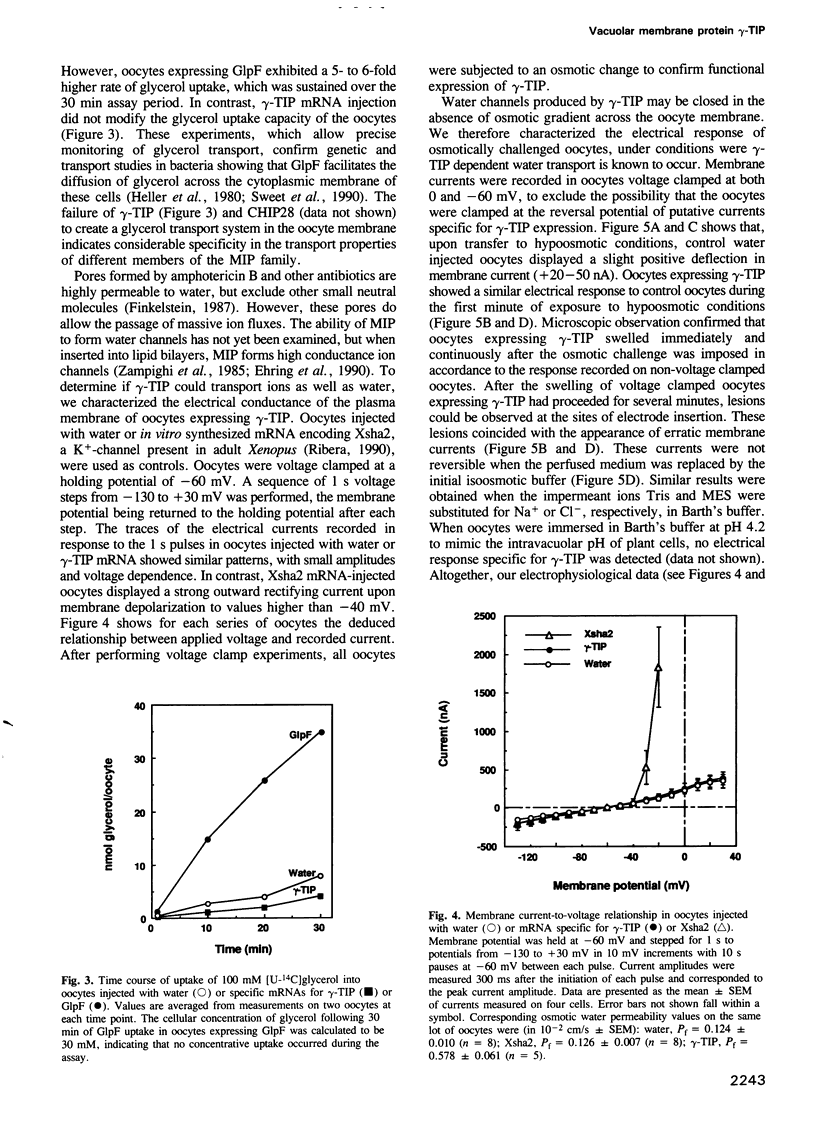
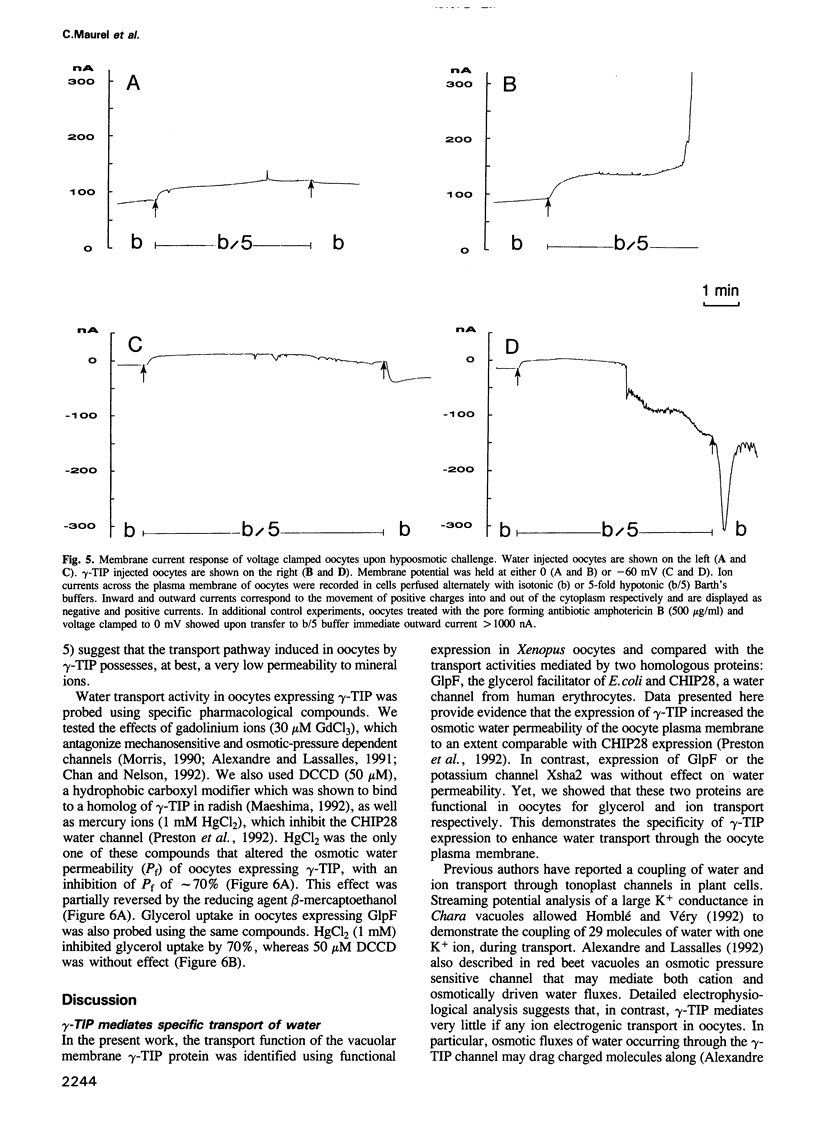
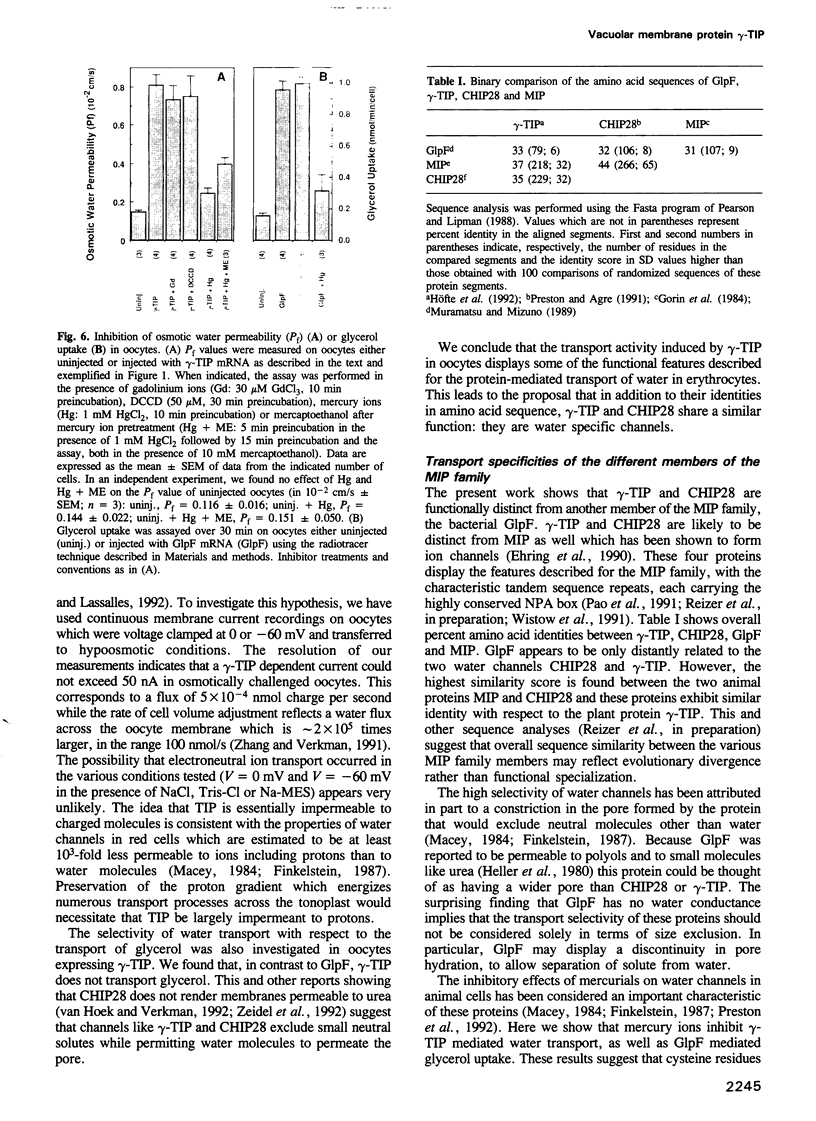
Abstract
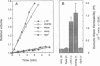
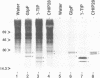
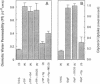
The vacuolar membrane (tonoplast) of higher plant cells contains an abundant 27 kDa protein called TIP (tonoplast intrinsic protein) that occurs in different isoforms and belongs to a large family of homologous channel-like proteins found in bacteria, plants and animals. In the present study, we identified and characterized the function of gamma-TIP from Arabidopsis thaliana by expression of the protein in Xenopus oocytes. gamma-TIP increased the osmotic water permeability of oocytes 6- to 8-fold, to values in the range 1-1.5 x 10(-2) cm/s. Similar results were obtained with the homologous human erythrocyte protein CHIP28, recently identified as the erythrocyte water channel. The bacterial homolog GlpF did not affect the osmotic water permeability of oocytes, but facilitated glycerol uptake, in accordance with its known function. By contrast, gamma-TIP did not promote glycerol permeability. Voltage clamp experiments provided evidence showing that gamma-TIP induced no electrogenic ion transport in oocytes, especially during osmotic challenge that resulted in massive transport of water. These results allow us to conclude that the various protein members of the MIP family have unique and specific transport functions and that the plant protein gamma-TIP likely functions as a water specific channel in the vacuolar membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre J., Lassalles J. P. Hydrostatic