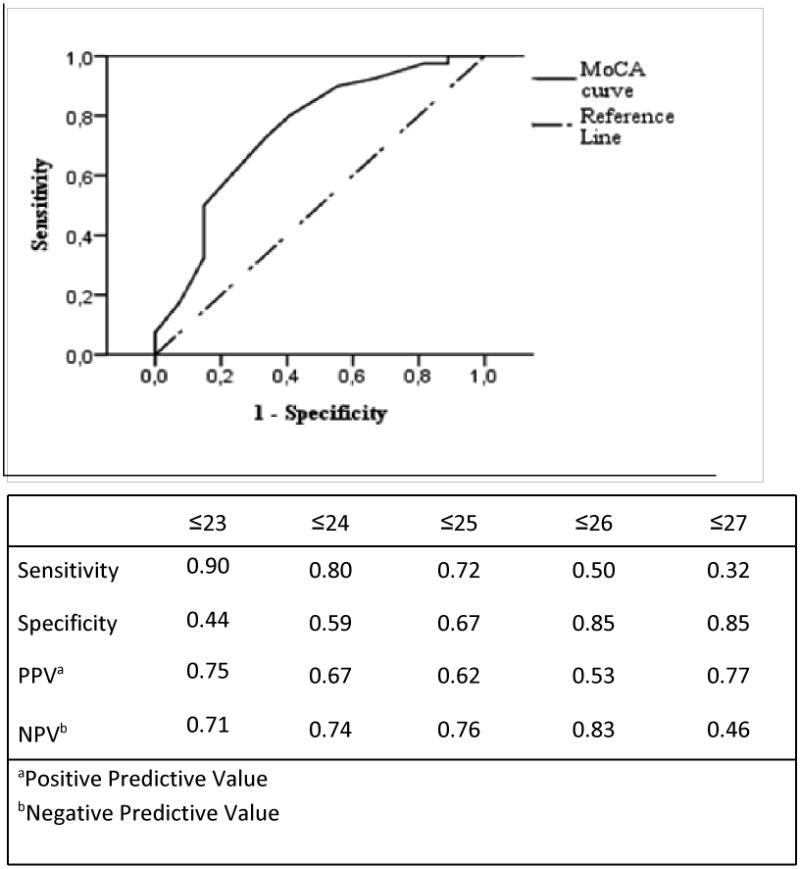
Analysis of ROC curve for the MoCA revealed that the best balance of sensitivity (72%) and specificity (67%) was at 25/30, with an area under the curve of 0.75 (95% confidence interval CI=0.63-0.88, p=0.001) (Figure 1). Alternatively, using a cut point of 23, the MoCA detected 90% of HAND cases, but specificity was reduced to 44%, while with a cut point of 26 (suggested in the literature as the ideal MoCA cut-off score to detect Mild Cognitive Impairment) the MoCA exhibited a poor sensitivity (50%) accompanied by an advantage in specificity (85%).12
Figure 1. ROC for the MoCA in identifying HAND. AUC=0.75, 95% CI=0.63-0.88.
Discussion
Several screening instruments have been developed to assess HAND in HIV-infected patients, including the HIV Dementia Scale (HDS) and its derivative form, the International HIV Dementia Scale (IDHS). Although they can adequately identify more severe forms of neurocognitive impairment, they are relatively insensitive in differentiating milder forms of HAND. The MMSE is traditionally used to detect cognitive impairment in a variety of dementing illnesses among HIV-uninfected elders, but it has consistently demonstrated poor sensitivity and specificity to detect HAND.22-24 By virtue of better assessment for attention, concentration, working memory, executive functioning and reasoning, the MoCA may be superior and further supportive data could justify use in this population.
Studies of cognitive aging in HIV-uninfected subjects that employ both Magnetic Resonance Imaging (MRI) and cognitive measures demonstrate age-related reductions in white matter integrity that are hypothesized to underlie age-related performance declines on tasks of information processing speed, episodic memory and executive functions.25 Older HIV-infected subjects with cognitive impairment appear to have greater deficits in executive functioning, postulated to be due to more cerebrovascular disease.26 This may suggest superior performance of the MoCA in older HIV-infected groups, but, to date, there are no data on the MoCA's capability among HIV-infected elders.24,27 In this work, we identified only moderate performance characteristics using our optimized cut off that is slightly lower than that previously published.12 Although the MMSE was also administered, most of the patients with HAND (74%) achieved a score above 28; thus, we deemed it a poor choice for further investigation. In agreement with other authors we postulate that adding additional tests could improve the psychometric performance of the MoCA, but we did not have a sufficient number of cases to investigate this in the present sample.13,14,27
Among our sample, all subjects were on cART and most were virologically suppressed, a strength of the work since this is most reflective of subjects who might undergo screening in older age. No patients in this sample met the criteria for HAD, consistent with published work describing a decreased incidence of HAD as a natural outcome of therapy.28 This may also be considered a strength, since patients with HAD are likely to be more obvious clinically and would not typically be subject to screening, such as the subject excluded from our study due to inability to perform the test sufficiently. Sample size and referral bias are limitations to the work. Further, we focused on a selected population of HIV-infected subjects enrolled in a parent study at our center, which may not be representative of the general HIV-infected population by virtue of older age (> 60 years) and relatively high level of education (mean years education = 16.3, SD = 2.32).
