Abstract
Neuropsychologists are developing more challenging and specific tests to detect early and subtle changes in cognition related to preclinical Alzheimer's disease (AD). The 16-item Face-Name Associative Memory Exam (FNAME-16) is a challenging paired associative memory test able to detect subtle memory changes associated with biomarker evidence of preclinical AD. However, as individuals progress along the AD trajectory, measures that are sensitive at the preclinical stage may become too challenging by the stage of Mild Cognitive Impairment (MCI). Our goal was to develop a modified version of the face-name and face-occupation paired associative memory task (FNAME-12) with fewer stimuli and additional learning trials suitable for use in MCI. We administered the FNAME-12A, an alternate version FNAME 12B, the original FNAME-16, and a series of other neuropsychological measures to 65 clinically normal (CN) older adults (aged 65 to 85) and a subsample characterized by MCI (n=18). The FNAME-12 exhibited psychometric equivalence with the FNAME-16 (r=0.77, p<.001) and was correlated with other measures of episodic and semantic memory. The alternate form, FNAME-12B, was highly correlated with FNAME-12A (r=0.76, p<.001). Mean performance on the FNAME 12A, stratified by education, was generated. The task was able to be completed by our MCI group yet remained challenging in the CN group, providing evidence of its utility along the AD trajectory.
Keywords: associative memory, preclinical Alzheimer's disease, Mild Cognitive Impairment, neuropsychology, test development
INTRODUCTION
Longitudinal and neuropathological studies indicate that Alzheimer's disease (AD) has a long protracted preclinical phase, where the pathological changes are occurring 10-15 years prior to the emergence of clinical symptoms (Pike et al., 2011, Price et al., 2009). As a result, clinical trials for AD have moved toward preventing decline in clinically normal (CN) older adults who have biomarker evidence of AD but still perform normally on traditional neuropsychological measures (Sperling et al., 2011b). As neuropsychologists are asked to diagnose people with preclinical AD, we may require different tests that are sensitive to this biomarker stage of AD. A number of experimental measures derived from translational neuroscience are now in development and specifically designed to be sensitive to these earliest and potentially very subtle cognitive and behavioral changes (Rentz et al., 2011, 2013).
Longitudinal and epidemiological studies identify changes in episodic memory such as delayed recall and paired associative learning as heralding preclinical AD (Blackwell et al., 2004; Elias et al. 2000). Paired associative memory tasks, such as the Free and Cued Selective Reminding Test (FCSRT) have been particularly successful in differentiating normal aged individuals from those who are at-risk for progression to MCI and AD (Amariglio et al. 2012, Parra, et al., 2010, Grober et al., 2008; Lindeboom et al. 2002). The success of the FCSRT is predicated on being able to differentiate AD from non-AD memory loss because it improves encoding specificity by means of pairing the word to be remembered with a category/ semantic cue (Wiggs, Weisberg, & Martin, 1998). As a result, the FCSRT induces deep semantic encoding which maximizes learning and recall. Individuals with MCI and AD have a remarkable reduction in sensitivity to cueing at the recall stage on the FCSRT (Rentz et al. 2013). This is thought to be associated with the pathological changes occurring in the hippocampus and temporolimbic networks responsible for memory consolidation involving conjunctions between unrelated stimuli (Konkel and Cohen, 1999) and semantic access, two essential features of the memory loss in AD.
The Face Name Associative Memory Exam (FNAME) (Rentz et al., 2011) designed by our group, is a behavioral version of a cross-modal associative memory test based on an fMRI task that pairs pictures of unfamiliar faces with common first names. The Face Name fMRI task has shown sensitivity to longitudinal clinical decline in MCI (O'Brien et al., 2010) as well as those at genetic risk for AD (Miller et al., 2008, Celone et al., 2006, Sperling et al., 2003) and is associated with beta-amyloid burden in CN older individuals (Sperling et al., 2009). Likewise, the neuropsychological measure FNAME-16, derived from Face Name fMRI tasks, has been shown to be related to beta-amyloid burden in CN elderly (Rentz et al., 2011).
The FNAME requires the participant to learn 16 novel, Face-Name and Face-Occupation pairs. This task is challenging in CN older adults and has proven too challenging in its length and attentional demands to longitudinally track those moving from CN to MCI. For this reason, we developed a modified 12-item version of the FNAME (FNAME-12) designed for use across the entire AD trajectory from CN older adults to MCI. This modified associative memory task, the FNAME-12, in contrast with the original FNAME-16, contains fewer stimuli, more learning trials, and a delayed recognition trial. However, the FNAME-12 continues to incorporate core features of the original FNAME: a paired associative learning paradigm and the ecologically valid complaint of many older adults, i.e., difficulty retrieving newly learned face-name pairs.