and osmotic pressure activated channel in plant vacuole. Biophys J. 1991 Dec;60(6):1326–1336. doi: 10.1016/S0006-3495(91)82170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Anderova M., Crawford N. M., Schroeder J. I. Expression of an outward-rectifying potassium channel from maize mRNA and complementary RNA in Xenopus oocytes. Plant Cell. 1992 Aug;4(8):961–969. doi: 10.1105/tpc.4.8.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. C., Nelson D. J. Chloride-dependent cation conductance activated during cellular shrinkage. Science. 1992 Jul 31;257(5070):669–671. doi: 10.1126/science.1379742. [DOI] [PubMed] [Google Scholar]
- Ehring G. R., Zampighi G., Horwitz J., Bok D., Hall J. E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Gen Physiol. 1990 Sep;96(3):631–664. doi: 10.1085/jgp.96.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M. G., Morrison N. A., Verma D. P. Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 1987 Jan 26;15(2):813–824. doi: 10.1093/nar/15.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Jones J. T., Mullet J. E. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol. 1990 Jul;15(1):11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homblé F., Véry A. A. Coupling of water and potassium ions in K channels of the tonoplast of Chara. Biophys J. 1992 Oct;63(4):996–999. doi: 10.1016/S0006-3495(92)81666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Hubbard L., Reizer J., Ludevid D., Herman E. M., Chrispeels M. J. Vegetative and Seed-Specific Forms of Tonoplast Intrinsic Protein in the Vacuolar Membrane of Arabidopsis thaliana. Plant Physiol. 1992 Jun;99(2):561–570. doi: 10.1104/pp.99.