Abstract
Summary
This multi-center, prospective, open-label, observational study evaluated the effects of once-monthly minodronate (50 mg) on treatment persistence, bone turnover markers, bone mineral density, low back pain, and upper gastrointestinal symptoms in outpatients with osteoporosis previously treated with daily or weekly bisphosphonate products.
Introduction
The purposes of this study were to investigate the effects of once-monthly oral minodronate (MIN 50 mg) on bone turnover markers and bone mineral density, low back pain, and upper gastrointestinal symptoms, as well as preference for and treatment persistence of MIN 50 mg among Japanese osteoporosis patients currently treated with daily or weekly bisphosphonates.
Methods
Study patients were allocated based on their preference to either the Switch group (patients willing to switch over to MIN 50 mg) or the Continue group (patients wanting to continue their current therapies). Patients’ treatment persistence and satisfaction levels with the therapies were assessed using a self-administered questionnaire. The study endpoints were serum TRACP-5b, serum P1NP, bone mineral density, upper gastrointestinal symptoms, and low back pain.
Results
In total, 264 and 133 patients were allocated into the Switch and Continue groups, respectively. Approximately, 65 % of patients were willing to switch to MIN 50 mg, with the predominant reason being “less frequent dosing more convenient.” Treatment persistence was significantly higher in the Switch group (MIN 50 mg) than the Continue group. Almost all patients with abnormal bone metabolism markers demonstrated normalization after switchover. MIN 50 mg alleviated low back pain and upper gastrointestinal symptoms induced by prior bisphosphonate use.
Conclusions
MIN 50 mg alleviates low back pain, reduces bone turnover markers and increases bone density, and induces fewer upper gastrointestinal symptoms after switchover from prior bisphosphonate products, and therefore, it may provide patients with a more convenient treatment option and enhance long-term treatment persistence.
Keywords: Bone mineral density, Minodronate, Once monthly, Persistence, Preference, TRACP-5b
Introduction
Bisphosphonates (BPs) are recommended as a first-line agent for osteoporosis treatment by many national and international guidelines [1–3]. However, the treatment persistence rate of BPs is far from satisfactory owing to its complex administration regimen and patients’ poor awareness levels of osteoporosis [4–9]. New drug formulations with less frequent dosing to resolve these problems have been developed, and weekly BP products have become mainstream in Japan, while monthly BPs are already familiar outside Japan. In September 2011, the first novel, once-monthly 50 mg formulation of minodronate (MIN 50 mg) was approved and marketed in Japan.
Minodronate is a third-generation bisphosphonate agent containing nitrogen [10] and approved for use for the treatment of osteoporosis in Japan [11]. Its mechanism of action involves inhibition of farnesyl pyrophosphate synthase activity [12], and therefore, MIN 50 mg shows strong inhibitory effects on bone resorption [13]. In an earlier clinical study, Okazaki et al. [14] reported that monthly minodronate at a dose of 30 or 50 mg had comparable efficacy to 1 mg daily administration in terms of change in bone mineral density (BMD) and bone turnover markers, with similar safety profiles.
The purposes of this multi-center, prospective, open-label, observational study were to investigate (1) the effects of once-monthly oral BP (MIN 50 mg) on bone turnover markers and bone mineral density (BMD), (2) analgesic effects of MIN 50 mg on low back pain (LBP) and upper gastrointestinal (GI) symptoms, and (3) preference for and treatment persistence of MIN 50 mg among Japanese patients with osteoporosis currently treated with daily or weekly bisphosphonates. Based on the findings of this study, we discuss the contribution of the once-monthly product to improving treatment persistence in outpatients with osteoporosis compared to treatment with conventional BP products.
Material and methods
This study was approved by the institutional review board at each participating study site. Prior to commencing any study procedure, the purposes and methods of this study were explained to all participants, and their written informed consent was obtained.
Study design
This observational study was conducted based on a multi-center, prospective, open-label, parallel-group comparison design, in outpatients with osteoporosis currently treated with daily or weekly BPs, at 11 institutions in Japan. A questionnaire form was used for data collection.