The goal of this study was to develop a psychometrically equivalent version of the original FNAME-16 for use in not only preclinical AD but also in individuals with more demonstrative cognitive difficulties (i.e., MCI). Specifically, we sought to create a test that was 1) feasible for individuals with MCI while remaining challenging in CN older adults, 2) demonstrated psychometric equivalence with the original FNAME-16 and other validated memory tests, 3) demonstrated internal consistency and 4) was able to be used longitudinally with a reliable alternate version.
METHODS
Participants
Sixty-five CN older adults were enrolled at the Center for Alzheimer's Research and Treatment at Brigham and Women's Hospital and at Massachusetts General Hospital. Participants were recruited from 3 longitudinal studies of aging designed to capture a spectrum of participants including CN individuals and those meeting criteria for MCI. Amongst other procedures in these longitudinal studies, individuals were screened by trained clinicians using the Clinical Dementia Rating Scale (CDR) involving participant and informant based report of cognitive functioning (Morris, 1993), review of medical history, and performance on neuropsychological screens (Mini Mental Status Exam MMSE, Logical Memory Delayed Recall- Wechsler Memory Scale-Revised WMS-R). Participants enrolled in the longitudinal studies were also required to consent to PET and MRI imaging. Our CN group was compromised of individuals who had previously participated in one of the longitudinal studies of aging (n=44), those who did not meet inclusion criteria for these longitudinal studies because of their inability to undergo MRI scans (i.e., metallic implants, claustrophobia, physical discomfort etc., n=6), and those who had been recruited but who were not willing to commit to the number and type of assessments and imaging required for participation (n=29). These individuals were classified as CN by cognitive performance and clinical judgment on CDR. CN participants scored above age and education adjusted cut-offs for MCI on Logical Memory II (which, for individuals over 65, is >8 points for those with >16 year of education, >4 points for those with 8-15 years of education, and >2 points for those with <7 years of education). In addition, CN met the following criteria: 1) obtaining 28/30 points or greater on the MMSE and 2) performing above the cut-off of 44/48 on the Total Recall of the FCSRT to ensure intact memory functioning. An exception to the MMSE cut-off was made for two participants whose scores fell below 28 but were included given their low educational achievement (9th and 11th grade) and otherwise normal performance on the FCSRT and other traditional neuropsychological measures.
A small sample of individuals with MCI were similarly recruited (n=18) from these longitudinal studies of aging to assess the feasibility of administering FNAME-12 in those with memory difficulties. These individuals were classified as having MCI based on clinical judgment and research criteria for early MCI used in ADNI, which includes 1) reports of subjective memory complaints corroborated by study partners and resulting in global scores ≥ 0.5 on the Memory Box Score of the CDR and 2) scores below age and education adjusted cut-offs on Logical Memory II. MCI participants additionally scored below the published cut-off for memory impairment of 44 on the Total Recall of the FCSRT. All participants were enrolled using informed consent protocols and procedures approved by the Partners Human Research Committee.
There were no differences between CN and MCI for age, education, or verbal IQ (see Table 1). The samples consisted generally of more females compared with males. In addition, the MCI group consisted of fewer males (27%) compared with the CN group (35%), however this is consistent with research showing higher incidence of AD in women (Andersen et al., 1999).
Table 1.
Demographic Characteristics and Performance on Traditional Neuropsychological Measures in the CN vs. MCI Groups
| Clinically Normal (CN) | Mild Cognitive Impairment (MCI) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| n | 65 | 18 | ||||
| Age | 73.82 | 6.14 | 64.93-85.40 | 70.48 | 7.25 | 64.90-81.42 |
| **Sex (% male) | 35.40 | 24.00 | ||||
| Education (years) | 16.68 | 2.66 | 9-20 | 17.28 | 2.16 | 12-20 |
| Verbal IQ (AmNART) | 121.82 | 8.05 | 95-131 | 119.50 | 9.45 | 96-130 |
| **MMSE (/30) | 29.02 | 1.11 | 25-30 | 26.40 | 2.41 | 22-30 |
| **FCSRT- Free Recall (/48) | 34.02 | 6.02 | 18-47 | 18.06 | 7.66 | 5-29 |
| **FCSRT- Total Recall (/48) | 47.63 | .64 | 45-48 | 37.65 | 9.45 | 21-48 |
| Verbal Fluency: F-A-S | 45.45 | 13.67 | 24-76 | 37.29 | 11.03 | 26-61 |
| *Verbal Fluency: 3 | 46.63 | 10.88 | 23-71 | 36.31 | 10.59 | 17-57 |
| Categories | ||||||
| VFDT (/32) | 30.29 | 2.38 | 21-32 | 29.07 | 3.56 | 20-32 |
| TMT A (secs) | 41.93 | 16.67 | 19-87 | 54.00 | 31.90 | 22-150 |
| TMT B (secs) | 98.45 | 52.98 | 28-277 | 126.67 | 80.70 | 41-300 |
Note: M=mean, SD=standard deviation, AmNART= American National Adult Reading Test, MMSE=mini mental state exam, FCSRT=Free and Cued Selective Reminding Test, VFDT=Visual Form Discrimination Test, TMT=Trail Making Test,
p<0.05
p<0.001 for independent t-test between CN and MCI
Neuropsychological evaluation
CN participants completed the following neuropsychological measures: FNAME-16 (Rentz et al., 2011, Amariglio et al., 2012), the FCSRT (Grober & Buschke, 1987), Verbal Fluency to letters F-A-S (Benton, Varney, Hamsher, & Spreen, 1983) and 3 Categories (Monsch et al., 1992), the Visual Form Discrimination Test (VFDT Benton et al., 1983), Trail Making Test A and B (Reitan, 1979) and FNAME-12A and/or B (described below) as well as questionnaires related to a secondary piloting project. Tests were administered to limit interference between memory tasks and no memory task was administered during a delay of another memory task. Individuals who received both versions A and B of FNAME-12 (n=34), completed the alternate version on a separate testing day. The MCI group completed an identical battery with the exception that they did not complete FNAME-16 and only received FNAME 12A.