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Herman E. M., Chrispeels M. J. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 1989 Nov;91(3):1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Höfte H., Chrispeels M. J. An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GIpF). Plant Cell. 1990 Jun;2(6):525–532. doi: 10.1105/tpc.2.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludevid D., Höfte H., Himelblau E., Chrispeels M. J. The Expression Pattern of the Tonoplast Intrinsic Protein gamma-TIP in Arabidopsis thaliana Is Correlated with Cell Enlargement. Plant Physiol. 1992 Dec;100(4):1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey R. I. Transport of water and urea in red blood cells. Am J Physiol. 1984 Mar;246(3 Pt 1):C195–C203. doi: 10.1152/ajpcell.1984.246.3.C195. [DOI] [PubMed] [Google Scholar]
- Maeshima M. Characterization of the major integral protein of vacuolar membrane. Plant Physiol. 1992 Apr;98(4):1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Ouyang L. J., Whelan J., Weaver C. D., Roberts D. M., Day D. A. Protein phosphorylation stimulates the rate of malate uptake across the peribacteroid membrane of soybean nodules. FEBS Lett. 1991 Nov 18;293(1-2):188–190. doi: 10.1016/0014-5793(91)81183-9. [DOI] [PubMed] [Google Scholar]
- Pao G. M., Wu L. F., Johnson K. D., Höfte H., Chrispeels M. J., Sweet G., Sandal N. N., Saier M. H., Jr Evolution of the MIP family of integral membrane transport proteins. Mol Microbiol. 1991 Jan;5(1):33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993 Jan 5;268(1):17–20. [PubMed] [Google Scholar]