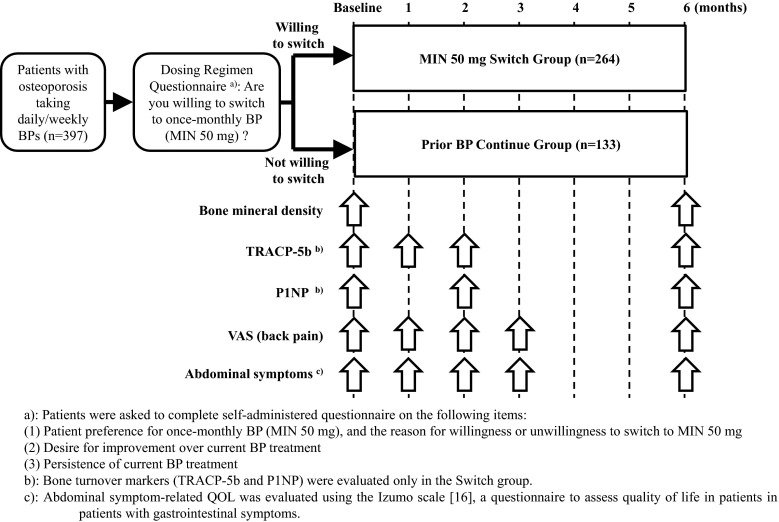
A total of 397 patients were enrolled in the study from October 2011 to April 2012 and asked their preference for once-monthly oral bisphosphonate for group allocation. Registered patients were allocated based on their answered preferences to either the “Switch” group (n = 264), consisting of patients who were willing to switch over to MIN 50 mg from their current therapies, or the “Continue” group (n = 133), consisting of patients who wanted to continue their current therapies (Fig. 1).
Fig. 1.
Study design and schedule. a Patients were asked to complete a self-administered questionnaire on the following items: (1) Patient preference for once-monthly BP (MIN 50 mg) and the reason for willingness or unwillingness to switch to MIN 50 mg. (2) Desire for improvement over current BP treatment. (3) Persistence of current BP treatment. b Bone turnover markers (TRACP-5b and P1NP) were evaluated only in the Switch group. c Abdominal symptom-related QOL was evaluated using the Izumo scale [16], a questionnaire to assess quality of life in patients with gastrointestinal symptoms
Study endpoints and schedule
The study endpoints were measured according to the following schedule: serum TRACP-5b (courtesy of DS Pharma Biomedical Co., Ltd., Tokyo, Japan), a bone resorption marker, at baseline and at 1, 2, and 6 months after the start of the study; serum P1NP, a bone formation marker, at baseline and at 2 and 6 months after the start of the study; and BMD of the lumbar spine, total hip, femoral neck, and/or 1/3 distal radius, at baseline and at 6 months after the start of the study. Upper GI symptoms were assessed by patients using a six-point symptom severity scale, and LBP was evaluated using a 100-mm visual analogue scale (VAS) and five items in the pain domain score of the Japanese Osteoporosis Quality of Life (JOQOL) Questionnaire [15]. Patients’ treatment persistence and satisfaction levels with the therapies were assessed using a self-administered questionnaire.
The self-administered questionnaire included the following items: (1) patient preference for once-monthly BP (MIN 50 mg) and the reason for willingness or unwillingness to switch to MIN 50 mg; (2) desire for improvement over current BP treatment; (3) patient persistence with current BP treatment; (4) patient persistence with MIN 50 mg over 6 months; and (5) satisfaction level in patients treated with MIN 50 mg and the reason for willingness to continue the medication.
Statistical analysis
All obtained data were statistically analyzed by one author (AS) using JMP software ver. 8.2 (SAS Institute, Cary, NC, USA). Continuous variables were summarized as mean ± standard deviation (SD), and categorical data were summarized as frequency and percentage (%). Time course comparisons from baseline were evaluated using the Wilcoxon signed-rank test, group comparisons at specific time points using the Wilcoxon rank-sum test or Student’s t test, and comparisons of incidences using the chi-square test or Fisher’s exact test. To assess treatment persistence, the data were illustrated as Kaplan-Meier survival curves according to treatment group and were analyzed using the log-rank test. All statistical tests were conducted with a significance level of α = 0.05, and no multiplicity adjustment was applied.
Results
A total of 397 patients using BPs for the treatment of osteoporosis were enrolled in the study. Study patients were divided into two study groups depending on their preference: patients in the Switch group (n = 264) were switched over to once-monthly minodronate (MIN 50 mg), and those in the Continue group (n = 133) continued treatment with their current daily or weekly BP.
Baseline characteristics of study patients
The demographic and baseline characteristics of study patients are summarized in Table 1. Overall, patients’ mean age was 76.0 years (range 54–98 y), and almost all study patients were female (94.2 %). The current bisphosphonate medication at baseline was predominantly a once-a-week product (78.8 %). Nearly half of patients (46.6 %) had concomitant diseases, including hypertension, hyperlipidemia, and diabetes mellitus. The most common concomitant medications were vitamin D analogues (57.7 %) and calcium agents (28.5 %).
Table 1.