Face-Name Associative Memory Exam (FNAME) Procedure
The FNAME-12A and its alternate form FNAME-12B have been designed as an abbreviated version of the original FNAME, which is described in depth elsewhere (Rentz et al., 2011, Amariglio et al., 2012). The FNAME-121 requires the participant to learn 12 unfamiliar face-name pairs and 12 face-occupation pairs (see Figure 1 for a schematic of the task). The test consists of an initial learning phase, immediate cued recall, delayed cued recall, facial recognition, and a multiple choice recognition trial (see Table 2).
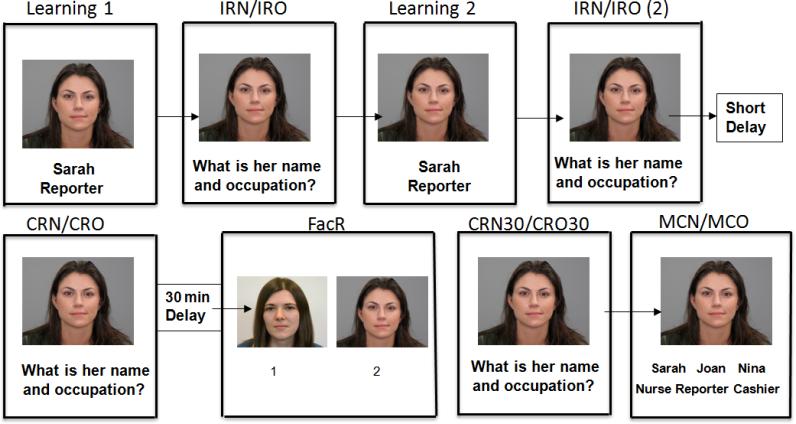
Figure 1.
Sample of 12-item FNAME procedure: Participants underwent 2 exposures to all 12 face, name, and occupation (Learning 1 and 2) groupings. Following each exposure, they were asked for name (IRN) and occupation (IRO) associated with each face. After a 5 minute short delay, they were asked for the name (CRN) and occupation (CRO) associated with each face. Following a 30-minute delay, they were asked to identify the previously learned face from 2 pictures (FacR). They were again asked for name (CRN30) and occupation (CRO30) associated with each face. For incorrect responses on CRN30 or CRO30, the participant was asked to select the name and/or occupation associated with the face amongst 3 items (MCN/MCO).
Table 2.
