Abstract
The association of longitudinal changes in LV structure and function with myocardial fibrosis is unclear. We relate temporal changes in body-size indexed LV mass (LVMi) and end-diastolic volume (EDVi), LV mass-to-volume ratio (MVR) and ejection fraction (LVEF) from cine CMR over 10 years, with replacement scar assessed from late-gadolinium enhancement, and lower post-contrast T1 times reflecting greater diffuse myocardial fibrosis measured at the end of the follow-up period. All participants (n=1813) who underwent CMR twice as part of the Multi-Ethnic Study of Atherosclerosis 10 years apart were included. Multivariable logistic and linear regression models adjusted for cardiovascular risk factors measured the association of 10-year changes in LV structure and function, with fibrosis measured at follow-up. The presence of LV scar at year-10 was cross-sectionally associated with higher LVMi (~10g/m2), higher MVR (0.1 – 0.2g/ml) but lower LVEF (~4%); and longitudinally with 3% decrease in LVEF and 0.7% greater EDVi in men over 10 years. Lower post-contrast T1 times at year-10 were associated cross-sectionally with lower LVMi (r=0.33), EDVi (r=0.25), and LVEF (in men only: r=0.14); and longitudinally with a decrease in LVMi (r=0.20) and reduction in LVEF (in men only: r=0.15). Sustained hypertension over 10 years was associated with increased LVMi, and higher diffuse and replacement fibrosis at follow-up. Over a 10-year period increased concentric hypertrophy in women and LV dilatation in men was associated with replacement fibrosis; while decreasing LVMi was associated with diffuse fibrosis. Hypertension induced remodeling was related to enhanced replacement and diffuse fibrosis as well as hypertrophy.
Keywords: MR imaging, LV volume, LV mass, Fibrosis, T1 Mapping, delayed enhancement, hypertension
INTRODUCTION
Left ventricular (LV) structural and functional changes in response to, or in association with, myocardial fibrosis are important components of cardiac disease. Indeed, myocardial fibrosis is considered as one of the chief factors that influence the process of cardiac remodeling1, 2, conceptualized as the structural and functional cardiac alterations that accompany exposure to pathogenic processes and cardiovascular risk factors 3. Previous studies have shown the association of myocardial fibrosis with alterations of myocardial structure and function, as well as with indices of impaired myocardial tissue deformation in varied cardiomyopathies4–7. Gender dependency of these alterations of structure and function has also been demonstrated,8 reflecting different mechanisms of cardiac morbidity and associated LV remodeling.
Cardiac Magnetic resonance (CMR) imaging can be used to accurately quantify LV mass and volume throughout the cardiac cycle, and is considered as the standard of reference for assessment of structure and function. CMR has also been used to characterize focal and diffuse myocardial fibrosis through late gadolinium enhancement (LGE) and longitudinal relaxation time (T1) mapping, respectively. In previous studies, T1 mapping times and related parameters have been shown to correlate well with collagen content of the myocardium as measured through biopsy9, 10, while LGE has long been used to measure replacement myocardial fibrosis11.
The goals of this study were to understand how LV structure and function and its 10-year change relate to diffuse and replacement myocardial fibrosis measured at the end of the follow-up period in a multi-ethnic population. We relate CMR measures of structure – LV mass, volume, and mass-to volume ratio, and function with measures of replacement fibrosis detected by myocardial scar from LGE, and diffuse myocardial fibrosis indexed by T1 mapping. We also study the variation LV structure and function, and fibrosis in conjunction with hypertension.
METHODS
Population characteristics
The design and population characteristics of the multi-ethnic study of atherosclerosis (MESA) have been described previously 12. Briefly, MESA is a prospective, population-based observational cohort study of 6814 men and women representing four racial/ethnic groups, aged 45–84 years and free of clinical cardiovascular disease at enrolment. As part of the baseline examination, between 2000 and 2002 (year 0), a total of 5004 (73%) participants received cine CMR exams at six field centers. Of the 5004 individuals who underwent CMR at baseline, 2981 participants underwent a follow-up CMR between 2010 and 2012 (year 10). The follow-up scan included LGE imaging and T1 mapping. Participants undergoing CMR scans were screened for gadolinium (Gd) eligibility. Participants with glomerular filtration rate (GFR) >=45 ml/min (60 ml/min for one site) and without history of allergic reaction to contrast agents were qualified to receive Gd. Of the eligible participants, 1814 agreed to a Gd injection, and underwent LGE imaging. 1320 of the participants who were injected Gd also had T1 mapping as part of the protocol. After exclusion for participants with either wrong or unavailable administered gadolinium dose, and for those where the images acquired had artifacts (51 participants); a total of 1223 participants were included in the T1 analysis. Over the 10-year follow-up period, a telephone interviewer contacted each participant (or representative) every 6 to 9 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Two physicians reviewed all records for independent end point classification (criteria provided in Supplement 2) and assignment of event dates. The institutional review boards of all MESA field centers approved the study protocol and all participants gave informed consent.
Cardiac Magnetic Resonance
LV mass and LV end diastolic volumes were indexed to body surface area (LVMi and EDVi). LV Mass to volume ratio (MVR) and ejection fraction (LVEF) were obtained as previously described13. In addition to indexing the LV mass by BSA, an allometric approach to indexing the LV mass as used in the same population was also implemented13. A detailed explanation of the acquisition methods is provided in the Supplement.