Demographic and baseline characteristics of study patients
| Items | Unit | Total (n = 397) | Switch (n = 264) | Continue (n = 133) | p value† |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age |
Years (min, max) |
76.0 ± 7.8 (54, 98) |
75.9 ± 7.6 (54, 96) |
76.0 ± 8.1 (55, 98) |
N. S. |
| Sex |
Female (%) |
374 (94.2 %) |
252 (95.5 %) |
122 (91.7 %) |
N. S. |
| Current bisphosphonate medication |
Daily regimen Alendronate Risedronate Minodronate |
84 (21.2 %) 11 5 68 |
54 (20.5 %) 7 2 45 |
30 (22.6 %) 4 3 23 |
N. S. |
|
Weekly regimen Alendronate Risedronate |
313 (78.8 %) 228 85 |
210 (79.5 %) 157 53 |
103 (77.4 %) 71 32 |
N. S. | |
| BMI |
(kg/m2) (min, max) |
22.9 ± 3.4 (14.4, 34.3) |
23.0 ± 3.5 (14.4, 34.3) |
22.5 ± 3.1 (16.9, 32.9) |
N. S. |
| Previous fracture | Yes | 170 (42.8 %) | 106 (40.2 %) | 63 (47.4 %) | N. S. |
|
Spine Hip joint Wrist joint Other |
126 17 15 22 |
80 8 12 14 |
46 9 3 8 |
N. S. | |
| Comorbidity | Yes | 185 (46.6 %) | 118 (44.7 %) | 67 (50.4 %) | N. S. |
|
Diabetes M. Renal disease Rheumatism Hyperlipidemia Hypertension Arteriosclerosis |
25 3 10 39 119 19 |
14 1 4 23 74 8 |
11 2 6 16 45 11 |
N. S. | |
| Concomitant medication | Yes | 310 (78.1 %) | 206 (78.0 %) | 104 (78.2 %) | N. S. |
|
Vitamin D Vitamin K Calcitonin Calcium agents |
229 22 8 113 |
154 13 8 84 |
75 9 0 29 |
N. S. | |
|
PPI H2 blocker Gastroprotectives PGE2 derivatives |
36 31 52 26 |
25 14 30 19 |
11 17 22 7 |
N. S. | |
| NSAIDs | 66 (16.6 %) | 45 (17.0 %) | 21 (15.8 %) | N. S. | |
| Glucocorticoids | 3 (0.8 %) | 3 (1.1 %) | 0 (0.0 %) | N. S. | |
| Baseline characteristics | |||||
| Back pain VAS score |
(mm) (min, max) |
26.1 ± 20.9 (0, 80) |
27.9 ± 20.8 (0, 79) |
22.6 ± 20.7 (0, 80) |
0.010* |
| Abdominal symptoms score |
Heartburn (min, max) Epigastralgia (min, max) Epigastric fullness (min, max) |
1.34 ± 2.01 (0, 9) 0.85 ± 1.75 (0, 13) 1.22 ± 1.97 (0, 10) |
1.38 ± 1.98 (0, 8) 0.89 ± 1.78 (0, 13) 1.24 ± 1.90 (0, 10) |
1.26 ± 2.06 (0, 9) 0.76 ± 1.68 (0, 8) 1.17 ± 2.11 (0, 10) |
N. S. N. S. N. S. |
| BMD (lumbar spine) |
(g/cm2) (n) |
0.768 ± 0.139 (247) |
0.767 ± 0.141 (167) |
0.770 ± 0.136 (80) |
N. S. |
|
(YAM%) (n) |
75.6 ± 13.4 (247) |
75.6 ± 13.7 (167) |
75.4 ± 12.8 (80) |
N. S. | |
| BMD (total hip) |
(g/cm2) (n) |
0.617 ± 0.098 (107) |
0.622 ± 0.088 (56) |
0.611 ± 0.110 (51) |
N. S. |
|
(YAM%) (n) |
70.7 ± 11.2 (107) |
71.8 ± 9.6 (56) |
70.3 ± 12.8 (51) |
N. S. | |
| BMD (femoral neck) |
(g/cm2) (n) |
0.534 ± 0.091 (150) |
0.527 ± 0.087 (88) |
0.545 ± 0.095 (62) |
N. S. |
|
(YAM%) (n) |
66.6 ± 10.3 (150) |
65.7 ± 9.6 (88) |
67.9 ± 11.1 (62) |
N. S. | |
| BMD (distal radius) |
(g/cm2) (n) |
0.455 ± 0.072 (60) |
0.455 ± 0.076 (45) |
0.453 ± 0.062 (15) |
N. S. |
|
(YAM%) (n) |
69.9 ± 11.1 (60) |
70.0 ± 11.7 (45) |
69.7 ± 9.6 (15) |
N. S. | |
Data are expressed as mean ± standard deviation unless otherwise indicated
BMI body mass index, Diabetes M. diabetes mellitus, PPI proton pump inhibitor, NSAID non-steroidal anti-inflammatory drug, VAS visual analogue scale, BMD bone mass density, YAM young adult mean
†Comparison between Switch group and Continue group
*Statistically significant (α = 0.05) as analyzed by Wilcoxon rank-sum test
N. S. statistically not significant as analyzed by Wilcoxon rank-sum test or chi-square test
No significant differences were observed in the demographic characteristics of the Switch and Control groups. Among baseline characteristics, no significant differences were observed between the Switch and the Continue groups, except the VAS score for back pain (27.9 ± 20.8 in the Switch group vs. 22.6 ± 20.7 in the Continue group; p = 0.010).