Definitions of Scales and Subscales for FNAME-12
| Abbreviation | Measure | Maximum Score | Definition |
|---|---|---|---|
| Subscales | |||
| IRN | Initial Name Recall | 24 | # of names learned over 2 trials |
| IRO | Initial Occupation Recall | 24 | # of occupations learned over 2 trials |
| CRN | Cued Recall of Names | 12 | # of names recalled |
| CRO | Cued Recall of Occupations | 12 | # of occupations recalled |
| FacR | Facial Recognition | 12 | # of faces correctly identified |
| CRN30 | Delayed Cued Recall of Names | 12 | # of names recalled |
| CRO30 | Delayed Cued Recall of Occupations | 12 | #of occupations recalled |
| MCN | Multiple Choice Recognition for Names | 12 | # Correct |
| MCO | Multiple Choice Recognition for Occupations | 12 | # Correct |
| Scales | |||
| FN-N | Name Learning & Retrieving Composite | 48 | IRN+CRN+ CRN30 |
| FN-O | Occupation Learning & Retrieving Composite | 48 | IRO+CRO+ CRO30 |
| Total | Composite of Name & Occupation Learning & Recall | 96 | FN-N + FN-O |
Initial learning phase
Participants are shown 12 faces, names, and occupations in succession on PowerPoint slides with one face per page. The examiner displays each stimulus for 8 seconds. Faces were obtained from consenting adults in the general public and all pictures were taken in color against a gray background. An equal number of men and women were included and efforts were made to include 4 minority faces per FNAME-12 version and to represent a broad range of ages (18+). Names were selected from the Social Security registry of the most popular names by year. Occupations were selected to cover a range of socioeconomic backgrounds (from plumber to lawyer). To ensure that the participant is attending to the items, the examiner points to the face and asks the participant to read the name and occupation associated with that face. After all 12 items are presented, the participant is shown each face and asked to recall the name and occupation associated with the face; they are allotted 15 seconds to produce an answer. The correct number of face-name pairs and the correct number of face-occupation pairs is recorded. This initial learning phase is repeated once using a different ordering of the faces but equivalent pairings. Items correctly learned for each trial are summed for a total score of initial recall of names (IRN) and a total score of initial recall of occupations (IRO).
Distracter Task
For a brief distracter task (approximately 5 minutes), participants are shown pictures of 12 well-known famous faces and asked to provide the names and occupations of the individuals and are allowed 20 seconds per face.
Cued Recall of face-name and face-occupation pairs
Participants are shown the previously learned 12 novel faces one at a time and asked to produce the name and occupation associated with each face resulting in scores for cued name recall (CRN) and cued occupation recall (CRO).
Delayed Recall and Recognition
Following a 30-minute delay, participants are shown slides of the previously learned face and age, race, and sex-matched distracter faces; they are asked to identify the target from the distracter (Facial Recognition). Participants are subsequently asked to provide the name and occupation associated with the previously learned face (CRN30, CRO30). If the participant is unable to produce the correct name or occupation, they are provided with multiple choice options and asked to select the correct name (MCN) and occupation (MCO) amongst: the correct name/occupation, a novel name/occupation, and a foil which is a name/occupation paired with a different face (see Figure 1).
FNAME Validation Procedure and Statistical Analysis
Previous work in a larger sample of older adults (n=210) showed 2 underlying factors comprising the original FNAME: face-name recall (IRN, CRN, CRN30) and face-occupation recall (IRO, CRO, CRO30) explaining 76% and 17% of the variance, respectively, in a Principal Components Analysis (Amariglio et al., 2012). Given these findings, we created equivalent summary scales for FNAME-12: FN-N items (IRN, CRN, CRN30) and FN-O items (IRO, CRO, CRO30) as well as a Total Score (FN-N + FN-O).
Form and Alternate Form Reliability
Chronbach's α was used to assess the internal consistency of both 12A and 12B. To assess for the equivalence and reliability of the alternate form FNAME-12B, a total of 34 CN participants completed both versions (at different visits). The test-retest time period was approximately 4.9 months so as to minimize practice effects while reducing the chance of capturing cognitive decline with longitudinal assessment in older adults (range of 1 to 36 weeks). To further address practice and order effects, Version A was administered on the first study visit for 61% of participants with Version B administered first in the remaining cases. Correlations (Pearson's r) were calculated for the scale and subscale scores in version A vs. B. Differences in performance between versions were assessed initially using pairwise t-tests for the Scale scores followed by pairwise t-tests on subscales. Correlations between versions were not calculated for recognition paradigms (FacR, MCN, MCO) given that most individuals performed at ceiling levels, resulting in minimal variance.
Psychometric Equivalence
To assess for convergent validity, we examined the relationship between FNAME-12A and FNAME-16 and the FCSRT (Grober & Buschke, 1987, Grober et al., 2008). We also examined the relationship between FNAME-12A and non-memory measures.
Mean CN Performance
We divided the sample using a median split in education and computed means and standard deviations for low and high education groups (>16 years and ≤ 16 years) for both Scale and individual Subscale scores.