LGE was used to detect presence of regional scar replacement. Delayed contrast enhancement images were obtained 15 min after an intravenous bolus injection of gadolinium-diethylenetriaminepenta-acetic acid (0.15 mmol/kg, Magnevist, Bayer Healthcare Pharmaceuticals, New Jersey, USA) to identify regional fibrosis. Short axis slices, one horizontal and one vertical long axis, all at the same positions as the cine images were acquired. The images were analyzed using QMass (Medis, Netherlands). The region of interest (ROI) for myocardium was manually placed on short-axis slices, the scar replacement area were then detected as the area with increased intensity manually by the user for each slice.
For evaluation of diffuse fibrosis, one short axis pre-contrast MOLLI14 image at the mid-slice position was acquired, repeated at 12 and 25 minutes after contrast injection (five of the six centers; all Siemens 1.5 T scanners). The imaging has been described in detail before15. All images were acquired with the same trigger delay time in end diastole. T1 maps were constructed offline using QMass research (Netherlands). On each T1-map (pre- and post-contrast), a region of interest was manually drawn around the core myocardium to calculate the myocardial T1 time. Lower post-contrast T1 times have been associated with greater diffuse myocardial fibrosis9, 15.
Statistical analysis
Statistical analysis was performed with STATA, version 12 (Stata Corp, TX). LV structure and function, T1 mapping parameters, presence of myocardial scar and the participants’ baseline data were evaluated using the Student’s t-test for continuous variables and the chi-square test for categorical variables. We stratified the cohort by gender in all analyses.
The associations of LV structure and function parameters from year-0 and year-10 exams with presence of myocardial scar at year-10 and T1 mapping parameters at year-10 were assessed using multivariable logistic and linear regression analyses respectively with fibrosis markers as dependent variables. Participants who had scar detected from the LGE assessment were excluded for analysis with T1 mapping parameters. The models adjusted for covariates - demographics (age, ethnicity) and traditional cardiovascular risk factors (BMI, systolic blood pressure, hypertension medication, diabetes, smoking, HDL and total cholesterol). In addition, when T1 times were the dependent variable, adjustment was performed for factors affecting acquisition – heart rate during CMR acquisition for pre-contrast T1 times; exact Gd dose and GFR16 for post-contrast T1 times. Additional to LV mass indexed by BSA, LV mass indexed using an allometric approach was also used, to clarify that the relationships were independent of the indexing method.
In the assessment of association with year 10 LV structure and function variables, the models adjusted for year-10 covariates. In the assessment of association with change in structure and function, the models adjusted for 10-year change in covariates and year-0 value of the parameter studied as well as year-0 value of the covariates. The associations explored with change include both categorical (coded as decrease of more than one standard deviation=0, change of less than one standard deviation=1, and increase of more than one standard deviation =2) and continuous change.
Additional analysis was performed to check for variation of hypertension status and LV structure and function and fibrosis. Hypertension categories were defined based on hypertension status based on JNC VI criteria at year-0 and year-10. Four categories were defined - without hypertension at year-0 and year-10; with hypertension at year-0 but no hypertension at year-10; with hypertension at year-10 but not at year-0; and those with hypertension at both year-0 and year-10. Values of LV structure and function and diffuse myocardial fibrosis were compared across categories using oneway ANOVA. Presence of scar was compared across categories using the chi-squared test.
RESULTS
Table 1 shows the baseline and follow up population characteristics by gender. The mean age of women and men at baseline were 58 and 59 years respectively. The number of participants on lipid-lowering and anti-hypertensive medication increased significantly over 10 years. The proportion of participants with impaired fasting glucose and treated diabetes increased significantly over the 10-year follow-up period in association with a significant increase in BMI. Over the 10 year follow-up period, 83 participants had a cardiovascular event (18 women, 65 men), 26 had myocardial infarction (3 women, 23 men) and 13 had congestive heart failure (4 women, 9 men).
Table 1.
Population Characteristics by Gender.
