Summary
Background
Chemoimmunotherapy has led to improved numbers of patients achieving disease response, and longer overall survival in young patients with chronic lymphocytic leukaemia; however, its application in elderly patients has been restricted by substantial myelosuppression and infection. We aimed to assess safety and activity of ibrutinib, an orally administered covalent inhibitor of Bruton tyrosine kinase (BTK), in treatment-naive patients aged 65 years and older with chronic lymphocytic leukaemia.
Methods
In our open-label phase 1b/2 trial, we enrolled previously untreated patients at clinical sites in the USA. Eligible patients were aged at least 65 years, and had symptomatic chronic lymphocytic leukaemia or small lymphocytic lymphoma requiring therapy. Patients received 28 day cycles of once-daily ibrutinib 420 mg or ibrutinib 840 mg. The 840 mg dose was discontinued after enrolment had begun because comparable activity of the doses has been shown. The primary endpoint was the safety of the dose-fixed regimen in terms of frequency and severity of adverse events for all patients who received treatment. This study is registered with ClinicalTrials.gov, number NCT01105247.
Findings
Between May 20, 2010, and Dec 18, 2012, we enrolled 29 patients with chronic lymphocytic leukaemia and two patients with small lymphocytic lymphoma. Median age was 71 years (range 65–84), and 23 (74%) patients were at least 70 years old. Toxicity was mainly of mild-to-moderate severity (grade 1–2). 21 (68%) patients had diarrhoea (grade 1 in 14 [45%] patients, grade 2 in three [10%] patients, and grade 3 in four [13%] patients). 15 (48%) patients developed nausea (grade 1 in 12 [39%] patients and grade 2 in three [10%] patients). Ten (32%) patients developed fatigue (grade 1 in five [16%] patients, grade 2 in four [13%] patients, and grade 3 in one [3%] patient). Three (10%) patients developed grade 3 infections, although no grade 4 or 5 infections occurred. One patient developed grade 3 neutropenia, and one developed grade 4 thrombocytopenia. After a median follow-up of 22·1 months (IQR 18·4–23·2), 22 (71%) of 31 patients achieved an objective response (95% CI 52·0–85·8); four patients (13%) had a complete response, one patient (3%) had a nodular partial response, and 17 (55%) patients had a partial response.
Interpretation
The safety and activity of ibrutinib in elderly, previously untreated patients with symptomatic chronic lymphocytic leukaemia, or small lymphocytic lymphoma is encouraging, and merits further investigation in phase 3 trials.
Funding
Pharmacyclics, Leukemia and Lymphoma Society, D Warren Brown Foundation, Mr and Mrs Michael Thomas, Harry Mangurian Foundation, P50 CA140158 to Prof J C Byrd MD.
Introduction
B-cell receptor signalling in both normal and malignant B-cells provides a strong proliferative and survival signal to the cell.1,2 Interfering with such signalling is therefore a logical approach to treatment of B-cell malignancies. 3,4 Ibrutinib (PCI-32765, Pharmacyclics, Sunnyvale, CA, USA) is a covalent inhibitor of Bruton tyrosine kinase (BTK), an important enzyme in the B-cell receptor signalling cascade.5 Patients who have inherited mutations in BTK have X-linked agamma globulinaemia (also known as Bruton agamma globulinaemia), a disease associated with diminished B-cell numbers, decreased serum immunoglobulin concentrations, and increased susceptibility to infections.
Ibrutinib forms a covalent bond with the BTK cysteine-481 residue, potently inhibiting enzyme activity inhibition even at nanomolar concentrations. 6 Several preclinical studies have shown the proapoptotic, antiproliferative, and stromal inhibitory properties of this drug in primary chronic lymphocytic leukaemia cells.7–9 Ibrutinib is orally bioavailable, and no maximum tolerated dose was reached when it was given once daily at doses of 2·5–12·5 mg/kg continuously until disease progression in a phase 1 trial of 56 patients with various relapsed or refractory B-cell cancers.10 Of the 50 assessable patients in the study, 60% achieved an objective response, with a median progression-free survival of 13·6 months.10 Phase 2 data for patients with relapsed or refractory chronic lymphocytic leukaemia treated with ibrutinib showed a high proportion of patients achieving an objective response and durable remissions, with an estimated progression-free survival of 75% and overall survival of 83% during the study of a heavily pretreated population of patients (patients had a median of four previous nonibrutinib regimens).11
Chemoimmunotherapy is the standard front-line approach for patients younger than 65 years with chronic lymphocytic leukaemia, with the combination of fludarabine, cyclophosphamide, and rituximab used most commonly.12–14 However, treatment with chemoimmunotherapy is associated with high rates of myelosuppression and infection; such complications are more frequent and more severe in patients older than 65 years because of reduced marrow reserve, and presence of comorbidities.15–17 The German Chronic Lymphocytic Leukaemia Study Group reported the first randomised study18 of chlorambucil versus single-agent fludarabine in a cohort of previously untreated patients who were older than 65 years and had chronic lymphocytic leukaemia. Although a greater proportion of patients who were treated with fludarabine achieved an objective response than those treated with chlorambucil, additional toxicity was noted with fludarabine and fludarabine did not lead to a benefit in overall survival. 18 Analysis of US Intergroup data similarly concluded that chlorambucil might be an acceptable treat ment for elderly patients (aged >65 years) with chronic lymphocytic leukaemia, and suggested that the addition of immunotherapy with the anti-CD20 monoclonal antibody rituximab might have a benefit irrespective of age.19 Addition of a CD20 monoclonal antibody to chlorambucil treatment in elderly patients with chronic lymphocytic leukaemia has been previously shown to be beneficial (median age of the study population 73 years),20 with early assessment of median progression-free survival for the combination reported as slightly less than 2 years, as compared with 10·9 months with chlorambucil alone.