With no statistically significant differences in the demographic and baseline characteristics between the groups, within-group comparisons were then conducted.
Patient preference and treatment persistence
Of 397 study patients, 264 (66.5 %) were willing to switch to MIN 50 mg, and 133 (33.5 %) were not willing to switch their current BP products. This trend, with two-thirds of patients willing to switch, was consistent across patients taking either daily or weekly BPs. In the daily dose subgroup, older patients showed a tendency to keep their current BP products. The most common reasons for willingness to switch to MIN 50 mg were “less frequent dosing more convenient” (87.3 %) followed by “easier to remember to take medication” (54.8 %). By contrast, the most common reasons for unwillingness to switch to MIN 50 mg were “being used to taking current product” (72.9 %) followed by “more chance to miss taking medication” (29.3 %) and “being satisfied with current BP” (27.8 %). The relationship between patients’ willingness to switch to MIN 50 mg and persistence of their prior BPs was investigated, revealing that patients who sometimes (n = 91) or often (n = 7) missed taking their current BPs (83.7 %, 82 of 98) were more willing to switch to MIN 50 mg than those who were completely persistent with their current BP regimen (60.8 %, 178 of 293) (p < 0.001).
Response to once-monthly minodronate
The early responses to once-monthly minodronate (MIN 50 mg) were evaluated by comparisons within the Switch group (change over time) or between the Switch and Continue groups.
Changes in BMD after 6 months of treatment (Fig. 2)
Fig. 2.
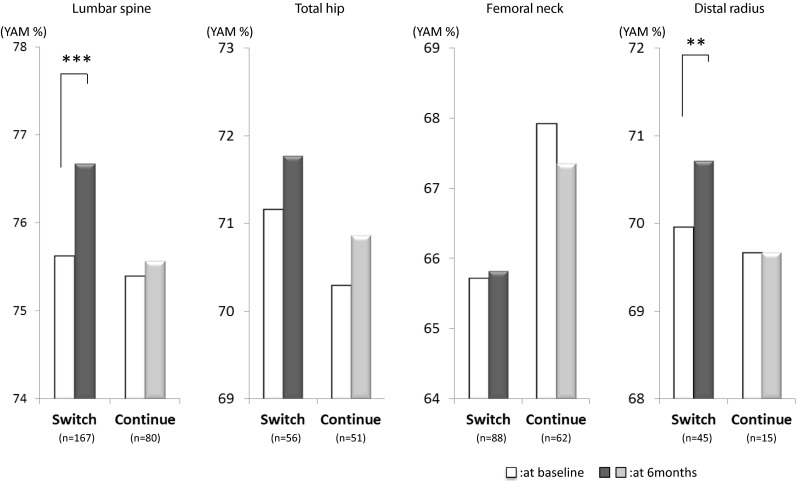
Changes in BMD after 6 months of treatment. YAM% young adult mean (%) **p < 0.01; ***p < 0.001, significantly different from baseline as analyzed by Wilcoxon signed-rank test
BMD in the Switch group, evaluated as the percentage of young adult mean values (YAM%), was significantly increased in the lumbar spine (1.5 %, p < 0.001) and distal radius (1.1 %, p < 0.01). In the subgroups of patients who switched from alendronate and risedronate, BMD was significantly increased in the lumbar spine (+1.1 %, p < 0.001 and +2.3 %, p < 0.01, respectively). In the subgroup of patients who switched from daily doses of minodronate, BMD was significantly increased in the lumbar spine (+3.2 %, p < 0.01). By contrast, no significant changes were observed in BMD in the total hip or the femoral neck in the Continue group or any of its subgroups.
Changes in bone metabolism markers after 6 months of treatment (Fig. 3)
Fig. 3.