| Characteristics | Women (n=874) | Men (n=939) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Year 0 | Year 10 | p-value | Year 0 | Year 10 | p-value | |
| Age(yrs) | 58.1±8.9 | 67.6±8.8 | <0.001 | 58.7±9.0 | 68.1±8.9 | <0.001 |
| Ethnicity(%) | ||||||
| Caucasian | 46 | 44 | ||||
| Chinese- American | 9 | 9 | ||||
| African- American | 26 | 23 | ||||
| Hispanic | 18 | 23 | ||||
| BMI(kg/m2) | 28.3±5.6 | 28.7±5.8 | <0.001 | 27.8±4.0 | 28.1±4.4 | <0.001 |
| SBP(mmHg) | 122±22 | 123±21 | 0.046 | 122±17 | 121±18 | 0.12 |
| HTN Med(%) | 29 | 50 | <0.001 | 27 | 50 | <0.001 |
| Chol(mg/dl) | 201±35 | 195±35 | <0.001 | 188±34 | 171±35 | <0.001 |
| HDL(mg(dl) | 57±15 | 60±17 | <0.001 | 44±11 | 49±13 | <0.001 |
| Lipid Med(%) | 14 | 36 | <0.001 | 16 | 40 | <0.001 |
| Smokers(%) | 12 | 7 | <0.001 | 13 | 9 | <0.001 |
| Diabetes(%) | 7 | 15 | <0.001 | 9 | 18 | <0.001 |
| LVMi(g/m2) | 58.9±8.8 | 59.4±9.6 | 0.10 | 69.3±10.7 | 73.9±12.7 | <0.001 |
| EDVi(ml/m2) | 67.4±10.4 | 62.2±10.9 | <0.001 | 72.0±13.4 | 69.2±15.1 | <0.001 |
| ESVi(ml/m2) | 24.4±5.1 | 22.6±6.1 | <0.001 | 28.0±6.7 | 28.4±9.5 | 0.15 |
| MVR(g/ml) | 0.89±0.14 | 0.98±0.19 | <0.001 | 0.98±0.19 | 1.10±0.24 | <0.001 |
| LVEF(%) | 63.7±5.3 | 63.7±6.3 | 0.98 | 61.1±5.8 | 59.3±7.2 | <0.001 |
| Scar present(% n) | 2.5 % | 12.9% | ||||
|
| ||||||
| T1 pre (ms)* | 986±45 | 968±43 | ||||
| T1 at 12′(ms) * | 440±41 | 466±35 | ||||
| T1 at 25′(ms) * | 504±41 | 530±36 | ||||
BMI: body mass index. SBP: systolic blood pressure. HTN Med: hypertension medication. Chol: total cholesterol. HDL: high-density lipoprotein. LVMi: LV mass indexed to body surface area (g/m2). EDVi: end-diastolic volume indexed to body surface area (ml/m2). MVR: LV mass to end-diastolic volume ratio (g/ml). LVEF: LV ejection fraction (%). T1 pre, T1 at 12′, T1 at 25′: T1 time at T1 time pre-contrast injection, 12 and 25 minutes post-contrast injection respectively (ms).
n=598 women and 625 men for T1 mapping parameters.
CMR defined LVMi increased significantly for men, while EDVi decreased significantly in both women and men over the 10 year follow-up period. In women, there was also a decrease in ESVi and no change in LVEF, while in men there was no significant change in ESVi and consequently a small but significant reduction in LVEF. The LV mass/volume ratio (MVR) increased between baseline and follow-up in both women and men, though men had a higher mean MVR as compared to women in both exams. Post-contrast T1 times both at 12 and 25 minutes were lower in women as compared to men. Conversely, the proportion of participants with myocardial scar in men (12.9%) was five times greater than that in women (2.5%).
Late-Gadolinium Enhancement
Table 2 shows the coefficients for logistic regression analyses of structural and functional variables for the presence of LGE-defined scar at the year-10 follow up examination. Cross-sectional analysis (vs year-10 structural and functional LV parameters) showed the presence of myocardial scar was associated with greater LVMi and MVR as well as lower LVEF with coefficients greater in women than in men. LGE-defined myocardial scar was also associated with higher EDVi in men but not in women. Greater LVMi and MVR at year-0 as well as their increase over 10 years were also associated with the presence of myocardial scar at year-10 after adjustment for covariates. A lower LVEF at year-0 and a further decrease over the 10-year follow-up period were also associated with the presence of LGE-defined myocardial scar. When LV mass was indexed using the allometric approach, the results remained consistent and similar with what was observed for LVMi (indexed to BSA) as shown in Supplemental Table S3.
Table 2.
Coefficients for multivariable logistic regression relating presence of scar defined by LGE at the follow up year-10 exam with CMR parameters.
| Variable | Cross-Sectional | Associations with Baseline and Change | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Uni | Multi | r2 | Uni | Multi | r2 | ||
|
| |||||||
| Δ | BL | Δ | |||||
| WOMEN | |||||||
|
| |||||||
| LVMi | 0.07† (0.04,0.10) | 0.07† (0.03,0.11) | 0.14 | 0.03 (−0.02,0.07) | 0.06* (0.01,0.11) | 0.08† (0.02,0.13) | 0.19 |
| EDVi | −0.01 (−0.05,0.03) | 0.001 (−0.04 0.04) | 0.07 | 0.003 (−0.04,0.05) | −0.001 (−0.05,0.04) | −0.002 (−0.05,0.05) | 0.07 |
| MVR | 3.45† (1.94,4.96) | 3.17† (1.51,4.83) | 0.14 | 1.9 (−0.25,4.04) | 4.54† (1.54,7.54) | 2.30* (0.26,4.34) | 0.2 |
| LVEF | −0.09† (−0.15,0.03) | −0.08* (−0.14, −0.02) | 0.11 | −0.06* (−0.12, −0.00) | −0.10* (−0.19, −0.01) | −0.08* (−0.15, −0.01) | 0.18 |
|
| |||||||
| MEN | |||||||
|
| |||||||
| LVMi | 0.05† (0.03,0.06) | 0.05† (0.04,0.07) | 0.12 | 0.04† (0.02,0.06) | 0.05† (0.03,0.07) | 0.05† (0.03,0.07) | 0.13 |
| EDVi | 0.02* (0.00,0.03) | 0.02† (0.01,0.03) | 0.08 | 0.02† (0.01,0.03) | 0.02* (0.01,0.04) | 0.02† (0.01,0.04) | 0.08 |
| MVR | 1.29† (0.58,2.01) | 0.93* (0.15,1.71) | 0.07 | 0.3 (−0.47,1.07) | 1.21* (0.06,2.35) | 0.81 (−0.02,1.64) | 0.07 |
| LVEF | −0.07† (−0.10, −0.04) | −0.06† (−0.09, −0.04) | 0.09 | −0.04† (−0.06, −0.02) | −0.09† (−0.13, −0.05) | −0.06† (−0.09, −0.03) | 0.1 |
Coefficients and 95% confidence intervals (in brackets) for multivariable logistic regression models to assess the cross-sectional associations, and baseline and change associations of CMR variables with the presence of scar as assessed by LGE at year 10. Multivariable models adjusted for risk factor values at year-10 for cross-sectional associations. For associations with baseline (BL) and change (Δ) in values, models adjusted for baseline value and change in value of covariates.
if p<0.05,
if p<0.001.