- Ribera A. B. A potassium channel gene is expressed at neural induction. Neuron. 1990 Nov;5(5):691–701. doi: 10.1016/0896-6273(90)90223-3. [DOI] [PubMed] [Google Scholar]
- Soreq H., Seidman S. Xenopus oocyte microinjection: from gene to protein. Methods Enzymol. 1992;207:225–265. doi: 10.1016/0076-6879(92)07016-h. [DOI] [PubMed] [Google Scholar]
- Sweet G., Gandor C., Voegele R., Wittekindt N., Beuerle J., Truniger V., Lin E. C., Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990 Jan;172(1):424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. T., Zhang R. B., Verkman A. S. High channel-mediated water permeability in rabbit erythrocytes: characterization in native cells and expression in Xenopus oocytes. Biochemistry. 1991 Feb 26;30(8):2087–2092. doi: 10.1021/bi00222a013. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Pisano M. M., Chepelinsky A. B. Tandem sequence repeats in transmembrane channel proteins. Trends Biochem Sci. 1991 May;16(5):170–171. doi: 10.1016/0968-0004(91)90065-4. [DOI] [PubMed] [Google Scholar]
- Zampighi G. A., Hall J. E., Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992 Aug 25;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zhang R. B., Logee K. A., Verkman A. S. Expression of mRNA coding for kidney and red cell water channels in Xenopus oocytes. J Biol Chem. 1990 Sep 15;265(26):15375–15378. [PubMed] [Google Scholar]
- Zhang R. B., Verkman A. S. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am J Physiol. 1991 Jan;260(1 Pt 1):C26–C34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]
- van Hoek A. N., Verkman A. S. Functional reconstitution of the isolated erythrocyte water channel CHIP28. J Biol Chem. 1992 Sep 15;267(26):18267–18269. [PubMed] [Google Scholar]