Abstract
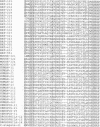
Using the polymerase chain reaction, we have isolated numerous rat and human cDNAs of which the deduced amino acid sequences are highly homologous to the sequences of the extracellular domain of cadherins. The entire putative coding sequences for two human proteins defined by two of these cDNAs have been determined. The overall structure of these molecules is very similar to that of classic cadherins, but they have some unique features. The extracellular domains are composed of six or seven subdomains that are very similar to those of cadherins, but have characteristic properties. The cytoplasmic domains, on the other hand, have no significant homology with those of classic cadherins. Since various cDNAs with almost identical features were obtained also from Xenopus, Drosophila and Caenorhabditis elegans, it appears that similar molecules are expressed in a variety of organisms. We have tentatively named these proteins protocadherins. They are highly expressed in brain and their expression appears to be developmentally regulated. The proteins expressed from the two full-length cDNAs in L cells were approximately 170 or 150 kDa in size, and were localized mainly at cell-cell contact sites. Moreover, the transfectants showed cell adhesion activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amagai M., Klaus-Kovtun V., Stanley J. R. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991 Nov 29;67(5):869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- Bertolotti R., Rutishauser U., Edelman G. M. A cell surface molecule involved in aggregation of embryonic liver cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4831–4835. doi: 10.1073/pnas.77.8.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
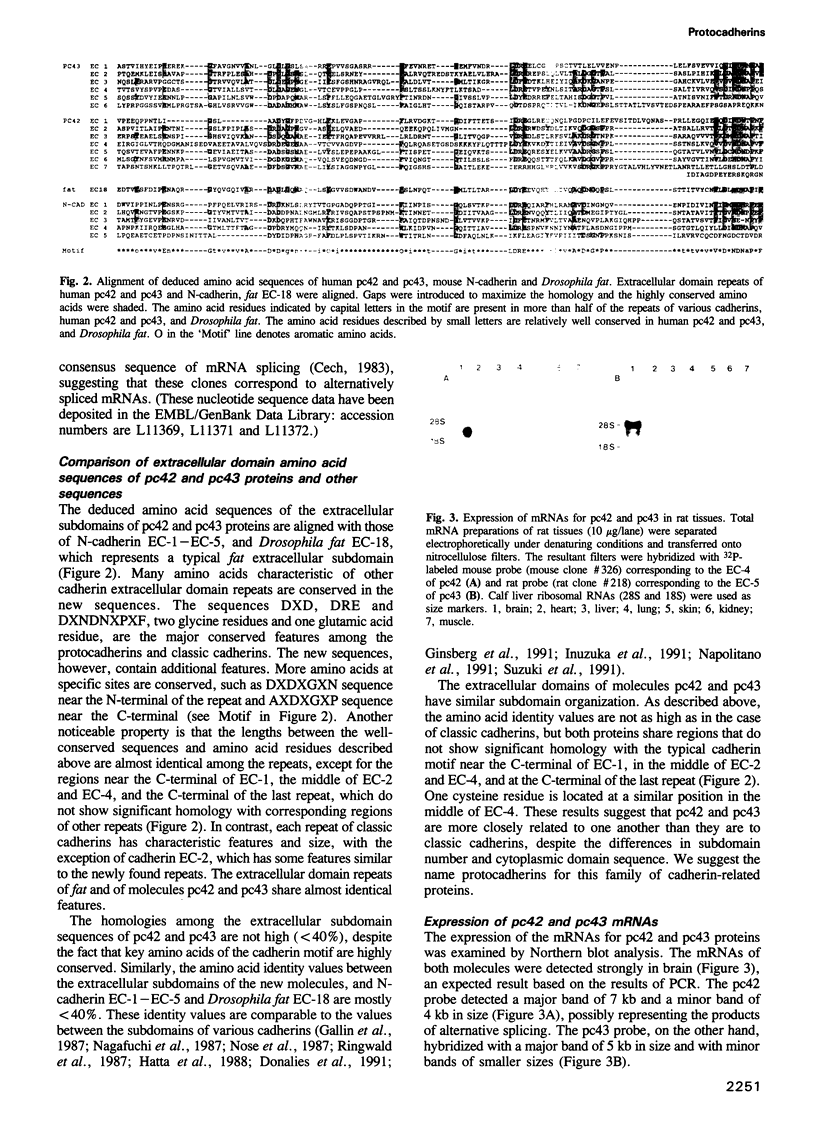
- Cech T. R. RNA splicing: three themes with variations. Cell. 1983 Oct;34(3):713–716. doi: 10.1016/0092-8674(83)90527-5. [DOI] [PubMed] [Google Scholar]
- Chen W. C., Obrink B. Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells. J Cell Biol. 1991 Jul;114(2):319–327. doi: 10.1083/jcb.114.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrick R. J., Dickey D., Kintner C. R. The effects of N-cadherin misexpression on morphogenesis in Xenopus embryos. Neuron. 1990 Apr;4(4):493–506. doi: 10.1016/0896-6273(90)90108-r. [DOI] [PubMed] [Google Scholar]
- Donalies M., Cramer M., Ringwald M., Starzinski-Powitz A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8024–8028. doi: 10.1073/pnas.88.18.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen U. H., Behrens J., Sachs M., Eberle G., Voss B., Warda A., Löchner D., Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991 Apr;113(1):173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T., Miyatani S., Takeichi M. Ectopic expression of N-cadherin perturbs histogenesis in Xenopus embryos. Development. 1990 Sep;110(1):97–104. doi: 10.1242/dev.110.1.97. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., DeSimone D., Geiger B. Expression of a novel cadherin (EP-cadherin) in unfertilized eggs and early Xenopus embryos. Development. 1991 Feb;111(2):315–325. doi: 10.1242/dev.111.2.315. [DOI] [PubMed] [Google Scholar]
- Goodwin L., Hill J. E., Raynor K., Raszi L., Manabe M., Cowin P. Desmoglein shows extensive homology to the cadherin family of cell adhesion molecules. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1224–1230. doi: 10.1016/s0006-291x(05)80917-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988 Oct;107(4):1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Nose A., Nagafuchi A., Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta M., Miyatani S., Copeland N. G., Gilbert D. J., Jenkins N. A., Takeichi M. Genomic organization and chromosomal mapping of the mouse P-cadherin gene. Nucleic Acids Res. 1991 Aug 25;19(16):4437–4441. doi: 10.1093/nar/19.16.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Kimoto N., Shimoyama Y., Hirohashi S., Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992 Jul 24;70(2):293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Holton J. L., Kenny T. P., Legan P. K., Collins J. E., Keen J. N., Sharma R., Garrod D. R. Desmosomal glycoproteins 2 and 3 (desmocollins) show N-terminal similarity to calcium-dependent cell-cell adhesion molecules. J Cell Sci. 1990 Oct;97(Pt 2):239–246. doi: 10.1242/jcs.97.2.239. [DOI] [PubMed] [Google Scholar]