LVMi: LV mass indexed to body surface area (g/m2). EDVi: end-diastolic volume indexed to body surface area (ml/m2). MVR: LV mass to end-diastolic volume ratio (g/ml). LVEF: LV ejection fraction (%).
Participants who had scar detected from the LGE assessment were excluded for analysis with T1 mapping parameters, as the presence of LGE-defined scar was significantly associated with pre- and post-contrast T1 times. Pre-contrast T1 times were significantly higher and post-contrast T1 times significantly lower in the group with CMR scar defined by LGE (supplemental Table S1).
Pre and Post-contrast T1 times
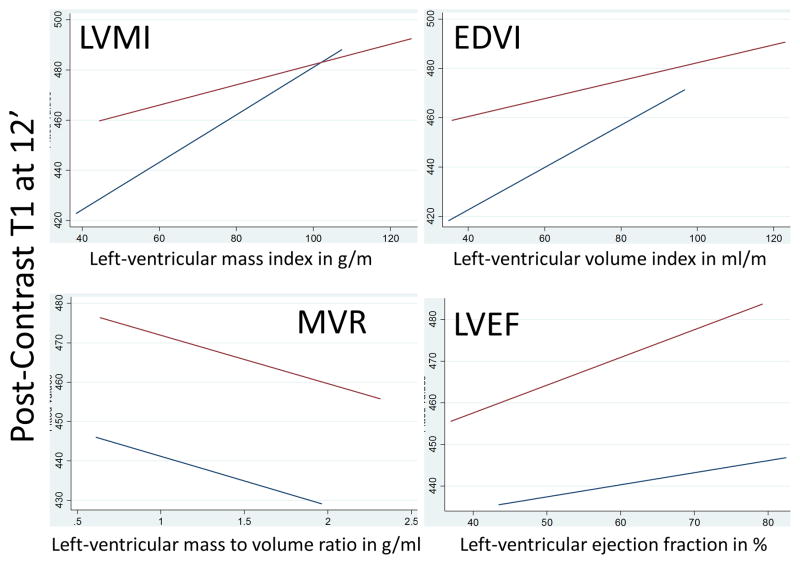
Table 3 (and Supplement Table S2) shows association of T1 times with LV structural and functional parameters. Cross-sectional analyses at follow up (year 10 exam) revealed that lower LVMi and EDVi were associated with lower post-contrast T1 times reflecting greater interstitial fibrosis. Regression coefficients were greater in women versus men as indicated in adjusted linear regression plots in Figure 1. Approximately 1 g/m2 lower LVMI was related to 1.0–1.2 ms lower post-contrast T1 times in women, while the corresponding value was 0.35–0.45 ms in men. In men also, lower LVEF was associated with lower post-contrast T1 reflecting greater fibrosis. Greater concentric remodeling indexed as higher MVR was related to higher pre-contrast T1 times (greater fibrosis) in both univariable (coef: 15.67, p=0.021) and multivariable (coef: 17.46, p=0.018) analyses but in men only. Pre-contrast T1 times showed weak inverse correlations with LVMi and EDVi but not with LVEF and only in univariate analyses. None of the other parameters showed any statistically significant relation with pre-contrast T1 times even after gender-specific analysis.
Table 3.
Coefficients for cross-sectional multivariable linear regression of T1 12′ times at year-10 with LV size and function at year-10, as well as T1 times at year-10 with 10-year change in LV size and function between the baseline (year 0) and follow up (year 10) CMR exams.