Effective agents with few toxic effects are needed for elderly patients. In view of the excellent tolerability and safety profile of ibrutinib, and the high proportion of relapsed patients who achieved an objective response, we aimed to assess the safety and efficacy of ibrutinib in previously untreated patients aged 65 years and older with chronic lymphocytic leukaemia or small lymphocytic lymphoma. In addition, the study sought to examine the effect of genomic features on response to ibrutinib.
Methods
Study design and participants
In our phase 1b/2, open-label, multicentre study, we enrolled participants at seven clinical sites in the USA (appendix). Study sites were selected on the basis of their participation in the first clinical study,10 with selection of additional sites based on a sponsor feasibility assessment. Eligible participants were previously untreated, were aged at least 65 years, and had a diagnosis of chronic lymphocytic leukaemia or small lymphocytic lymphoma as defined by the International Workshop on Chronic Lymphocytic Leukaemia (IWCLL)21 and WHO22 classifications, a current requirement for treatment according to US National Cancer Institute or IWCLL guidelines, Eastern Cooperative Oncology Group (ECOG) performance statuses of 2 or lower, adequate organ function (including creatinine 1·5 times the upper limit of normal or lower, and alanine aminotransferase 2·5 times the upper limit of normal or lower), and an absence of active infection. Exclusion criteria included any cancer restricting expected survival to less than 2 years, gastrointestinal disease that could inhibit absorption of ibrutinib, and medicines associated with torsades de pointes.
An institutional review board approved the protocol at the respective sites, and the study was conducted according to the principles of the Declaration of Helsinki, the International Conference on Harmonisation, and the Guidelines for Good Clinical Practice. All patients provided written informed consent.
Procedures
Eligible participants received 28 day cycles of once-daily ibrutinib 420 mg (three 140 mg capsules) or once-daily ibrutinib 840 mg (six 140 mg capsules). Patients were assigned sequentially by enrolment. Although we initially planned to assess two fixed doses of ibrutinib, we closed the 840 mg per day cohort before full accrual after comparable activity of the doses was shown elsewhere in relapsed or refractory patients with chronic lymphocytic leukaemia.11 Ibrutinib treatment was delayed in the event of any uncontrolled, potentially drug-related grade 3 or worse toxicity. Doses could also be held in the event of grade 4 neutropenia occurring for more than 7 days, grade 3 or worse drop in platelet count, and grade 3 or worse diarrhoea, nausea, or vomiting. Ibrutinib treatment could be resumed when the toxicity resolved, and if toxicity re-occurred a dose reduction was mandated. Extensive dose holds (longer than 28 days) for toxicity resulted in permanent discontinuation of the study drug. Ibrutinib was to be given continuously, until disease progression or toxic effects led to discontinuation.
The primary endpoint was the safety of the fixed-dose regimen assessed by the frequency and severity of adverse events. The study was designed to terminate when the safety endpoints were validated. Patients were followed up for at least 12 cycles in the study, and then could continue ibrutinib treatment in a long-term extension study. Patients were monitored by history, physical examination, and peripheral blood drawn every week for the first month, every other week for the second month, and monthly thereafter. Adverse events were graded according to the Common Toxicity Criteria of the National Cancer Institute, Version 4.0. We graded haematological toxicities according to IWCLL 2008 criteria.21
Secondary endpoints were the proportion of patients achieving an overall response, progression-free survival, long-term tolerability, and pharmacodynamics. We did a response assessment, including a CT radiological examination at the end of cycles 2, 5, 8, 12, 15, and 24. We took a bone marrow biopsy sample to confirm complete responses.21 All patients had baseline assessments of blood count; however, baseline bone marrow biopsy was not required or collected at study entry. We assessed response in patients with chronic lymphocytic leukaemia on the basis of IWCLL guidelines,21 with the exception that lymphocytosis was not a sole criterion for progression (as per Hallek and colleagues23). A partial response in all other parameters in the setting of persistent lymphocytosis was characterised as a partial response with lymphocytosis.24 Response in patients with small lymphocytic lymphoma was assessed on the basis of the International Working Group for non-Hodgkin lymphoma 2007 criteria.25 We assessed haematological improvement in the subset of patients with cytopenia (haemoglobin ≤110 g/L, platelet counts of ≤100 × 109 platelets per L or absolute neutrophil counts ≤1·5 × 109 cells per L) at baseline. Haematological improvement was defined as haemoglobin of more than 110 g/L or an increase of at least 50% from baseline, platelet counts of more than 100 × 109 platelets per L or an increase of at least 50% from baseline, absolute neutrophil counts of more than 1·5 × 109 cells per L or an increase of at least 50% from baseline. We defined sustained haematological improvement as duration of response lasting for at least 56 days (two cycles) without transfusions or provision of growth factors. Exploratory endpoints included identification of putative biomarkers of response, and overall survival. We required all patients to have an IWCLL indication for therapy as part of eligibility, but the exact criteria for which they met this indication were not collected.
We assessed ZAP-70 methylation as previously described;26 ZAP-70 was regarded as methylated at CpG3 if scores were at least 15%. We assessed IgG, IgA, and IgM levels for patients who did not receive intravenous immunoglobulins as concomitant therapy during ibrutinib treatment. We assessed levels at baseline and at cycles 3, 6, and 12. Testing for interphase cytogenetics, immunoglobulin gene (IgHV) mutational analysis, and β2 microglobulin concentrations was done at baseline by a central laboratory (Ohio State University central laboratory, Columbus, OH, USA).