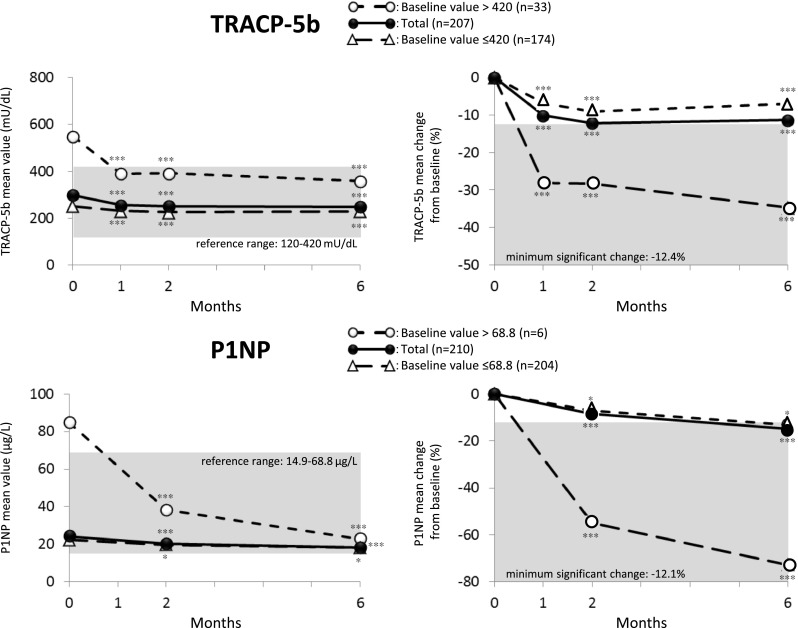
Time course of change in TRACP-5b (upper) and P1NP (bottom) after switchover to MIN 50 mg. Data were evaluated in patients who completed the 6-month measurements. ***p < 0.001; *p < 0.05, significantly different from baseline as analyzed by Wilcoxon signed-rank test
In the Switch group, the levels of the bone marker TRACP-5b (reference range 120 to 420 mU/dL) significantly reduced within 1 month after treatment (−10.2 %, p < 0.001) and at subsequent time points. Especially in the subgroup of patients with a higher baseline TRACP-5b (over 420 mU/dL), TRACP-5b decreased rapidly (by approximately 30 %) into the reference range within 1 month after treatment. By contrast, in the subgroup of patients with normal baseline TRACP-5b levels, values were maintained within the reference range without any unwanted reductions throughout the study period.
A subgroup analysis was conducted for each prior BP medication. Patients with a high baseline TRACP-5b level (>420 mU/dL) in the subgroup switched over from alendronate showed the same trend as the Switch group overall, with a reduction of approximately 25 %. In the subgroup switched from risedronate, a larger reduction of approximately 35 % was observed. In the subgroup switched from a daily dose of minodronate, a marked reduction of approximately 45 % was observed but was not statistically significant due to the small number of patients.
The bone marker P1NP (reference range 14.9 to 68.8 μg/L) was also significantly reduced within 2 months after treatment (−8.4 %, p < 0.001) and at subsequent time points. Especially in the subgroup of patients with a higher baseline P1NP (over 68.8 μg/L), P1NP decreased rapidly (approximately 55 %) into the reference range within 2 months after treatment. Similar to TRACP-5b, in the subgroup of patients with a normal baseline P1NP, values were maintained in the reference range without any unwanted reductions throughout the study period.
Changes in low back pain and upper gastrointestinal symptoms
Low back pain is well known as a common symptom of osteoporosis, and upper GI symptoms are common adverse drug reactions to BPs.
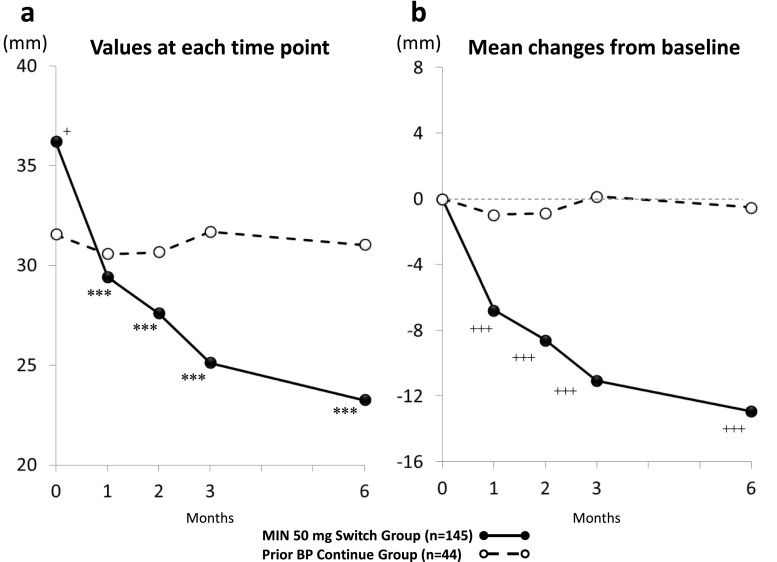
Changes in low back pain after 6 months of treatment (Fig. 4)
Fig. 4.