| Variable | Cross-sectional | Longitudinal | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Uni | Multi | r2 | Uni | Multi | r2 | ||
|
| |||||||
| Δ | BL | Δ | |||||
| WOMEN | |||||||
|
| |||||||
| LVMi | 0.99† (0.61,1.36) | 1.16† (0.83,1.48) | 0.48 | 0.68† (0.28, 1.09) | 1.10† (0.72, 1.48) | 1.13† (0.75, 1.53) | 0.48 |
| EDVi | 0.86† (0.56,1.15) | 0.63† (0.38,0.88) | 0.45 | 0.30 (−0.04, 0.65) | 0.64† (0.35, 0.93) | 0.60† (0.29, 0.90) | 0.47 |
| MVR | −12.60 (−31.38,6.17) | 2.63 (−13.77,19.02) | 0.43 | 5.86 (−13.60, 25.31) | −2.35 (−25.82, 20.79) | 3.06 (−14.61, 20.72) | 0.43 |
| LVEF | 0.55* (0.01,1.09) | 0.25 (−0.19,0.68) | 0.43 | 0.35 (−0.14, 0.83) | 0.08 (−0.51, 0.67) | 0.27 (−0.19, 0.72) | 0.44 |
|
| |||||||
| MEN | |||||||
|
| |||||||
| LVMi | 0.38† (0.14,0.63) | 0.35* (0.09,0.61) | 0.17 | 0.43† (0.15,0.71) | 0.22 (−0.08,0.51) | 0.64† (0.34,0.91) | 0.30 |
| EDVi | 0.32† (0.13,0.51) | 0.27** (0.07,0.47) | 0.17 | 0.01 (−0.20,0.22) | 0.31* (0.09, 0.53) | 0.22 (−0.00,0.43) | 0.30 |
| MVR | −8.61 (−20.32,3.09) | −8.02 (−20.49,4.46) | 0.16 | 8.25 (−2.99,19.49) | −20.84* (−36.89, − 4.79) | −0.24 (−12.38,11.89) | 0.29 |
| LVEF | 0.786† (0.39,1.18) | 0.48* (0.07,0.90) | 0.17 | 0.67† (0.31,1.02) | 0.02 (−0.52,0.56) | 0.55* (0.14,0.95) | 0.29 |
Coefficients and 95% confidence intervals (in brackets) for multivariable linear regression models to assess the cross-sectional associations, and baseline and change associations of CMR variables with T1 post-contrast 12′ times at year 10. Multivariable models adjusted for risk factor values at year-10 for cross-sectional associations. For associations with baseline (BL) and change (Δ) in values, models adjusted for baseline value and change in value of covariates. Additionally, models adjusted for gadolinium dose and glomerular filtration rate at year-10 for post-contrast T1 times.
if p<0.05,
if p<0.001.
LVMi: LV mass indexed to body surface area (g/m2). EDVi: end-diastolic volume indexed to body surface area (ml/m2). MVR: LV mass to end-diastolic volume ratio (g/ml). LVEF: LV ejection fraction (%).
Figure 1.
Plots showing adjusted linear regression fits for men (red line) and women (blue line) with LV structure and function parameters at year-10 on the x-axis and post-contrast T1 times in ms at 12 minutes at year-10 on the y-axis. Linear regression fits obtained after adjustment for traditional cardiovascular risk factors.
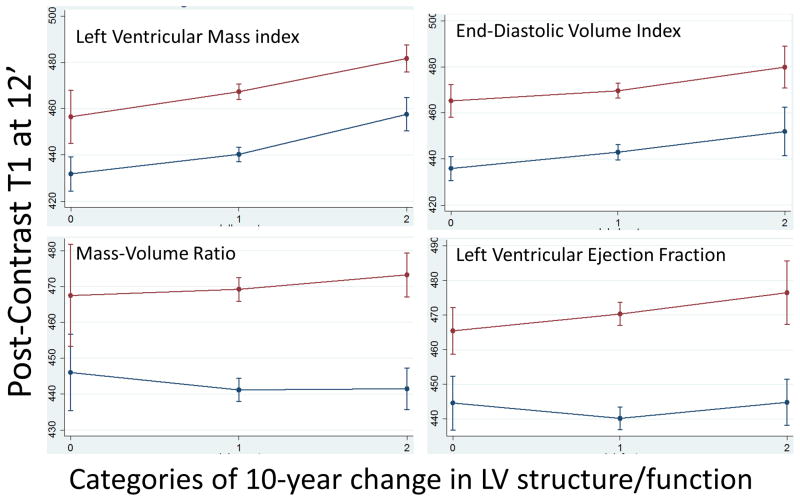
Longitudinal analyses revealed that a decrease in LVMi (in both men and women), EDVi (in women only) and LVEF (in men only) was related to a lower post-contrast T1 at follow-up as shown in Table 3 (and supplemental Table S2). Categorical analyses revealed similar results as shown in Figure 2. Baseline concentric remodeling as indexed by higher MVR corresponded to lower post-contrast T1 times measured at the 10-year follow-up exam reflecting greater fibrosis, but this was significant only in men. Lower baseline values of LVMi, EDVi and LVEF were related to lower post-contrast T1 times. These relationships remained significant after repeating the analysis stratified by gender. In men only, higher baseline MVR (coef: 28.95, p=0.005) but not its’ change was related to increased pre-contrast T1 times. None of the other baseline parameters or their change showed statistically significant relation with pre-contrast T1 times. When LV mass was indexed using the allometric approach, the results remained consistent and similar with what was observed for LVMi (indexed to BSA) as shown in Supplemental Table S4.
Figure 2.
Plots showing marginal means and standard deviations for men (red line) and women (blue line) with categories of 10-year change in LV structure and function parameters on the x-axis and post-contrast T1 times at 12 minutes in ms at year-10 on the y-axis. Categories represent: 0 – decrease of over one standard deviation, 1 – change of parameter within one standard deviation, 2 - increase of over one standard deviation. Marginal means obtained after adjusting for traditional cardiovascular risk factors.
Hypertension status, fibrosis and LV structure and function
LVMi and MVR were greater in those with hypertension at both the baseline (year-0) and follow up (year-10) MESA examinations. LVEF was greater in women with hypertension as compared to those without hypertension. The percentage of participants with myocardial scar was significantly higher in those with hypertension as compared to those without hypertension. Post-contrast T1 times were also lower reflecting greater interstitial fibrosis in those with hypertension as compared to those without hypertension. However, this was statistically significant in men only (Table 4). 8 women and 24 women were categorized as having hypertension at year-0 but not at year-10. Since the number of participants in this group was small, it was omitted from analysis.