For pharmacodynamic analyses, we assessed the level of BTK occupancy in blood mononuclear cells following treatment at both dose levels. A cell-permeable, fluorescently tagged derivative of ibrutinib, PCI-33380, was used to visualise BTK binding before treatment and at 4 h and 24 h after treatment.6
Statistical analysis
A sample size of 12–24 patients per group for the two fixed-dose daily regimens of ibrutinib was considered to define the preliminary safety profile of ibrutinib, without power considerations. No hypothesis testing was planned in this study. We planned to enrol 36 patients: 24 in the 420 mg cohort and 12 in the 840 mg cohort. The 840 mg cohort, however, closed before full accrual after comparable activity of the doses was shown elsewhere in relapsed or refractory patients with chronic lymphocytic leukaemia.11 We report all subgroup analyses undertaken.
We report descriptive statistics. Because few patients received an ibrutinib dose of 840 mg and the results for the two dose cohorts were similar, we pooled data for all patients. We did the analyses for all patients who received any dose of study drug. We calculated the proportion of patients achieving an objective response using an exact binomial 95% CI. We used the Kaplan-Meier method for time-to-event analysis (curves and corresponding quartiles). Patients were censored for progression-free survival at the last clinical assessment before receipt of new anticancer therapy, or after loss to follow-up, whichever occurred first. Patients were censored for overall survival at the last known alive date. No imputation was done for missing data. Exploratory analysis to identify characteristics associated with response in each subgroup was compared by patients achieving a response with an exact binomial 95% CI, which is only used as indicative. We did not adjust for multiplicity. This analysis shows the final data from our study, extracted on Feb 27, 2013. All statistical analyses were done with SAS version 9.3 under the Windows server 2008 R2 enterprise service pack 1 on a validated and secured computing system.
This study is registered with ClinicalTrials.gov, number NCT01105247.
Role of the funding source
This study was sponsored by Pharmacyclics. Clinical investigators were responsible for design of the study protocol and analysis plan together with Pharmacyclics. The investigators and their respective research teams collected all of the data, and Pharmacyclics confirmed the accuracy of the data and compiled the data for summation and analysis. Investigators had full access to the data and analyses for compilation of this report. SO’B wrote the first draft of the manuscript; all authors carefully reviewed the manuscript and approved the final version. The corresponding author had full access to all of the data and had final responsibility to submit for publication.
Results
Between May 20, 2010, and Dec 18, 2012, we enrolled 29 patients with symptomatic chronic lymphocytic leukaemia and two patients with symptomatic small lymphocytic lymphoma (table 1). The median age was 71 years and 23 (74%) patients were older than 70 years (table 1). At the time of analysis, median follow-up for all patients was 22·1 months (IQR 18·4–23·2). 27 patients were treated with ibrutinib 420 mg and four were treated with ibrutinib 840 mg. Median treatment duration was 21·0 months (14·8–22·6). The relative dose intensity (percentage of expected dose received) was 98·9% (range 49·9–100·0; IQR 96·9–99·9). Only one patient required dose modification for nausea (grade 1 nausea from day 30 to day 322, which was regarded as possibly related to treatment), and was maintained on a lower dose of 280 mg after day 176.
Table 1.
Baseline characteristics
| Patients (N=31) | |
|---|---|
| Median age, years (range) | 71 (65–84) |
| Aged 70 years or older | 23 (74%) |
|
| |
| Sex | |
| Male | 19 (61%) |
| Female | 12 (39%) |
|
| |
| Diagnosis | |
| Chronic lymphocytic leukaemia | 29 (94%) |
| Small lymphocytic lymphoma | 2 (6%) |
|
| |
| Time from initial diagnosis to study entry, months | 57·3 (0·7–369·8, 24·8–89·3) |
|
| |
| Rai stage at treatment | |
| I | 8 (26%) |
| II | 5 (16%) |
| III | 6 (19%) |
| IV | 11 (35%) |
| Missing data | 1 (3%) |
|
| |
| ECOG performance status | |
| 0 | 23 (74%) |
| 1 | 8 (26%) |
|
| |
| Bulky nodes (≥5 cm) | 6 (19%) |
|
| |
| Unmutated IgHV | 15 (48%) |
|
| |
| Interphase cytogenetics* | |
| Del(17p13.1) | 2 (6%) |
| Del(11q22.3) | 1 (3%) |
| Del(13q14) | 17 (55%) |
| Trisomy 12 | 8 (26%) |
|
| |
| β2 microglobulin concentration | |
| >3 mg/L | 8 (26%) |
| ≤3 mg/L | 23 (74%) |
|
| |
| Cytopenia | |
| Absolute neutrophil count <1500 cells per μL | 1 (3%) |
| Haemoglobin <110 g/L | 11 (35%) |
| <100 000 platelets per μL | 12 (39%) |
|
| |
| Lactate dehydrogenase | |
| <350 U/L | 22 (71%) |
| ≥350 U/L | 9 (29%) |
|
| |
| Zap-70 | |
| Methylated | 14 (45%) |
| Non-methylated | 16 (52%) |
| Missing | 1 (3%) |
Data are median (range, IQR) or n (%), unless otherwise stated. ECOG=Eastern Cooperative Oncology Group. IgHV=immunoglobulin variable heavy chain. Del=deletion.
Cutoffs were defined per the assay specifications as done in the central laboratory.
Two patients discontinued treatment because of adverse events, including grade 3 fatigue in one patient and grade 2 viral infection in a second patient. Two patients withdrew consent (one patient withdrew consent to start a new treatment option because of an insufficient improvement; the second patient withdrew consent to continue in this clinical trial and further data were not provided). Only one patient progressed; the patient, who had del(17p13.1), achieved an initial response after 3·7 months of ibrutinib therapy, and later developed Richter’s transformation at 9·6 months. After discontinuation of ibrutinib, this patient received salvage therapy with oxaliplatin, fludarabine, cytarabine, and rituximab, and died of progressive disease 113 days after the last dose of ibrutinib; this patient was the only participant who died on the study. 26 (84%) of 31 patients continued treatment in the long-term extension study. Nine (29%) of 31 patients required ibrutinib treatment to be held due to grade 3 or greater toxicity.