RESULTS
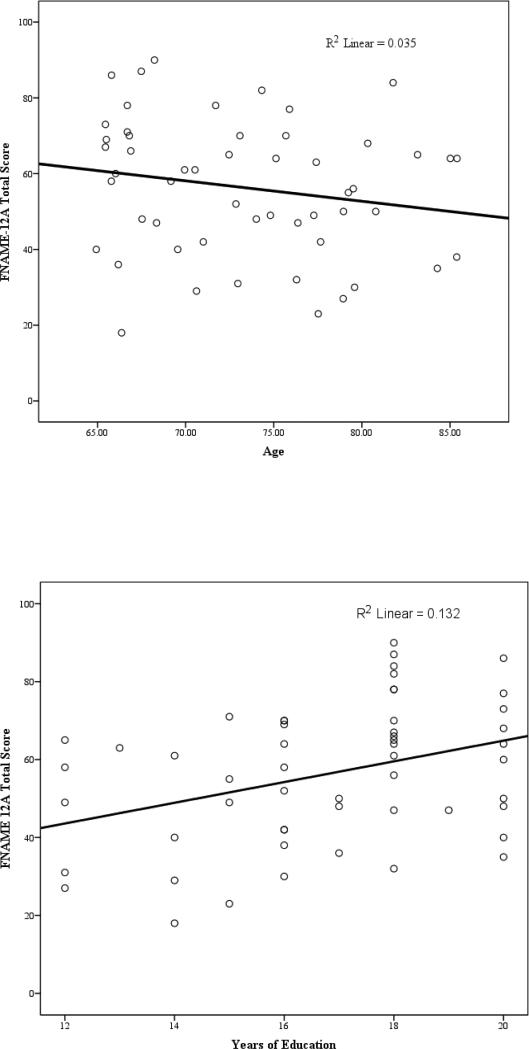
The total mean score for FNAME-12A in CN individuals was 56.70 out of 96; occupations were more frequently learned and remembered (34.76/48) compared with names (21.94/48). Performance on FNAME-12 was positively related to years of education for 12A (r=0.36, p=0.004) and 12B (r=0.33, p=0.013) and positively related to estimated premorbid IQ (AmNART) for 12A (r=.28, p=.023) and 12B (r=.40, p=.005). There was a non-significant trend for 12A and 12B to be related to age (p=.09). The relationship between age and 12A became significant when we examined either version 12A or 12B to account for cases where individuals did not complete both versions (see figure 2). Performance was not related to sex (for 12A, r=.150, p=.141 and for 12B, r=.138, p=.183). Versions 12A and 12B exhibited good internal consistency: α= 0.81 and 0.87 respectively. As expected, the total scores for 12A and 12B were highly correlated with the total score for the original FNAME-16 (see Table 3). Similarly, FN-N and FN-O were highly correlated for 12A, 12B, and FNAME-16 (see Table 3).
Figure 2.
Total Score on FNAME-12A and its relationship to Education and Age in CN Older Adults
Table 3.
Correlations (Pearson‘s r) between FNAME-16 and FNAME-12A and B in CN Older Adults
| 12A | 12B | ||||||
|---|---|---|---|---|---|---|---|
| FNAME Scales | FN-N | FN-O | Total | FN-N | FN-O | Total | |
| FNAME | FN-N | 0.75** | 0.71** | ||||
| 16 | FN-O | 0.69** | 0.57** | ||||
| Total | 0.77** | 0.68** | |||||
Note: FN-N=composite score for name learning, FN-O= composite score for occupation learning,
p<.001
Performance in CN vs. MCI
The task was able to be completed by individuals diagnosed with MCI with no basal scores or discontinuations of administration, yet it remained challenging in the CN group. CN participants scored better on FNAME-12 [t(68)=6.42, p=.000] compared with those classified as exhibiting MCI. More specifically, the total score for 12A in CN individuals was 56.28 out of 96 compared with 25.24 out of 96 in MCI (see Table 6). No individuals in either group performed at basal levels, although 1 CN older adult performed at the ceiling level (96/96).
Table 6.
Average Performance (means and standard deviations) for CN Older Adults and MCI (aged 65-85) for FNAME 12- A and 12-B by Education Level
| CN | MCI | |||||
|---|---|---|---|---|---|---|
| FNAME Scales & Subscales | ≤16 years of education | >16 years of education | ||||
| n=24 | n=30 | n=18 | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Scales | ||||||
| Total | 48.92 | 16.55 | 62.38 | 16.73 | 25.24 | 15.60 |
| FN-N | 15.87 | 9.52 | 24.55 | 10.83 | 6.63 | 4.731 |
| FN-O | 33.04 | 9.42 | 37.83 | 7.34 | 19.00 | 11.84 |
| Subscales | ||||||
| IRN | 6.88 | 4.36 | 10.86 | 4.76 | 2.94 | 2.33 |
| IRO | 15.96 | 4.23 | 17.79 | 4.06 | 9.65 | 5.17 |
| CRN | 4.96 | 3.21 | 7.25 | 3.23 | 1.76 | 1.64 |
| CRO | 9.00 | 2.80 | 10.43 | 1.71 | 5.00 | 3. 20 |
| FacR | 11.96 | 0.21 | 12.00 | 0.00 | 11.82 | 0.39 |
| CRN30 | 4.22 | 2.78 | 6.69 | 3.51 | 1.53 | 1.63 |
| CRO30 | 8.43 | 2.91 | 9.97 | 2.23 | 4.35 | 3.84 |
| MCN | 8.20 | 2.33 | 9.67 | 2.18 | 6.65 | 1.77 |
| MCO | 11.05 | 1.32 | 11.42 | 1.28 | 9.29 | 2.66 |
Note: SD=standard deviation, FN-N=composite score for name learning, FN-O= composite score for occupation learning, IRN=initial name learning, IRO=initial occupation learning, CRN=cued recall of names, CRO=cued recall of occupations, FacR= Facial Recognition, MCN=multiple choice names, MCO=multiple choice occupations
Alternate Form Reliability
The means, standard deviations, and correlation coefficients between Scale and Subscale scores for FNAME-12A vs.12B in CN older adults are provided in Table 4. While Total Scores between 12A and 12B were highly correlated (r=0.76, p=.000), participants scored better on 12A (M=56.70/96) versus 12B (mean=46.18/96). Upon further inspection, we found that participants exhibited more difficulty learning and retrieving face-occupation pairs in 12B vs. 12A, but performed equivalently for name learning and recall between versions. More specifically, participants initially learned an average of 13.58 on 12B compared with an average of 16.85 face-occupation pairs in 12A. This pattern was similarly observed in subscales of immediate and delayed recall of occupations (see Table 4).