Table 4.
Hypertension status, fibrosis and LV hypertrophy.
| Variable | Women (n=866) | Men (n=915) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 0(n=377) | 1(n=196) | 2(n=293) | p | 0(n=415) | 1(n=208) | 2(n=292) | P | |
| Year-10 | ||||||||
|
|
||||||||
| LVMi | 56±8 | 60±10 | 63±10 | <0.001 | 71±11 | 75±12 | 76±15 | <0.001 |
| EDVi | 63±11 | 61±11 | 62±11 | 0.18 | 70±14 | 68±15 | 68±16 | 0.08 |
| MVR | 0.92±0.2 | 1.01±0.2 | 1.03±0.2 | <0.001 | 1.04±0.2 | 1.14±0.3 | 1.16±0.3 | <0.001 |
| LVEF | 63.1±6 | 63.8±7 | 64.5±7 | 0.018 | 59.3±6 | 59.5±8 | 59.4±8 | 0.95 |
|
|
||||||||
| Year-0 | ||||||||
|
|
||||||||
| LVMi | 56±8 | 58±8 | 63±9 | <0.001 | 67±9 | 70±10 | 72±12 | <0.001 |
| EDVi | 67±10 | 67±11 | 68±10 | 0.34 | 73±13 | 72±14 | 71±14 | 0.08 |
| MVR | 0.84±0.1 | 0.89±0.1 | 0.94±0.1 | <0.001 | 0.93±0.2 | 1.00±0.2 | 1.05±0.2 | <0.001 |
| LVEF | 63.1±5 | 63.6±6 | 64.6±5 | 0.002 | 61±5 | 60.7±6 | 61.5±6 | 0.31 |
|
|
||||||||
| Fibrosis Measures | ||||||||
|
|
||||||||
| T1 pre | 986±45 | 984±48 | 989±45 | 0.74 | 966±37 | 970±38 | 969±53 | 0.65 |
| T1 12′ | 444±43 | 437±40 | 438±39 | 0.11 | 471±33 | 467±32 | 460±37 | 0.002 |
| T1 25′ | 508±42 | 501±41 | 501±39 | 0.11 | 536±35 | 530±37 | 523±37 | <0.001 |
| Scar % | 1.06 | 2.55 | 4.44 | 0.022 | 8.67 | 14.42 | 18.49 | 0.001 |
Mean±SD for CMR variables between 3 groups for men and women in - those without hypertension at year-0 and year-10 (Group 0); those with hypertension at year-10 but not at year-0 (Group 1); and those with hypertension at both year-0 and year-10 (Group 2). Hypertension was determined based on JNC VI criteria. P-value shown is for oneway ANOVA based on the three defined categories. LVMi: LV mass indexed to body surface area (g/m2). EDVi: end-diastolic volume indexed to body surface area (ml/m2). MVR: LV mass to end-diastolic volume ratio (g/ml). LVEF: LV ejection fraction (%). T1 pre, T1 at 12′, T1 at 25′: T1 time at T1 time pre-contrast injection, 12 and 25 minutes post-contrast injection respectively (ms).
DISCUSSION
This study investigates the relationship between LV remodeling characterized by alterations of LV structure and function that occurred over a 10-year period, with CMR-derived parameters of replacement and interstitial myocardial fibrosis measured at follow up. Our study shows that in a large multi-ethnic population of middle-to-older aged women and men followed over 10 years: (a) LV structural and functional correlates of replacement fibrosis from LGE are different from those of diffuse myocardial fibrosis from T1 mapping; (b) there are differences in these correlations of myocardial fibrosis with LV remodeling in women versus men; and (c) hypertension is related to myocardial hypertrophy, and both replacement and interstitial fibrosis.
Replacement myocardial fibrosis
Previous studies have shown cross-sectional associations of replacement myocardial fibrosis with systolic and diastolic dysfunction, adverse LV remodeling and mortality4, 17. Our study shows similar cross-sectional associations of replacement fibrosis with LV structural and functional indices in the MESA follow up exam. In addition, LV structural and functional parameters measured 10 years previously, as well as their 10-year change, were related to presence of replacement fibrosis in the MESA follow up exam. Increased myocardial mass and concentricity, and decreased function were associated with the presence of LGE-defined scar defined follow up. Importantly, in men only, presence of replacement fibrosis was also associated with LV dilatation during the follow up period.
Progressive LV remodeling arising from hypertension, diabetes and other cardiovascular risk factors may lead to hypertrophied myocytes and increased LV concentric remodeling. Myocardial overload coupled with impaired microvascular circulation can result in focal myocyte necrosis leading to focal replacement fibrosis4, 18. On the other hand, sudden loss of myocardial tissue due to myocardial infarction, as well as progressive replacement fibrosis induced by the mechanisms mentioned above may induce eccentric remodeling characterized by lengthened myocytes, greater volume and reduced LVEF. Therefore, as demonstrated, focal/replacement fibrosis as cause or consequence of myocardial injury, is associated with compensatory mechanisms known to influence the residual myocardium leading to concentric or eccentric hypertrophy accompanied by dilatation or LV volume reduction.