22 (71%, 95% CI 52·0–85·8) of 31 patients achieved an objective response (defined as complete and partial responses, table 2, figure 1), with four (13%) achieving a complete response. Four patients (13%) had a partial response with lymphocytosis, and three patients (10%) had stable disease; median follow-up for these seven patients was 20·3 months (IQR 14·7–22·8), as compared with 22·1 months (20·3–23·2) for the 22 patients who achieved partial or complete response. Of the 22 patients who achieved a complete or partial response, the initial responses were ten partial responses with lymphocytosis, and 12 true partial responses. For the ten patients who initially responded with partial response with lymphocytosis, the median time to true partial response or complete response was 7·3 months (IQR 4·6–7·4).
Table 2.
Overall best response to treatment
| Patients (N=31) | |
|---|---|
| CR | 4 (13%) |
|
| |
| CR with incomplete blood count recovery | 0 |
|
| |
| Nodular PR | 1 (3%) |
|
| |
| PR | 17 (55%) |
|
| |
| PR with lymphocytosis | 4 (13%) |
|
| |
| Stable disease | 3 (10%) |
|
| |
| Progressive disease | 0 |
|
| |
| Not assessable or missing | 2 (7%) |
| Overall response rate | 22 (71·0%, 52·0–85·8) |
| Time to initial response | |
| Patients | 22 |
| Mean (SD) time, months | 2·7 (1·6) |
| Median (range) time, months | 1·9 (1·5–7·4) |
Data are n (%) or n (%, 95% CI), unless otherwise stated. CR=complete response. PR=partial response.
Figure 1. Treatment duration by best response.
CR=complete response. PR=partial response. AE=adverse event. *Discontinued treatment. †Patient with small lymphocytic lymphoma.
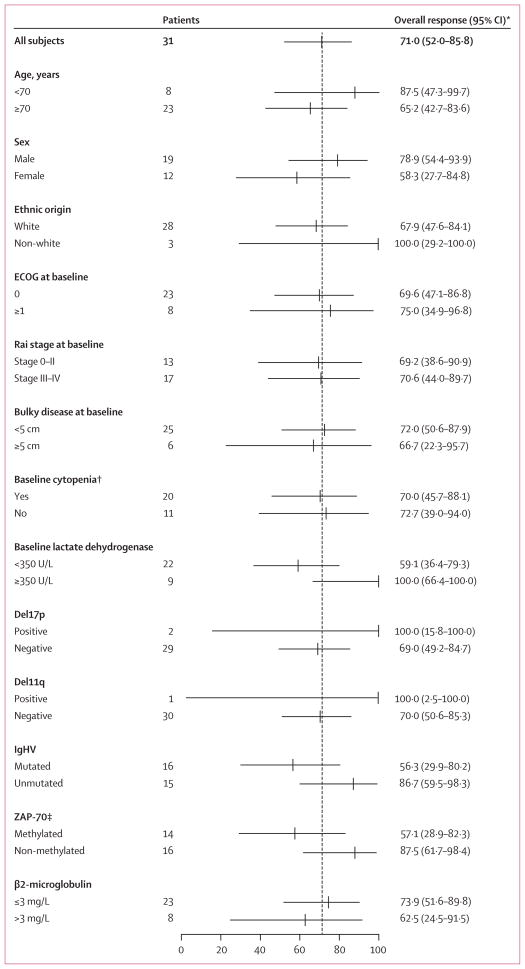
We did subgroup analyses for proportion of patients achieving an objective response for various predetermined prognostic variables (figure 2). In every comparison, the 95% CI overlapped, so none of these differences was regarded as significant. Patients with unmutated IgHV might have been predisposed to achieve a partial response or complete response, due to more rapid resolution of lymphocytosis. However, if partial response with lymphocytosis is included, we noted no apparent difference in the proportion of patients achieving an objective response based on mutational status (data not shown), which supports previous findings for relapsed patients receiving ibrutinib.11
Figure 2. Overall responses by subgroup analyses.
Overall responses defined as complete or partial responses according to International Workshop on Chronic Lymphocytic Leukemia criteria. ECOG=Eastern Cooperative Oncology Group. IgHV=immunoglobulin variable heavy chain. *95% exact binomial CI. †Haemoglobin ≤110 g/L or platelet counts of ≤100 × 109 platelets per L or absolute neutrophil count ≤1·5 × 109 cells per L. ‡ZAP-70 methylated refers to CpG3 ≥15%, non-methylated refers to CpG3 <15%; data were not available for one patient.
Baseline absolute lymphocyte count in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma was high, with a median of 41 × 109 cells per L (IQR 25·8 × 109–67·9 × 109). Ibrutinib led to lymphocytosis in 17 (55%) of 31 patients, peaking at a median of 1·1 weeks (IQR 1·1–3·9) and then slowly declining (figure 3). The lymphocytosis occurred together with a notable reduction in lymph node size that was sustained over the course of treatment (figure 3). Most patients normalised (absolute lymphocyte count <4 × 109 cells per L) or achieved greater than 50% reduction from baseline in their absolute lymphocyte count during follow-up. Of 13 patients who achieved a partial response with lymphocytosis at some time during their treatment, two ultimately achieved a complete response and seven achieved a partial response with resolution of lymphocytosis.
Figure 3. Median absolute lymphocyte counts (ALC) and the sum of the products of lymph node diameters (SPD).
Data are median (95% CI).