Time course of change in back pain VAS score: a mean values at each evaluation point and b mean changes from baseline. VAS scores were evaluated in patients with baseline VAS scores ≥10 mm who completed 6-month VAS measurements. +p < 0.05; +++p < 0.001, significantly different from values in the Continue group as analyzed by Wilcoxon rank-sum test, ***p < 0.001, significantly different from baseline as analyzed by Wilcoxon signed-rank test
Low back pain was evaluated in patients with a baseline VAS score of 10 mm or more who completed all scheduled VAS measurements. The Switch and Continue groups consisted of 145 and 44 patients, respectively. The baseline mean VAS scores were 36.0 ± 16.0 mm in the Switch group and 32.0 ± 18.0 mm in the Continue group, which were significantly different (p = 0.046).
In the Switch group, VAS scores for low back pain significantly improved 1 month after treatment and at subsequent time points (p < 0.001). Also, the Switch group showed significantly greater decreases from baseline than the Continue group at 1 month after treatment and subsequent time points (p < 0.001). By contrast, in the Continue group, the VAS scores were stable, and therefore, the mean changes from baseline were not statistically significant throughout the study.
Subgroup analysis was conducted for each prior BP treatment (alendronate, risedronate, or daily dose of minodronate). In all subgroups, VAS scores demonstrated a decreasing trend after switching, with the decreases in the subgroups of patients who switched from alendronate and daily dose of minodronate statistically significant at 1 month after treatment and subsequent time points (p < 0.05). The Switch group showed significantly greater decreases from baseline than the Continue group only for the subgroup of patients who switched from alendronate.
Changes in upper gastrointestinal symptoms after 6 months of treatment (data not shown)
Upper GI symptoms (heartburn, epigastralgia, and epigastric fullness) were evaluated using the Izumo scale questionnaire [16].
At baseline, a higher percentage of patients who switched over from alendronate had GI symptoms compared to those who switched over from risedronate or daily doses of minodronate. The use of concomitant gastrointestinal agents for GI symptoms was similar among the prior BP treatment subgroups, with no significant differences.
In the Switch group, the mean Izumo scale scores for the three GI symptoms significantly improved 1 month after treatment and at subsequent time points (p < 0.05), with the exception of epigastric fullness at 2 months after treatment. By contrast, in the Continue group, the mean Izumo scale scores for epigastralgia and epigastric fullness were stable over the 6-month study period, while the mean scores for heartburn tended to improve, with statistically significant differences at 3 and 6 months after treatment compared to baseline (p < 0.05 and p < 0.01, respectively).
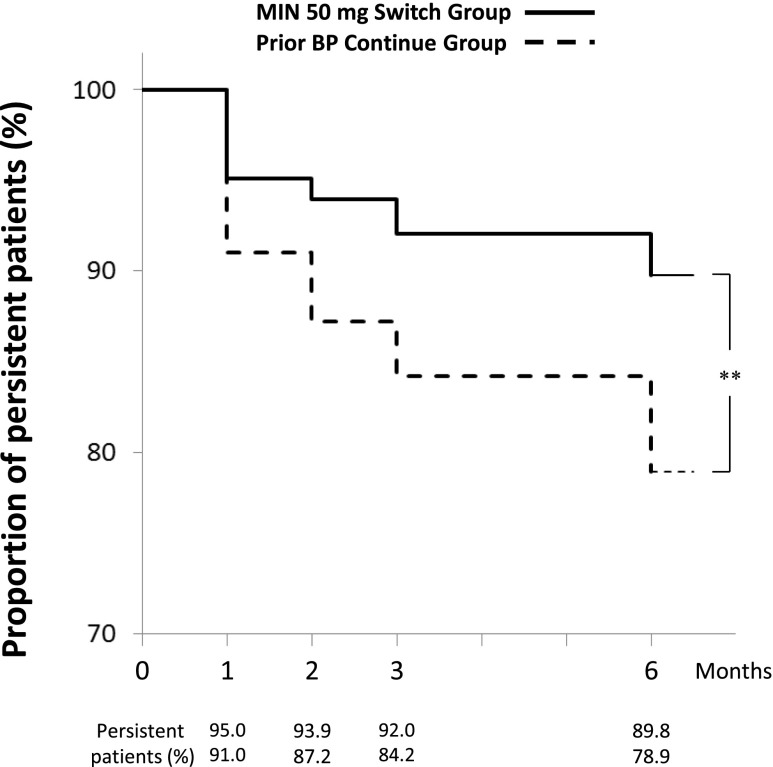
Treatment persistence of MIN 50 mg
Treatment persistence in the Switch and Continue groups during the 6-month study period in summarized using survival curves in Fig. 5. Overall, the persistence rate after 6 months of treatment was higher in the Switch group (89.8 %) than that in the Continue group (78.9 %; p < 0.003, log-rank test). During the study period, 9 patients (3.4 %) in the Switch group hoped to switch back to their previous BP products, while 19 patients (14.3 %) in the Continue group hoped to switch over to MIN 50 mg. Regarding expectations (baseline) and actual impressions (after 6 months of treatment) of the once-monthly regimen, 87.3 % of patients (220 of 252) expected less frequent dosing to be more convenient, and 77.9 % of patients (180 of 231) actually had such an impression. However, the impression that “monthly schedule fits lifestyle better” drastically increased from baseline (5.6 %, 14 of 252) to the end of the study (32.5 %, 75 of 231).