Diffuse myocardial fibrosis
In our study, post-contrast T1 mapping of the myocardium, with lower values indicating greater fibrosis, showed the strongest and most consistent relationships with parameters of LV remodeling. In women, lower LV mass and chamber volumes at baseline, and their further longitudinal decrease over a 10-year follow-up period, were related to greater interstitial fibrosis. In men, lower LV chamber volume at baseline, and a reduction over the 10-year follow-up period of time in LV mass and LVEF were consistently related to greater diffuse interstitial fibrosis. Interestingly, in men, concentric remodeling at baseline was associated with greater interstitial fibrosis 10 years later but the cross-sectional association at the end of the follow up period was not statistically significant. Moreover, pre-contrast T1 times were positively associated with MVR only. This is perhaps indicative of pre-contrast T1 times being representative of both intracellular and extracellular alterations that become prominent only with advanced cardiac disease and greater hypertrophic concentricity.
The process of death and regeneration of myocytes continues through the entire cardiac life. Studies have shown that with aging, the process of cell regeneration slows down leading to noncompensated myocyte loss19 and progressively diffuse myocardial fibrosis20. The relationship of aging with diffuse myocardial fibrosis in the same population was studied in detail previously15. Ventricular remodeling secondary to exposure to cardiovascular risk factors places increased demands on this process of myocyte death and regeneration, and shifts this homeostasis further, in addition to the effects of structural and functional changes associated with aging alone20. This is evidenced in this study by the relation of baseline concentric remodeling, as well as decreased LVMi and chamber volume with diffuse interstitial fibrosis. Therefore, while focal/replacement fibrosis, indicative of more extensive myocyte loss, is easily detectable using LGE and is associated with increased LV mass and chamber size, cardiac aging, characterized by diffuse interstitial fibrosis detected by T1 mapping, may be associated with both decreased LV mass and chamber size.
Gender differences in myocardial fibrosis and ventricular remodeling
Gender-related differences in adaptations to increased load and myocardial dysfunction have been studied before21. These results support the concept that in population studies women and men undergo different patterns of LV remodeling either due to different exposures to injurious mechanisms or different responses to cardiovascular risk factors over time. For associations with replacement fibrosis, subtle gender differences were observed (higher magnitude of coefficients in women) indicating that replacement fibrosis is associated with larger changes in structure and function in women as compared to men. Figure 1 illustrates associations of LV structure and function with diffuse fibrosis. Higher coefficients in women as compared to men were observed in relation with LV mass. The magnitude of concentric remodeling was also considerably lower among women versus men. This perhaps results from a combination of processes: (a) adverse remodeling is more common in men21; (b) reduction in the number of myocytes is faster in men as compared to women, and myocyte turnover is also slower in men than women19; (c) however, the size of myocytes is in average larger in men as compared to women19; finally, (d) the coefficients also reflect the fact that average LV mass and LV volumes are higher in men as compared to women even after body-size indexing. Lower LV chamber volume over a 10-year period was strongly related to diffuse interstitial fibrosis in women, perhaps indicating restrictive filling with eventual diastolic dysfunction due to a stiffer LV. This process of increased diffuse myocardial fibrosis with fewer myocytes (related to aging and remodeling) could explain the enhanced prevalence of diastolic heart failure seen in older men, and especially women22. On the other hand, while there was a non-significant relationship between diffuse fibrosis and LVEF in women, in men, increasing fibrosis had a strong association with reduced LVEF, reflecting greater systolic dysfunction.
Hypertension, fibrosis and hypertrophy
We also demonstrate in this study that in those with continued hypertension (baseline and follow-up exam), there is significantly greater concentric remodeling and greater fibrosis (both replacement and diffuse). Increased blood pressure is related to increased myocyte hypertrophy as well as concentric remodeling so that LV chamber performance can be maintained (as evidenced by either maintained or increased LVEF). The process of increased reactive fibrosis linked to the activation of the renin-angiotensin aldosterone system might potentially be at play enhancing alterations in those with hypertension23. The relationship of the presence of replacement fibrosis assessed with delayed enhancement in particular, was strong. There was a two (men) to four (women) fold increase in the percent of participants who had replacement fibrosis in the group with sustained hypertension as compared to those without hypertension.
Those with sustained hypertension also had increased diffuse myocardial fibrosis compared to those without hypertension in this population; however, this relationship was weaker than that observed for replacement fibrosis and hypertension. The quantification of diffuse or interstitial fibrosis using T1 times provides us with a measure of diffuse fibrosis/volume of extracellular matrix relative to that of the volume of the whole myocardium. This definition has an inherent disadvantage - with increasing myocyte hypertrophy (and hence total myocardial volume), as seen in hypertension, the sensitivity of this method of quantification to detect small changes in diffuse fibrosis might be reduced. This could potentially be among the reasons that interstitial fibrosis was only modestly increased in those with sustained hypertension compared to those without hypertension. The effective change in the amount of diffuse fibrosis in addition to hypertrophy, concurrently with hypertension status in a longitudinal fashion would perhaps shed more light on this relationship.
Perspective
Temporal changes in left ventricular structure and function help in studying the complex process of remodeling of the left ventricle with aging and hypertension. Myocardial fibrosis is considered as one of the chief factors that influence the process of cardiac disease and remodeling. This study explores the relationship between temporal changes in left ventricular structure and function, and diffuse and interstitial myocardial fibrosis.