Median time to initial response was 1·9 months (IQR 1·8–4·6); median time to best response was 5·9 months (1·9–8·3) and median time to complete response was 12·4 months (9·1–14·7). For all 31 patients at 24 months, the Kaplan-Meier estimate of progression-free survival was 96·3% (95% CI 76·5–99·5) and overall survival was 96·6% (77·9–99·5; figure 4). There was no difference by dose, although numbers were small (appendix). Median progression-free survival was not reached, with only one patient progressing during follow-up, providing a 24 month progression-free survival of 96·3% (95% CI 76·5–99·5).
Figure 4. Kaplan-Meier curves of progression-free survival (A) and overall survival (B).
+=censored.
Pharmacodynamic assessments showed that the median BTK occupancy of cycle 1 was more than 90% for both treatment groups, before or after drug administration on days 1 and 8 (figure 5). Of the four patients who had less than 90% BTK occupancy (in one of whom BTK occupancy was less than 90% at two timepoints), two achieved partial responses, and two achieved complete responses. All four patients remained progression free and continued on ibrutinib until study closure, and continued therapy on the long-term extension study.
Figure 5. Percentage of Bruton tyrosine kinase occupancy in peripheral blood mononuclear cells of patients before ibrutinib, and at 4 h and 24 h after ibrutinib treatment.
Boxplots include the 25–75th percentile of the distribution of the population. Line within the boxplot shows the median and the diamond shows the mean of the population. Line bars show the standard deviation and circles show outliers.
The most common adverse event was diarrhoea, which was reported for most patients (table 3). This adverse event was often self-limited and resolved without discontinuation of the drug. No episodes of grade 4 diarrhoea occurred, and no treatment discontinuation resulted from this adverse event. Other adverse events that were commonly reported in patients included nausea (mainly grade 1, with a maximum grade of 2), up to grade 3 fatigue, and hypertension (table 3). Three patients had grade 3 infections, but no higher grade infections were reported. One patient had grade 3 neutropenia and one had grade 4 thrombocytopenia; no anaemia of grade 3 or greater was reported. No patients died from causes related to treatment or, within 30 days of last treatment of study drug. We noted no differences in the safety profiles of patients who received once-daily ibrutinib 420 mg and those who received 840 mg (data not shown). However, because of the imbalance in patients (27 received ibrutinib 420 mg vs four patients received ibrutinib 840 mg), a conclusive statement cannot be made about these safety profiles.
Table 3.
Adverse events that occurred in 10% or more of patients
| All grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|---|
| Diarrhoea | 21 (68%) | 14 (45%) | 3 (10%) | 4 (13%) | 0 | 0 |
| Nausea | 15 (48%) | 12 (39%) | 3 (10%) | 0 | 0 | 0 |
| Fatigue | 10 (32%) | 5 (16%) | 4 (13%) | 1 (3%) | 0 | 0 |
| Hypertension | 9 (29%) | 2 (6%) | 5 (16%) | 2 (6%) | 0 | 0 |
| Peripheral oedema | 9 (29%) | 8 (26%) | 1 (3%) | 0 | 0 | 0 |
| Dizziness | 8 (26%) | 7 (23%) | 0 | 1 (3%) | 0 | 0 |
| Dyspepsia | 8 (26%) | 7 (23%) | 1 (3%) | 0 | 0 | 0 |
| Upper respiratory tract infection | 8 (26%) | 2 (6%) | 6 (19%) | 0 | 0 | 0 |
| Arthralgia | 7 (23%) | 5 (16%) | 2 (6%) | 0 | 0 | 0 |
| Constipation | 7 (23%) | 7 (23%) | 0 | 0 | 0 | 0 |
| Urinary tract infection | 7 (23%) | 0 | 6 (19%) | 1 (3%) | 0 | 0 |
| Vomiting | 7 (23%) | 4 (13%) | 3 (10%) | 0 | 0 | 0 |
| Abdominal pain | 6 (19%) | 6 (19%) | 0 | 0 | 0 | 0 |
| Gastro-oesophageal reflux disease | 6 (19%) | 4 (13%) | 2 (6%) | 0 | 0 | 0 |
| Headache | 6 (19%) | 3 (10%) | 2 (6%) | 1 (3%) | 0 | 0 |
| Anaemia | 5 (16%) | 2 (6%) | 3 (10%) | 0 | 0 | 0 |
| Anxiety | 5 (16%) | 5 (16%) | 0 | 0 | 0 | 0 |
| Contusion | 5 (16%) | 5 (16%) | 0 | 0 | 0 | 0 |
| Epistaxis | 5 (16%) | 5 (16%) | 0 | 0 | 0 | 0 |
| Insomnia | 5 (16%) | 4 (13%) | 1 (3%) | 0 | 0 | 0 |
| Petechiae | 5 (16%) | 5 (16%) | 0 | 0 | 0 | 0 |
| Stomatitis | 5 (16%) | 4 (13%) | 1 (3%) | 0 | 0 | 0 |
| Aphthous stomatitis | 4 (13%) | 4 (13%) | 0 | 0 | 0 | 0 |
| Back pain | 4 (13%) | 1 (3%) | 2 (6%) | 1 (3%) | 0 | 0 |
| Cellulitis | 4 (13%) | 2 (6%) | 2 (6%) | 0 | 0 | 0 |
| Dry eye | 4 (13%) | 4 (13%) | 0 | 0 | 0 | 0 |
| Erythema | 4 (13%) | 4 (13%) | 0 | 0 | 0 | 0 |
| Muscle spasms | 4 (13%) | 3 (10%) | 1 (3%) | 0 | 0 | 0 |
| Peripheral sensory neuropathy | 4 (13%) | 4 (13%) | 0 | 0 | 0 | 0 |
| Pruritus | 4 (13%) | 4 (13%) | 0 | 0 | 0 | 0 |
| Rash pruritic | 4 (13%) | 4 (13%) | 0 | 0 | 0 | 0 |
| Sinusitis | 4 (13%) | 2 (6%) | 2 (6%) | 0 | 0 | 0 |
| Thrombocytopenia | 4 (13%) | 1 (3%) | 2 (6%) | 0 | 1 (3%) | 0 |
Data are n (%). Adverse events reported from first dose up to 30 days of last dose of ibrutinib, not including the extension study.