Fig. 5.
Treatment persistence in the Switch and Continue groups. Proportion of persistent patients for each treatment was evaluated at 1, 2, 3, and 6 months of treatment. **p < 0.01, significantly different between groups as analyzed by log-rank test
Discussion
We conducted a questionnaire-based study on drug preference for once-monthly oral bisphosphonate (MIN 50 mg) and persistence of MIN 50 mg in Japanese patients with osteoporosis currently treated with daily or weekly BPs. Based on patients’ preference, study patients were allocated to either the “Switch” group, consisting of patients who were willing to switch over to MIN 50 mg from their current therapies, or the “Continue” group, consisting of patients who wanted to continue their current therapies. The bone mineral densities, bone turnover markers, time courses of low back pain and upper GI symptoms, and treatment persistence were compared between the treatment groups to evaluate the usefulness of once-monthly MIN 50 mg treatment.
About 65 % of study patients were willing to switch to MIN 50 mg among both patients treated with daily BPs and weekly BPs. In addition, at the end of the study, almost all of the study patients in the Switch group responded via questionnaire that they were “willing to continue MIN 50 mg.” Once-monthly treatments such as MIN 50 mg will be a powerful option for these patients. Indeed, the treatment persistence of MIN 50 mg was significantly higher than that in the Continue group consisting of patients treated with daily or weekly BP products, and therefore, the once-monthly treatment should improve patient persistence.
Almost all the patients treated with BP products whose bone turnover marker levels exceeded the normal range at baseline achieved normal levels after switching to MIN 50 mg (Fig. 3). This finding suggests a superior bone resorption inhibitory effect of MIN 50 mg. In addition, MIN 50 mg was not associated with an unwanted reduction in bone turnover markers among patients whose marker levels were already normal, indicating that MIN 50 mg treatment is an ideal solution for improving bone turnover markers.
While many reports have indicated the bone density-increasing effects of switching to daily minodronate from other BPs [11, 17–19], our study further confirmed that switching to once-monthly minodronate from other BPs significantly increased the bone density of the lumbar spine and distal radius (Fig. 2), suggesting that once-monthly minodronate 50 mg will be a powerful alternative for osteoporosis treatment.
While daily minodronate has been reported to alleviate low back pain [19], this study revealed that once-monthly MIN 50 mg also achieved positive results. Prompt effects of MIN 50 mg on low back pain after switchover from prior BPs (Fig. 4) suggest the contribution of minodronate-specific inhibitory action on the P2X2/3 receptor [20, 21], which is expressed in primary sensory neurons that mediate nociception and are implicated in neuropathic and inflammatory pain responses [22].
We have already reported that daily minodronate treatment induced fewer GI symptoms in comparison with weekly alendronate treatment [19]. As expected from this prior report, the GI symptoms induced by weekly alendronate were resolved statistically significantly within 1 month after switchover from alendronate to MIN 50 mg (data not shown), suggesting that once-monthly minodronate may also induce fewer GI symptoms (heartburn, epigastralgia, and epigastric fullness). MIN 50 mg is highly likely to improve both convenience and treatment persistence because it alleviates low back pain and results in fewer GI symptoms after switchover from conventional BP products.
There are limitations in this study. This is an open-label observational study involving small number of patients, and the patients are not randomized but are assigned to two groups by their preference. Therefore, there is a selection bias in being enrolled in the two groups.
Because MIN 50 mg alleviates low back pain, reduces bone turnover markers, increases bone mineral density and treatment persistence, and induces fewer GI symptoms after switchover from conventional BP products, the drug may provide patients with a more convenient treatment option and enhance long-term treatment persistence with the therapy.
Acknowledgments
We thank DS Pharma Biomedical Co., Ltd for technical support.
Conflicts of interest
Akinori Sakai, Satoshi Ikeda, Nobukazu Okimoto, Hidehiro Matsumoto, Kitau Teshima, Yuichi Okazaki, Fumio Fukuda, Shinobu Arita, Hiroshi Tsurukami, Masato Nagashima, and Toru Yoshioka received research support from Astellas Pharma Inc. (Tokyo, Japan); the sponsor was not involved in the design of study, the enrollment of patients, or the collection, analysis, or interpretation of data. The authors were supported in the editing and writing of this manuscript by Dr. Tsumura with EPS Corporation (Tokyo, Japan) and funded by Astellas. The authors are fully responsible for the content and editorial decisions of this manuscript.