Table 4.
Alternate-Form Reliability: Means, Standard Deviations, paired sample t-tests, and Correlations between FNAME Versions 12A and 12B in CN Older Adults
| FNAME-12 Scales & Subscales | Version A | Version B | Correlation (r) between A & B | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Scales | |||||
| **Total (/96) | 56.70 | 19.29 | 46.18 | 20.03 | .76** |
| FN-N (/48) | 21.94 | 11.35 | 19.18 | 11.97 | .77** |
| **FN-O (/48) | 34.76 | 9.00 | 27.00 | 9.55 | .69** |
| Subscales | |||||
| IRN (/24) | 9.91 | 5.17 | 9.15 | 5.65 | .76** |
| **IRO (/24) | 16.85 | 4.24 | 13.58 | 4.73 | .73** |
| CRN (/12) | 6.42 | 3.54 | 5.45 | 3.59 | .79** |
| **CRO (/12) | 9.45 | 2.72 | 7.77 | 2.59 | .61** |
| FacR(/12) | 11.97 | 0.18 | 11.90 | 0.54 | n/a |
| CRN30 (/12) | 5.52 | 3.39 | 4.87 | 3.65 | .70** |
| **CRO30 (/12) | 8.86 | 2.90 | 6.55 | 3.24 | .70** |
| MCN (/12) | 9.14 | 2.42 | 9.14 | 3.04 | n/a |
| MCO (/12) | 11.00 | 1.45 | 11.36 | 3.20 | n/a |
Note:SD=standard deviation, FN-N=composite score for name learning, FN-O= composite score for occupation learning, IRN=initial name recall, IRO=initial occupation recall, CRN=cued recall of names, CRO=cued recall of occupations, FacR= facial recognition, MCN=multiple choice names, MCO=multiples choice occupations, Pearson's r
p<.001, n/a see methods section
Psychometric Equivalence
Table 1 shows summary scores for performance on traditional neuropsychological measures for both CN and MCI. The MCI group performed worse on the FCSRT, verbal fluency, and TMT A but equivalently to the CN on TMT B and VFDT. Table 5 shows the relationship between traditional neuropsychological measures and performance on FNAME-12 in CN. Total scores on 12A and 12B were positively related to Free Recall on the FCSRT but not the Total Recall component (see Table 5). FNAME-12 scores were also positively related to category fluency but not to phonemic/letter fluency (F-A-S). FNAME -12A and 12B were not related to TMT A but performance on 12B was positively related to TMT B.
Table 5.
Correlations (Pearson’s r) between FNAME 12 Versions A and B and Traditional Neuropsychological Measures in CN Older Adults
| Cognitive Domain | Measure | FNAME 12A | FNAME 12B |
|---|---|---|---|
| Total Score | Total Score | ||
| Estimated Premorbid IQ | AmNART | .28* | .40* |
| Memory | FCSRT-Free Recall | .32* | .33* |
| FCSRT-Total Recall | .10 | .13 | |
| Executive Functions | Verbal Fluency: F-A-S | .19 | .19 |
| TMT A | −.16 | −.22 | |
| TMT B | −.17 | −.33* | |
| Semantic/Executive | Verbal Fluency: 3 Categories | .45* | .44* |
| Visuospatial Processing | VFDT | .01 | .04 |
Note: AmNART=American National Adult Reading Test, FNAME= face-name associative memory exam, FCSRT= Free and Cuec Selective Reminding Test, VFDT=Visual Form Discrimination Test, TMT=Trail Making Test,
p<.05
Sample Data
Mean performance for CN was provided. It was arranged by education level (>16 or ≤ 16 years of education) given the positive relationship between performance on FNAME-12 and years of schooling (see Table 6).
