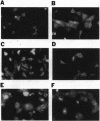
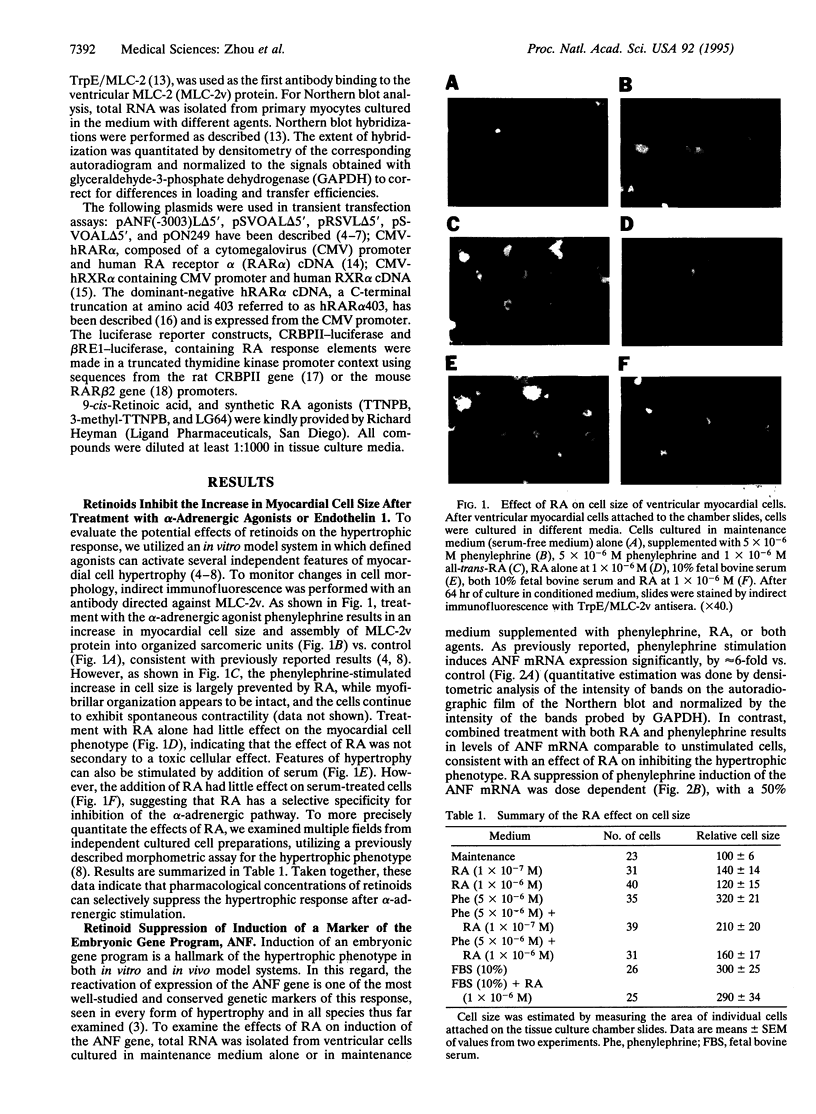
Abstract
Utilizing an in vitro model system of cardiac muscle cell hypertrophy, we have identified a retinoic acid (RA)-mediated pathway that suppresses the acquisition of specific features of the hypertrophic phenotype after exposure to the alpha-adrenergic receptor agonist phenylephrine. RA at physiological concentrations suppresses the increase in cell size and induction of a genetic marker for hypertrophy, the atrial natriuretic factor (ANF) gene. RA also suppresses endothelin 1 pathways for cardiac muscle cell hypertrophy, but it does not affect the increase in cell size and ANF expression induced by serum stimulation. A trans-activation analysis using a transient transfection assay reveals that neonatal rat ventricular myocardial cells express functional RA receptors of both the retinoic acid receptor and retinoid X receptor (RAR and RXR) subtypes. Using synthetic agonists of RA, which selectively bind to RXR or RAR, our data indicate that RAR/RXR heterodimers mediate suppression of alpha-adrenergic receptor-dependent hypertrophy. These results suggest the possibility that a pathway for suppression of hypertrophy may exist in vivo, which may have potential therapeutic value.