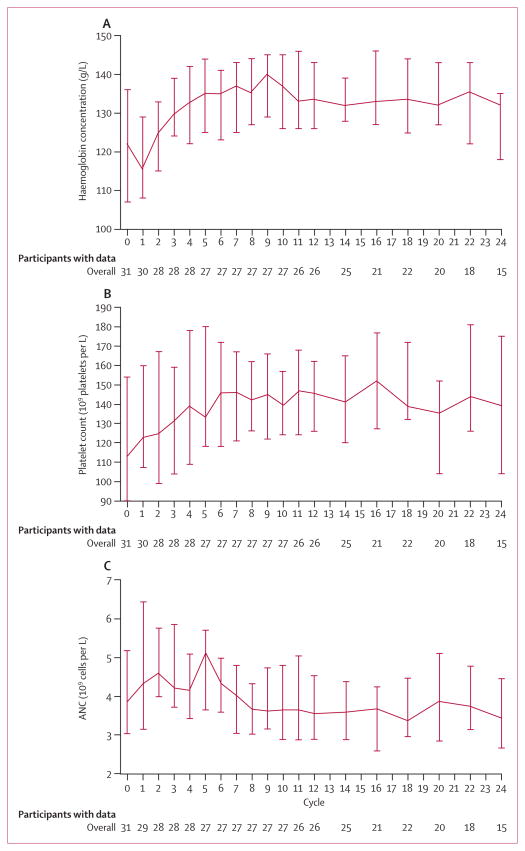
Notable improvements in baseline cytopenias were frequent (figure 6). 12 (39%) of 31 patients started treatment with a platelet count of fewer than 100 × 109 platelets per L and seven (58%) of these 12 patients had sustained improvement to counts of more than 100 × 109 platelets per L. 11 (35%) of 31 patients started treatment with haemoglobin concentrations of less than 110 g/L, and seven (64%) of these 11 patients had sustained improvement in the haemoglobin.
Figure 6. Median blood counts for all treated patients.
Data are median (95% CI). (A) Haemoglobin concentrations. (B) Platelet counts. (C) Absolute neutrophil counts (ANC).
Median serum IgA increased between baseline measure ment and measurement at 12 cycles; no changes were seen in serum IgM and serum IgG concentrations over time (table 4; figure 7).
Table 4.
Serum immunoglobulin changes for patients who did not receive immunoglobulins during treatment
| IgA
|
IgM
|
IgG
|
||||
|---|---|---|---|---|---|---|
| Data for all four timepoints | All available data | Data for all four timepoints | All available data | Data for all four timepoints | All available data | |
|
Baseline
| ||||||
| Patients | 16 | 26 | 13 | 23 | 15 | 25 |
| Median (95% CI), mg/dL | 52·0 (32·0–115·0) | 46·0 (29·0–74·0) | 25·1 (9·0–45·0) | 25·0 (11·5–40·0) | 594 (440–685) | 625 (462–685) |
|
| ||||||
|
Cycle 3
| ||||||
| Patients | 16 | 22 | 13 | 18 | 15 | 21 |
| Median (95% CI), mg/dL | 74·0 (52·0–159·0) | 72·5 (53·0–144·0) | 25·8 (11·0–46·0) | 28·4 (13·0–44·0) | 541 (366–701) | 593 (403–701) |
|
| ||||||
|
Cycle 6
| ||||||
| Patients | 16 | 23 | 13 | 20 | 15 | 22 |
| Median (95% CI), mg/dL | 90·5 (70·0–155·0) | 94·0 (70·0–135·0) | 34·0 (21·0–50·0) | 34·5 (23·0–43·0) | 579 (450–734) | 623 (556–783) |
|
| ||||||
|
Cycle 12
| ||||||
| Patients | 16 | 19 | 13 | 16 | 15 | 18 |
| Median (95% CI), mg/dL | 101·0 (71·0–175·0) | 104·0 (75·0–175·0) | 37·0 (23·0–43·0) | 36·0 (23·0–43·0) | 596 (505–747) | 613 (517–747) |
Figure 7. Serum immunoglobulin changes for previously untreated patients who did not receive immunoglobulins during treatment.
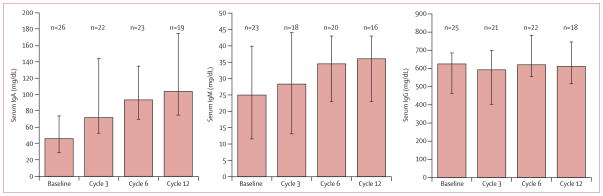
Data show median (95% CIs).
Discussion
Our study suggests that orally administered ibrutinib, a potent, covalent inhibitor of BTK, is well tolerated and effective in a previously untreated population of elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma.