- Inuzuka H., Miyatani S., Takeichi M. R-cadherin: a novel Ca(2+)-dependent cell-cell adhesion molecule expressed in the retina. Neuron. 1991 Jul;7(1):69–79. doi: 10.1016/0896-6273(91)90075-b. [DOI] [PubMed] [Google Scholar]
- Kemler R., Babinet C., Eisen H., Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4449–4452. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R., Ozawa M., Ringwald M. Calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1989 Oct;1(5):892–897. doi: 10.1016/0955-0674(89)90055-0. [DOI] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992 Apr 17;69(2):225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Koch P. J., Walsh M. J., Schmelz M., Goldschmidt M. D., Zimbelmann R., Franke W. W. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990 Oct;53(1):1–12. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney P. A., Weber U., Onofrechuk P., Biessmann H., Bryant P. J., Goodman C. S. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991 Nov 29;67(5):853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- McCrea P. D., Gumbiner B. M. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem. 1991 Mar 5;266(7):4514–4520. [PubMed] [Google Scholar]
- Miyatani S., Shimamura K., Hatta M., Nagafuchi A., Nose A., Matsunaga M., Hatta K., Takeichi M. Neural cadherin: role in selective cell-cell adhesion. Science. 1989 Aug 11;245(4918):631–635. doi: 10.1126/science.2762814. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano E. W., Venstrom K., Wheeler E. F., Reichardt L. F. Molecular cloning and characterization of B-cadherin, a novel chick cadherin. J Cell Biol. 1991 May;113(4):893–905. doi: 10.1083/jcb.113.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO J. 1987 Dec 1;6(12):3655–3661. doi: 10.1002/j.1460-2075.1987.tb02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Engel J., Kemler R. Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function. Cell. 1990 Nov 30;63(5):1033–1038. doi: 10.1016/0092-8674(90)90506-a. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Dours-Zimmermann M. T. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991 Sep;7(3):391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- Ringwald M., Baribault H., Schmidt C., Kemler R. The structure of the gene coding for the mouse cell adhesion molecule uvomorulin. Nucleic Acids Res. 1991 Dec 11;19(23):6533–6539. doi: 10.1093/nar/19.23.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y., Yoshida T., Terada M., Shimosato Y., Abe O., Hirohashi S. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989 Oct;109(4 Pt 1):1787–1794. doi: 10.1083/jcb.109.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin B. C., Gallin W. J., Edelman G. M., Cunningham B. A. Genes for two calcium-dependent cell adhesion molecules have similar structures and are arranged in tandem in the chicken genome. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11545–11549. doi: 10.1073/pnas.88.24.11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Sano K., Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 1991 Apr;2(4):261–270. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara H., Ozaki H. S., Takeichi M. Immunological detection of cell surface components related with aggregation of Chinese hamster and chick embryonic cells. Dev Biol. 1979 May;70(1):206–216. doi: 10.1016/0012-1606(79)90017-4. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet L., Jr, Mareel M., Fiers W., van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991 Jul 12;66(1):107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol. 1986 Oct;103(4):1441–1450. doi: 10.1083/jcb.103.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]