Full text
PDF
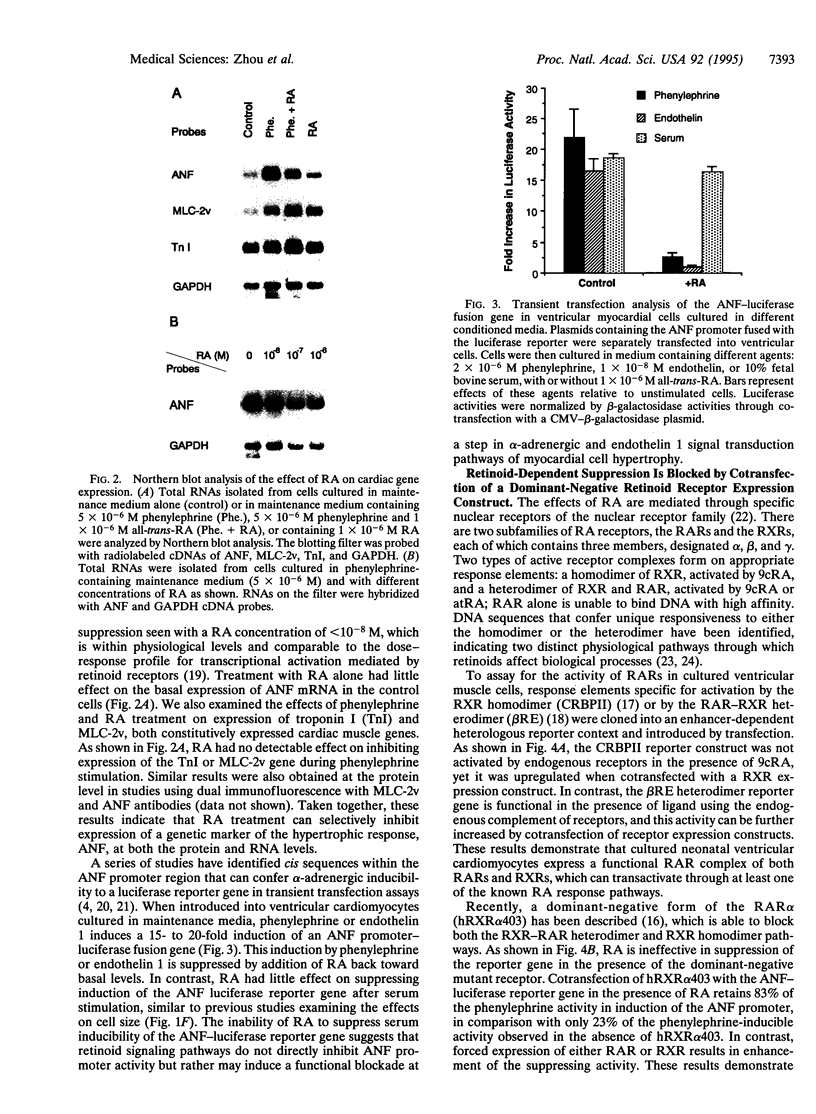

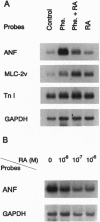
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argentin S., Sun Y. L., Lihrmann I., Schmidt T. J., Drouin J., Nemer M. Distal cis-acting promoter sequences mediate glucocorticoid stimulation of cardiac atrial natriuretic factor gene transcription. J Biol Chem. 1991 Dec 5;266(34):23315–23322. [PubMed] [Google Scholar]
- Boehm M. F., McClurg M. R., Pathirana C., Mangelsdorf D., White S. K., Hebert J., Winn D., Goldman M. E., Heyman R. A. Synthesis of high specific activity [3H]-9-cis-retinoic acid and its application for identifying retinoids with unusual binding properties. J Med Chem. 1994 Feb 4;37(3):408–414. doi: 10.1021/jm00029a013. [DOI] [PubMed] [Google Scholar]
- Boehm M. F., Zhang L., Badea B. A., White S. K., Mais D. E., Berger E., Suto C. M., Goldman M. E., Heyman R. A. Synthesis and structure-activity relationships of novel retinoid X receptor-selective retinoids. J Med Chem. 1994 Sep 2;37(18):2930–2941. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Knowlton K. U., Zhu H., Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991 Dec;5(15):3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Zhu H., Knowlton K. U., Miller-Hance W., van-Bilsen M., O'Brien T. X., Evans S. M. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- Cox L. R., Motz J., Troll W., Garte S. J. Effects of retinoic acid on NIH3T3 cell transformation by the H-ras oncogene. J Cancer Res Clin Oncol. 1991;117(2):102–108. doi: 10.1007/BF01613132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Heyman R. A., Umesono K., Evans R. M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson E., Sucov H. M., Kubalak S. W., Schmid-Schönbein G. W., DeLano F. A., Evans R. M., Ross J., Jr, Chien K. R. Atrial-like phenotype is associated with embryonic ventricular failure in retinoid X receptor alpha -/- mice. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7386–7390. doi: 10.1073/pnas.92.16.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B. Ras C-terminal processing enzymes--new drug targets? Cell. 1991 Apr 5;65(1):1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Grépin C., Dagnino L., Robitaille L., Haberstroh L., Antakly T., Nemer M. A hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcription. Mol Cell Biol. 1994 May;14(5):3115–3129. doi: 10.1128/mcb.14.5.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Iwaki K., Sukhatme V. P., Shubeita H. E., Chien K. R. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990 Aug 15;265(23):13809–13817. [PubMed] [Google Scholar]