References
- 1.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP, Petak SM, Osteoporosis Task Force AACE. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Suppl 3):1–37. doi: 10.4158/EP.16.S3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North American Menopause Society Management of osteoporosis in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17(1):25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 3.Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S. Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos. 2012;7(1–2):3–20. doi: 10.1007/s11657-012-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yood RA, Emani S, Reed JI, Lewis BE, Charpentier M, Lydick E. Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int. 2003;14(12):965–968. doi: 10.1007/s00198-003-1502-4. [DOI] [PubMed] [Google Scholar]
- 5.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18(3):271–277. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 6.Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19(6):811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- 7.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. Benefit of adherence with bisphosphonates depends on the age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008;23(9):1435–1441. doi: 10.1359/jbmr.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman SL, Gold DT. Compliance and persistence with osteoporosis therapies. Curr Rheumatol Rep. 2008;10(2):118–122. doi: 10.1007/s11926-008-0021-x. [DOI] [PubMed] [Google Scholar]
- 10.Chatani Y. Minodronic acid hydrate as a new therapeutic agent for osteoporosis. Clin Calcium. 2005;15(1):9–14. [PubMed] [Google Scholar]
- 11.Kubo T, Shimose S, Matsuo T, Fujimori J, Ochi M. Minodronate for the treatment of osteoporosis. Drugs Today (Barc) 2010;46(1):33–37. doi: 10.1358/dot.2010.46.1.1437707. [DOI] [PubMed] [Google Scholar]
- 12.Sorbera LA, Castañer J, Leeson PA. Minodronic acid. Drugs Future. 2002;27(10):935–941. doi: 10.1358/dof.2002.027.10.701186. [DOI] [Google Scholar]
- 13.Tanishima S, Morio Y. A review of minodronic acid hydrate for the treatment of osteoporosis. Clin Interv Aging. 2013;8:185–189. doi: 10.2147/CIA.S23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, Fukunaga M, Shiraki M, Nishizawa Y, Ohashi Y, Matsumoto T. Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int. 2012;23(6):1737–1745. doi: 10.1007/s00198-011-1782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumamoto K, Nakamura T, Suzuki T, Gorai I, Fujinawa O, Ohta H, Shiraki M, Yoh K, Fujiwara S, Endo N, Matsumoto T. Validation of the Japanese Osteoporosis Quality of Life Questionnaire. J Bone Miner Metab. 2010;28(1):1–7. doi: 10.1007/s00774-009-0125-z. [DOI] [PubMed] [Google Scholar]
- 16.Furuta K, Ishihara S, Sato S, Miyake T, Ishimura N, Koshino K, Tobita H, Moriyama I, Amano Y, Adachi K, Ohta A, Kinoshita Y. Development and verification of the Izumo Scale, new questionnaire for quality of life assessment of patients with gastrointestinal symptoms. Nihon Shokakibyo Gakkai Zasshi. 2009;106(10):1478–1487. [PubMed] [Google Scholar]
- 17.Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Ohashi Y, Nakamura T, Matsumoto T. Three years of treatment with minodronate in patients with postmenopausal osteoporosis. J Bone Miner Metab. 2012;30(4):439–446. doi: 10.1007/s00774-011-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshioka T, Okimoto N, Okamoto K, Sakai A. A comparative study of the effects of daily minodronate and weekly alendronate on upper gastrointestinal symptoms, bone resorption, and back pain in postmenopausal osteoporosis patients. J Bone Miner Metab. 2013;31(2):153–160. doi: 10.1007/s00774-012-0393-x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int. 2009;20(8):1429–1437. doi: 10.1007/s00198-008-0816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakimoto S, Nagakura Y, Tamura S, Watabiki T, Shibasaki K, Tanaka S, Mori M, Sasamata M, Okada M. Minodronic acid, a third-generation bisphosphonate, antagonizes purinergic P2X2/3 receptor function and exerts an analgesic effect in pain models. Eur J Pharmacol. 2008;589(1–3):98–101. doi: 10.1016/j.ejphar.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Yamagami Y, Mashiba T, Iwata K, Tanaka M, Nozaki K, Yamamoto T. Effects of minodronic acid and alendronate on bone remodeling, microdamage accumulation, degree of mineralization and bone mechanical properties in ovariectomized cynomolgus monkeys. Bone. 2013;54(1):1–7. doi: 10.1016/j.bone.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Mo G, Bernier LP, Zhao Q, Chabot-Doré AJ, Ase AR, Logothetis D, Cao CQ, Séguéla P. Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol Pain. 2009;5:47. doi: 10.1186/1744-8069-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]