Our elderly and treatment-naive patient population was selected to receive ibrutinib investigational therapy based on previous data from relapsed patients with chronic lymphocytic leukaemia who were treated with ibrutinib; the data showed that ibrutinib showed good tolerability and led to a high proportion of patients achieving an objective response (panel).11 Although chemo immunotherapy is the standard of care for fit patients with chronic lymphocytic leukaemia, equivalent treatment in older patients or in those with significant comorbidities is often not well tolerated.15,16 Ibrutinib was well tolerated in our study, although mild diarrhoea was a common, albeit self-limiting, adverse event early in the treatment course. Because the drug is minimally myelosuppressive in patients with chronic lymphocytic leukaemia, haematological toxicity was rare and infections were uncommon, despite only four patients receiving replacement intravenous immunoglobulins while on study. This finding is contrary to typical side-effects noted with chemoimmunotherapy for this elderly population of previously untreated patients, in whom myelosuppression is common, and often associated with infections. Future studies looking at subsets of immune cells in patients treated with ibrutinib will be informative about the effect of ibrutinib on other normal lymphocytes, including T cells and natural killer cells, as ibrutinib also inhibits interleukin-inducible kinase (which has immune-modulating potential).35 A systematic assessment of constitutional or B symptoms in patients receiving ibrutinib would be a worthwhile analysis, but was not done in the context of this study.
Time to initial response (complete response, partial response, or partial response with lymphocytosis) and time to best response (complete or partial response) were based on the 22 patients who achieved complete or partial response as assessed by IWCLL guidelines. The difference between initial and best response is due to many patients achieving an initial response at about 2 months, and then achieving a better response later (at about 6 months). The four patients who achieved complete response took a median of around 12 months to achieve it: the longest of all response times.
22 (71%) of 31 patients achieved an objective response, with an additional four (13%) achieving a partial response with lymphocytosis. Not only did a high proportion of patients achieve an objective response in this study, but the times to these responses were rapid (median time to response 1·9 months) and durable during the conduct of the study (median duration of response not estimable). We noted resolution of lymphocytosis and subsequent partial or complete response in nine (69%) of 13 of these patients during follow-up on this study. Four patients achieved partial response with lymphocytosis, and did not convert to a true partial or complete response during follow-up on this study; these patients continue on ibrutinib therapy in the setting of a long-term extension study. Although only four (13%) patients achieved a complete response, this finding might be partly explained by the lack of requirement for periodic bone marrow biopsies apart from when confirming complete response. This requirement was inconsistently followed by the investigators. As such, two patients who appeared in clinical complete remission, but who did not have a bone marrow biopsy to assess complete response, were classified as only achieving a partial response.
As previously reported in a relapsed population of patients with chronic lymphocytic leukaemia who were treated with ibrutinib, responses did not vary with the presence or absence of high-risk prognostic factors in our study.11 However, the frequency of patients with the high-risk genetic abnormalities del(17p13.1) or del(11q22.3), or with increased β2-microglobulin was relatively low in this study. Large future studies in high-risk populations will be needed to definitively assess the effect of ibrutinib on these high-risk subgroups.
Median progression-free survival was not reached, with only one patient progressing during follow-up. Of the four patients who were censored before 6 months for progression-free survival, two were also censored for overall survival before 6 months, and the other two were censored for overall survival at 25·3 months and 28·9 months, respectively. The differences in censoring times were due to the different censoring rules we used for progression-free survival and overall survival.
Chlorambucil is often regarded as a standard treatment option for elderly patients, with most published trials suggesting a median progression-free survival of 8–18 months.17,27,28 Combination treatments with a chlorambucil backbone and an anti-CD20 monoclonal antibody (eg, rituximab, ofatumumab, or obinutuzumab) have been reported to achieve longer progression-free survival than chlorambucil alone, with reported median progression-free survival of 15·7–24·0 months with the chemoimmunotherapy combin ations.20,29,30 Recently, lenalidomide has been used as initial therapy in patients older than 65 years with chronic lymphocytic leukaemia,31 with 65% of patients achieving an objective response (including 10% achieving a complete response). 83% of patients in the study had at least one episode of grade 3–4 neutropenia and the estimated 2 year progression-free survival was 60%.
An unresolved question is whether improvements in outcome are associated with rapid achievement of responses. The low degree of myelosuppression reported with ibrutinib in patients with chronic lymphocytic leukaemia, and the low levels of non-haematological toxicities, make this a useful agent to use in combination with other therapies, especially other non-cytotoxic therapies, including B-cell-depleting monoclonal antibodies such as rituximab. This combination has led to relapsed patients with chronic lymphocytic leukaemia achieving a high frequency of objective response (17 [85%] of 20 patients assessable for early response assessment at 3 months) together with an early resolution of lymphocytosis compared with single-agent ibrutinib.32 Additional ibrutinib com binations, including with other targeted agents such as PI3K inhibitors, agents that target anti-apoptotic proteins, and cyclin-dependent kinase inhibitors, could be explored. However, combination with other drugs might lead to additional toxicities, potentially an important factor to consider in an elderly population of patients. Furthermore, the long-term benefit of such combinations in view of the durability of remission reported to date with ibrutinib is unknown.
Panel: Research in context.
Systematic review
We searched PubMed for articles published in English after Jan 1, 2005, to identify agents used to treat chronic lymphocytic leukaemia (rituximab, ofatumumab, obinutuzumab, chlorambucil, fludarabine, cyclophosphamide, and lenalidomide). We identified primary publications for these agents,12–20,27–34 which show that, in a chemotherapy and lenalidomide regimen, myelosuppression and infections are the major problems. Only one trial has been published to date on ibrutinib.11 To our knowledge, no other data are available for ibrutinib treatment in previously untreated elderly patients.
Interpretation
We have shown that ibrutinib is active and safe for the treatment of patients older than 65 years with chronic lymphocytic leukaemia. Larger phase 3 trials are required to confirm these findings.