Limitations
To our knowledge, this is the first study associating longitudinal changes in LV structure and function to myocardial fibrosis in a large multi-ethnic population. This population was free of any cardiovascular disease at baseline and hence the conclusions from this study may be limited by survival bias. An analysis of changes in myocardial fibrosis concomitantly with LV remodeling could not be performed as fibrosis indices were available only at follow-up. In this study, we assess the association of temporal changes of LV structure and function with cross-sectional data with respect to fibrosis measures, inferences with respect to longitudinal changes in fibrosis with changes in LV structure and function could not be made. In the process of a 10–12 year longitudinal study such as one in our study, it is a reality that there would be changes in personnel, technology and methodology. To calibrate for and account for changes that occur because of changes in pulse sequences and readers between the baseline and follow-up exams, utmost care was taken. A detailed explanation of this process has been provided in the supplemental document; however, we do acknowledge that this introduces additional variability in the measurement of temporal changes.
Conclusions
This study shows that while increasing LV hypertrophy and decreasing ejection fraction over 10 years are associated with replacement fibrosis, decreasing LV mass over 10 years, perhaps related to age and risk factor mediated myocyte loss, were associated with diffuse myocardial fibrosis. Increased concentricity in women only and LV dilatation in men only were associated with replacement fibrosis indicating gender differences in remodeling mechanisms. In addition, ejection fraction was preserved with increased diffuse myocardial fibrosis in women but was reduced in men. Finally, hypertension induced remodeling is related to enhanced replacement and interstitial fibrosis as well as hypertrophy in a multi-ethnic population of free-living individuals.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Exploration of the relationship of changes in LV structure and function over 10 years with fibrosis at the end of follow-up in multi-ethnic free-living individuals.
The LV structural and functional correlates of replacement and diffuse interstitial fibrosis are different.
Gender-specific associations.
What is relevant?
The longitudinal study of LV remodeling, an important component in hypertensive heart disease.
The relationship of LV remodeling and sustained hypertension with fibrosis.
Summary.
Over a 10-year period increased concentric hypertrophy in women and LV dilatation in men was associated with replacement fibrosis; while decreasing LVMi was associated with diffuse fibrosis. Hypertension induced remodeling is related to enhanced replacement and diffuse fibrosis as well as hypertrophy.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/ (UID: NCT00005487).
SOURCES OF FUNDING
This research was supported by contracts N01-HC-95159 through N01-HC-95168 from the National Heart, Lung, and Blood Institute.
Footnotes
DISCLOSURES
None.
References
- 1.Kramer CM, Lima J, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, Yeh I-T, Tallant B, Axel L. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation. 1993;88:1279–1288. doi: 10.1161/01.cir.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 2.Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, Becker LC, Lima JA. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101:2734–2741. doi: 10.1161/01.cir.101.23.2734. [DOI] [PubMed] [Google Scholar]
- 3.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level--risk factors, screening, and outcomes. Nature reviews. 2011;8:673–685. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 4.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 5.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen E-L, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 6.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 7.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced t1 mapping. Journal of the American College of Cardiology. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, Liu CY, McClelland RL, Bluemke DA, Lima JA. Age, gender and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: The multi-ethnic study of atherosclerosis. Circulation. 2012;126:2481–2490. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, Mudd JO, van der Geest RJ, Lima JA, Halushka MK. T1 mapping in cardiomyopathy at cardiac MR: Comparison with endomyocardial biopsy. Radiology. 2012;265:724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messroghli D, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, Oh-Ici D, Klein C, Berger F, Kuehne T. Assessment of diffuse myocardial fibrosis in rats using small animal look-locker inversion recovery t1 mapping. Circ Cardiovasc Imaging. 2011;4:636–640. doi: 10.1161/CIRCIMAGING.111.966796. [DOI] [PubMed] [Google Scholar]
- 11.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K. Multi-ethnic study of atherosclerosis: Objectives and design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (multi-ethnic study of atherosclerosis) study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messroghli D, Plein S, Higgins D, Walters K, Jones T, Ridgway J, Sivananthan M. Human myocardium: Single-breath-hold MR t1 mapping with high spatial resolution-reproducibility study. Radiology. 2006;238:1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 15.Liu C-Y, Liu Y-C, Wu C, Armstrong A, Volpe GJ, Van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced t1 mapping: MESA (multi-ethnic study of atherosclerosis) Journal of the American College of Cardiology. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gai N, Turkbey EB, Nazarian S, van der Geest RJ, Liu CY, Lima JA, Bluemke DA. T1 mapping of the gadolinium-enhanced myocardium: Adjustment for factors affecting interpatient comparison. Magnetic resonance in medicine. 2011;65:1407–1415. doi: 10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. Journal of the American College of Cardiology. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophyrelation to remodeling. Journal of the American College of Cardiology. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 19.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: Effects on the human heart. Journal of the American College of Cardiology. 1995;26:1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 20.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 21.Luchner A, Bröckel U, Muscholl M, Hense H-W, Döring A, Riegger GAJ, Schunkert H. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: A population-based study. Cardiovascular Research. 2002;53:720–727. doi: 10.1016/s0008-6363(01)00510-7. [DOI] [PubMed] [Google Scholar]
- 22.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. Journal of the American College of Cardiology. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 23.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.