Both advanced age and HIV have been found to impact cognitive performance, and several groups have explored HIV-age interaction effects on neuropsychological performance with mixed findings. Some studies argue that HIV accelerates age-related cognitive changes through mechanisms common to the aging process, while others postulate that HIV and aging might be additive in the expression of cognitive decline due, in part, to comorbidities that increase with age.20-23 Regardless of etiology or potential interactions, routine cognitive screening could help clinicians better understand and manage functional consequences of cognitive impairment (e.g. medication adherence) in older HIV-infected subjects, but optimal screening instruments are needed. We conclude that the MoCA has moderate performance characteristics for HAND in elderly HIV-infected patients, but additional work is needed to optimize screening in this vulnerable population.
Acknowledgments
We thank our study participants.
Conflicts of Interest and Source of support: This study was supported by the following grants from the National Institutes of Health: K24-MH-098759 (VV), P50-AG-023501 (ADRC, PI: Bruce Miller); P30-AI-027763 (UCSF/GIVI Center for AIDS Research), and the UL1-RR-024131 (UCSF GCRC). Additional support received from the Larry L. Hillblom Foundation and the UCSF AIDS Research Institute.
References
- 1.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 2.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16:94–98. [PubMed] [Google Scholar]
- 6.Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012;61:341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker JT, Lopez OL, Dew MA, et al. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. Aids. 2004;18(Suppl 1):S11–18. [PubMed] [Google Scholar]
- 9.Valcour VG, Shikuma CM, Watters MR, et al. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. Aids. 2004;18(Suppl 1):S79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacktor N, Skolasky R, Selnes OA, et al. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol. 2007;13:203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- 11.Valcour V, Paul R, Chiao S, et al. Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis. 2011;53:836–842. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 13.Overton ET, Azad TD, Parker N, et al. The Alzheimer's disease-8 and Montreal Cognitive Assessment as screening tools for neurocognitive impairment in HIV-infected persons. J Neurovirol. 2013;19:109–116. doi: 10.1007/s13365-012-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koski L, Brouillette MJ, Lalonde R, et al. Computerized testing augments pencil-and-paper tasks in measuring HIV-associated mild cognitive impairment(*) HIV Med. 2011;12:472–480. doi: 10.1111/j.1468-1293.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan LG, K N, Chua A. HIV-associated neurocognitive disorders (HAND) in a South Asian population - contextual application of the 2007 criteria. BMJ Ope. 2012 doi: 10.1136/bmjopen2011-000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiao S, Rosen HJ, Nicolas K, et al. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retroviruses. 2013;29:949–956. doi: 10.1089/aid.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delis DC, K J, Kaplan E, Ober BA. California Verbal Learning Test. 2nd. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 18.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 21.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 22.Ganasen KA, Fincham D, Smit J, et al. Utility of the HIV Dementia Scale (HDS) in identifying HIV dementia in a South African sample. J Neurol Sci. 2008;269:62–64. doi: 10.1016/j.jns.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Skinner S, Adewale AJ, DeBlock L, et al. Neurocognitive screening tools in HIV/AIDS: comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009;10:246–252. doi: 10.1111/j.1468-1293.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamminga J, Cysique LA, Lu G, et al. Validity of cognitive screens for HIV-associated neurocognitive disorder: a systematic review and an informed screen selection guide. Curr HIV/AIDS Rep. 2013;10:342–355. doi: 10.1007/s11904-013-0176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunning-Dixon FM, Brickman AM, Cheng JC, et al. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valcour V, Paul R, Neuhaus J, et al. The Effects of Age and HIV on Neuropsychological Performance. J Int Neuropsychol So. 2011;17:190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valcour VG. Evaluating cognitive impairment in the clinical setting: practical screening and assessment tools. Top Antivir Med. 2011;19:175–180. [PMC free article] [PubMed] [Google Scholar]
- 28.Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990-1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]