The elderly population of previously untreated patients assessed in this trial had several comorbidities. Comorbidites are common in elderly individuals, and are increasingly severe and frequent as people age. A trial16 of fludarabine and cyclophosphamide alone or with rituximab enrolled patients older than 70 years (but not >81 years); notably, all participants had to have a creatinine clearance of more than 70 mL/min and a comorbidity score of less than 6. However, only 8% of all patients older than 65 years who attended the MD Anderson Leukemia Clinic (Houston, TX, USA) in 1 month met such criteria (O’Brien S; unpublished data). Treatment of all patients older than 70 years with fludarabine-based therapy showed that such patients had increased toxicity compared with younger patients and were often unable to complete the planned six cycles of therapy, whereas in the present study the median treatment duration for the 23 patients 70 years and older was 21·7 months (IQR 14·8–23·1) with only two of these patients requiring treatment discontinuation for an adverse event.33,34 In conclusion, our data support continued assessment of ibrutinib in this population of patients. Two phase 3 trials of ibrutinib in this population examining progression-free survival are ongoing or planned: RESONATE-2, comparing efficacy with chlorambucil or ibrutinib (NCT01722487), and NCT01886872 examining bendamustine-rituximab versus ibrutinib-rituximab versus ibrutinib.
Acknowledgments
We thank the investigators and coordinators at each of the clinical sites; patients who participated in this trial and their families; employees of Pharmacyclics who contributed to the design and implementation of this trial; Cathy Zhou (Pharmacyclics, Sunnyvale, CA, USA) for assistance with statistical data analysis; Anh Tran (Pharmacyclics) for study management; Namit Ghildyal, Janssen Research & Development, Titusville, NJ, USA, for editorial assistance of the manuscript after the first draft that was generated by SO’B and edited by JCB.
Footnotes
Contributors
SO’B, RRF, SEC, JPS, JAB, KAB, DAR, MC, WGW, WZ, NAH, AJJ, RI, AH, BYC, FC, JJB, DFJ, and JCB were involved in the study conception and design, provision of study materials or patients, collection and assembly of data, data analysis, and interpretation, manuscript writing, and manuscript approval. BG and JAJ were involved in provision of study materials or patients, collection and assembly of data, and manuscript approval. TG was involved in collection and assembly of data, data analysis and interpretation, manuscript writing, and manuscript approval.
Conflicts of interest
RRF has received an honorarium for advisory board participation from Pharmacyclics. SEC has received consultancy fees from Pharmacyclics. JPS has received research funding from Pharmacyclics, Gilead, Genentech, Seattle Genetics, and Celgene. JAB has received research funding from Pharmacyclics. JAJ has received consultancy fees and is an advisory board member for Pharmacyclics. BYC, TG, FC, and DFJ are employees and stockholders of Pharmacyclics. AH is a former employee of Pharmacyclics. JJB is a former employee and is a stockholder of Pharmacyclics. All other authors declare that they have no conflicts of interest.
References
- 1.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenkre VP, Kahl BS. The future of B-cell lymphoma therapy: the B-cell receptor and its downstream pathways. Curr Hematol Malig Rep. 2012;7:216–20. doi: 10.1007/s11899-012-0127-0. [DOI] [PubMed] [Google Scholar]
- 3.Wiestner A. Targeting B-cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31:128–30. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- 4.Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006;176:5715–19. doi: 10.4049/jimmunol.176.10.5715. [DOI] [PubMed] [Google Scholar]
- 5.Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, Macewan DJ. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-kappaB. Cell Signal. 2013;25:106–12. doi: 10.1016/j.cellsig.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–94. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 8.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–89. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gribben JG, O’Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–50. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 14.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–43. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 16.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 17.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–39. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 18.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–91. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 19.Woyach JA, Ruppert AS, Rai K, et al. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential cancer and leukemia group B studies. J Clin Oncol. 2013;31:440–47. doi: 10.1200/JCO.2011.41.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goede V, Fischer K, Busch R, et al. Chemoimmunotherapy with GA101 plus chlorambucil in patients with chronic lymphocytic leukemia and comorbidity: results of the CLL11 (BO21004) safety run-in. Leukemia. 2013;27:1172–74. doi: 10.1038/leu.2012.252. [DOI] [PubMed] [Google Scholar]
- 21.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–99. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, et al. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes. [accessed Oct 11, 2013];Blood. 2012 eUpdate June 4, 2012. http://bloodjournal.hematologylibrary.org/content/111/12/5446.full/reply#bloodjournal_el_6920.
- 24.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2820–22. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Claus R, Lucas DM, Stilgenbauer S, et al. Quantitative DNA methylation analysis identifies a single CpG dinucleotide important for ZAP-70 expression and predictive of prognosis in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2483–91. doi: 10.1200/JCO.2011.39.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 28.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–23. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 29.Goede V, Fischer K, Humphrey K, et al. Obinutuzumab (GA101) plus chlorambucil (Clb) or rituximab versus Clb alone in patients with chronic lymphocytic leukemia and preexisting medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase III trial. Proc Am Soc Clin Oncol. 2013;31(suppl):abstr 7004. [Google Scholar]
- 30.Goede V, Fischer K, Kreuzer K-A, et al. Kinetics of blood cell subpopulations during treatment with obinutuzumab (GA101) + chlorambucil (GClb), rituximab +Clb (RClb) versus Clb alone in patients with chronic lymphocytic leukemia and coexisting medical conditions: stage I results of the CLL11 (BO21004) trial. Biennial International Workshop on CLL (iwCLL); Sept 9–11, 2013; Cologne, Germany. 2013. p. abstr 4.14. [Google Scholar]
- 31.Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–98. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA, Keating MJ, Wierda WG, et al. The Btk inhibitor ibrutinib (PCI-32765) in combination with rituximab is well tolerated and displays profound activity in high-risk chronic lymphocytic leukemia (CLL) patients. Blood. 2012;120:187. [Google Scholar]
- 33.Ferrajoli A, O’Brien S, Wierda WG, et al. Treatment of patients with CLL 70 years old and older: a single center experience of 142 patients. Leuk Lymphoma. 2005;46:S87. [Google Scholar]
- 34.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 35.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]