DISCUSSION
The Face Name Memory Exam (FNAME-12) exhibits promise as a measure of paired associative memory in CN older adults and those classified as having MCI. More specifically, the FNAME-12A exhibited psychometric equivalence with the original FNAME-16 and was related to other measures of memory. The alternate forms of FNAME-12 were highly correlated with each other, suggesting suitability for serial assessments. The FNAME-12 was well-tolerated by our MCI group with no basal scores or discontinuations of administration, yet it remained challenging in the CN group.
As researchers move toward treating individuals in the preclinical stage of AD, neuropsychologists are being asked to participate in identifying individuals earlier along the AD trajectory, where cognitive symptoms are potentially very subtle (Rentz et al., 2011, 2013). Traditional neuropsychological measures were not originally designed to detect or track these subtle changes but a number of measures have been developed to meet these goals. For example, the Memory Capacity Test from Herman Buschke and the FCSRT were designed to detect associative and semantic memory changes specific to AD (Rentz et al., 2010, Grober et al., 2008). Other examples of strategies to enhance the sensitivity of measures to AD include using change-detection or pattern-separation tasks such as The Short-Term Memory Binding (Parra et al., 2010, Didic et al., 2011) and The Behavioral Pattern Separation-Object (Stark, Yassa, Lacy & Stark, 2013). Given the changing demands of the field, neuropsychologists have been proactive in developing cognitive measures both capable of differentiating healthy aging from preclinical AD and capable of tracking symptom progression along the AD trajectory. The FNAME-12 is cross-modal, given evidence that memory tests requiring activation of multiple domains may be more sensitive to AD-related decline compared with domain-specific (i.e. verbal or visual) memory measures (Werheid & Clare, 2007). It exhibited psychometric equivalence with the original FNAME-16 as well as evidence of convergent validity with an established paired associative memory task, the FCSRT. The FNAME-12 was designed to incorporate semantic processing to enhance its specificity (Grober et al., 1987, Dudas, Clague, Thompson, Graham, & Hodges, 2005) and interestingly FNAME-12 performance was related to category fluency. Previous fMRI versions of FNAME require the coordinated activity of regions also activated during semantic processing (Binder & Desai, 2011). Extra learning trials and cued (rather than free) recall trials reduce the attentional burden of the task and can therefore clarify the cause of poor performance. As such, it was able to be completed by participants classified as exhibiting MCI. In addition, the task exhibits ecological validity given that associating names with new faces is a frequent occurrence in everyday life and declines in this ability are a common complaint in older adults.
The FNAME-12 is presented with a valid alternate form, increasing its potential to track cognitive progression, a necessity in neurodegenerative diseases. Internal consistency and alternate form reliability between FNAME-12A and 12B was exhibited. While the versions were highly correlated with one another, we did find that occupation learning was more challenging in version B versus A. One possible explanation for this finding is that version B included more subcategories of occupations; for example, florist rather than shopkeeper or economist rather than professor. Conceptualizing the semantic attributes of subcategories (i.e., florist and economist) has been shown to be vulnerable earlier in the AD trajectory compared with conceptualizing attributes of super-ordinate categories (i.e., shop-keeper and professor) (Giffard et al., 2002).
Finally, performance on the FNAME-12 was related to education level, but there was only a trend relationship with age. This could be considered a limitation of the study as we would expect memory to be related to age, but this finding may be an artifact of our relatively small sample size and abbreviated age range. Furthermore, a larger population size would be useful in determining the discriminant validity of the measure and providing normative data. Means and standard deviations for low and high education groups are provided, but should not be used independently to make diagnostic decisions given our sample size and given the fact that the FNAME-12 continues to be in development. However, we encourage use which further refines the measure and our understanding of its utility.
The next step in this line of research is to test performance across a larger sample and to establish criterion validity for this test i.e., to examine the relationship between FNAME-12 and AD-specific biomarkers. We are also developing Spanish versions of the FNAME to address the growing need for measures reflecting the demographics of the United States. As research and clinical trials move towards preventative treatment, it is imperative that neuropsychologists develop tools to both detect early cognitive signs of AD and to track symptom progression. Simple and cost effective neuropsychological measures will not only contribute tremendously to research and clinical care, but will enable neuropsychologists to adapt to evolving roles.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the NIH grants P01AG036694, P50AG005134, NIA grants 5T32AG023480 and 5R01AG027435 and the Alzheimer's Association SG COG-13-282201.
The authors declare that they have not financial interest or benefit arising from direct applications of this research.
We would like to acknowledge all of the research participants who make this work possible.
Footnotes
Test forms, scoring guidelines, and stimuli for FNAME-12 can be obtained for clinical and research use by emailing the corresponding author.