- Knowlton K. U., Baracchini E., Ross R. S., Harris A. N., Henderson S. A., Evans S. M., Glembotski C. C., Chien K. R. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991 Apr 25;266(12):7759–7768. [PubMed] [Google Scholar]
- Knowlton K. U., Michel M. C., Itani M., Shubeita H. E., Ishihara K., Brown J. H., Chien K. R. The alpha 1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem. 1993 Jul 25;268(21):15374–15380. [PubMed] [Google Scholar]
- LaMorte V. J., Thorburn J., Absher D., Spiegel A., Brown J. H., Chien K. R., Feramisco J. R., Knowlton K. U. Gq- and ras-dependent pathways mediate hypertrophy of neonatal rat ventricular myocytes following alpha 1-adrenergic stimulation. J Biol Chem. 1994 May 6;269(18):13490–13496. [PubMed] [Google Scholar]
- Leder A., Kuo A., Cardiff R. D., Sinn E., Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Kliewer S. A., Kakizuka A., Umesono K., Evans R. M. Retinoid receptors. Recent Prog Horm Res. 1993;48:99–121. doi: 10.1016/b978-0-12-571148-7.50008-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Milano C. A., Allen L. F., Rockman H. A., Dolber P. C., McMinn T. R., Chien K. R., Johnson T. D., Bond R. A., Lefkowitz R. J. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994 Apr 22;264(5158):582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- Rohrer D. K., Hartong R., Dillmann W. H. Influence of thyroid hormone and retinoic acid on slow sarcoplasmic reticulum Ca2+ ATPase and myosin heavy chain alpha gene expression in cardiac myocytes. Delineation of cis-active DNA elements that confer responsiveness to thyroid hormone but not to retinoic acid. J Biol Chem. 1991 May 5;266(13):8638–8646. [PubMed] [Google Scholar]
- Salbert G., Fanjul A., Piedrafita F. J., Lu X. P., Kim S. J., Tran P., Pfahl M. Retinoic acid receptors and retinoid X receptor-alpha down-regulate the transforming growth factor-beta 1 promoter by antagonizing AP-1 activity. Mol Endocrinol. 1993 Oct;7(10):1347–1356. doi: 10.1210/mend.7.10.8264664. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Sucov H. M., Dyson E., Gumeringer C. L., Price J., Chien K. R., Evans R. M. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994 May 1;8(9):1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A., Thorburn J., Chen S. Y., Powers S., Shubeita H. E., Feramisco J. R., Chien K. R. HRas-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993 Jan 25;268(3):2244–2249. [PubMed] [Google Scholar]