REFERENCES
- Alzheimer's Association Alzheimer's Disease Facts and Figures. Alzheimers Dement. 2013;9(2) doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Amariglio RE, Frishe K, Olson LE, Wadsworth LP, Lorius N, Sperling RA, Rentz DM. Validation of the Face Name Associative Memory Exam in cognitively normal older individuals. J Clin Exp Neuropsychol. 2012;34(6):580–587. doi: 10.1080/13803395.2012.666230. doi: 10.1080/13803395.2012.666230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JRM, Hofman A. Gender differences in the incidence of AD and vascular dementia The EURODEM Studies. Neurology. 1999;53(9):1992. doi: 10.1212/wnl.53.9.1992. doi: 10.1212/WNL.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Benton A, Varney N, desS.Hamsher K, Spreen O. Contributions to neuropsychological assessment. Oxford University Press; Oxford: 1983. [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends CognSci. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer's disease. Dement GeriatrCogn. 2004;17(1–2):42–48. doi: 10.1159/000074081. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deJager CA, Milwain E, Budge M. Early detection of isolated memory deficits in the elderly: The need for more sensitive neuropsychological tests. Psychol Med. 2002;32(3):483–491. doi: 10.1017/s003329170200524x. doi: 10.1017/S003329170200524X. [DOI] [PubMed] [Google Scholar]
- Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M, Ceccaldi M. Which memory system is impaired first in Alzheimer's disease? J Alzheimers Dis. 2011;27:11– 22. doi: 10.3233/JAD-2011-110557. doi: 10.3233/JAD-2011-110557. [DOI] [PubMed] [Google Scholar]
- Dudas RB, Clague F, Thompson SA, Graham KS, Hodges JR. Episodic and semantic memory in mild cognitive impairment. Neuropsychologia. 2005;43(9):1266–1276. doi: 10.1016/j.neuropsychologia.2004.12.005. doi:10.1016/j.neuropsychologia.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Archives of Neurology. 2000;57(6):808. doi: 10.1001/archneur.57.6.808. doi:10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. doi: 0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsych Soc. 2002;8(1):58–71. doi: 10.1017/S1355617701020069. [PubMed] [Google Scholar]
- Giffard B, Desgranges B, Nore-Mary F, Lalevee C, Beaunieux H, de la Sayette V, Eustache F. The dynamic time course of semantic memory impairment in Alzheimer's disease: clues from hyperpriming and hypopriming effects. Brain. 2002;125(9):2044–2057. doi: 10.1093/brain/awf209. doi: 10.1093/brain/awf209. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H. Genuine memory deficits in dementia. DevNeuropsychol. 1987;3(1):13–36. doi:10.1080/87565648709540361. [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C, Gorlyn M. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsych Soc. 2008;14(2):266. doi: 10.1017/S1355617708080302. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Frontiers in neuroscience. 2009;3(2):166. doi: 10.3389/neuro.01.023.2009. doi:10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73(2):126–133. doi: 10.1136/jnnp.73.2.126. doi:10.1136/jnnp.73.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the selective reminding test. J Clin Exp Neuropsychol. 1989;11(5):615–630. doi: 10.1080/01688638908400920. doi: 10.1080/01688638908400920. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. doi:10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133(9):2702–2713. doi: 10.1093/brain/awq148. doi:10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Pike KE, Ellis KA, Villemagne VL, Good N, Chételat G, Ames D, Rowe CC. Cognition and beta-amyloid in preclinical Alzheimer's disease: data from the AIBL study. Neuropsychologia. 2011;49(9):2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Manual for administration of neuropsychological test batteries for adults and children. Reitan Neuropsychology Laboratories; Tuscon, AZ: 1979. [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. doi:10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Parra M, Amariglio RE, Stern Y, Sperling RA, Ferris S. Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer's disease: A selective review. Alzheimer's Research & Therapy. 2013;5:58. doi: 10.1186/alzrt222. Doi:10.1186/alzrt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011a;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. doi: S1552-5260(11)00099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Aisen PS. Testing the Right Target and Right Drug at the Right Stage. Sci. Transl. Med. 2011b;3(111) doi: 10.1126/scitranslmed.3002609. 111cm33.doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51(12):2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. doi: 10.1016/j.neuropsych. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werheid K, Clare L. Are faces special in Alzheimer's disease? Cognitive conceptualization, neural correlates, and diagnostic relevance of impaired memory for faces and names. Cortex. 2007;43(7):898–906. doi: 10.1016/s0010-9452(08)70689-0. doi: 10.1016/S0010-9452(08)70689-0. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1998;37(1):103–118. doi: 10.1016/s0028-3932(98)00044-x. doi:/10.1016/S0028-3932(98)00044-X. [DOI] [PubMed] [Google Scholar]