Abstract
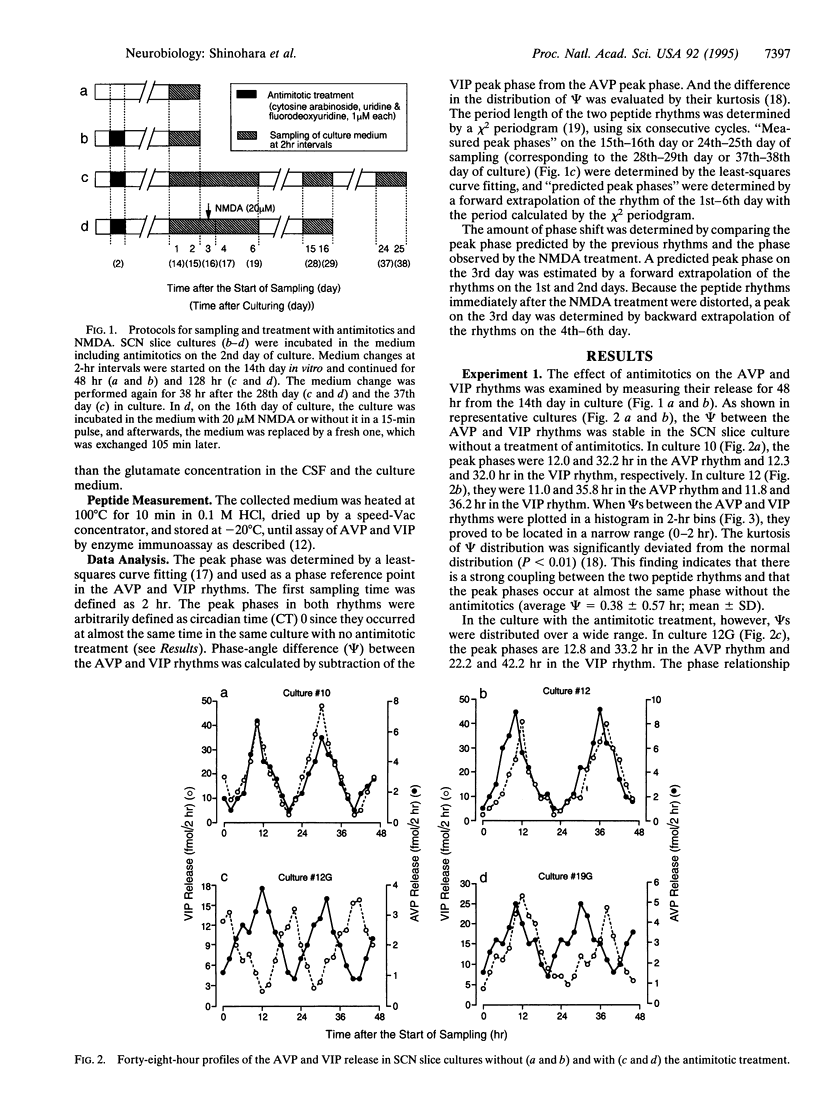
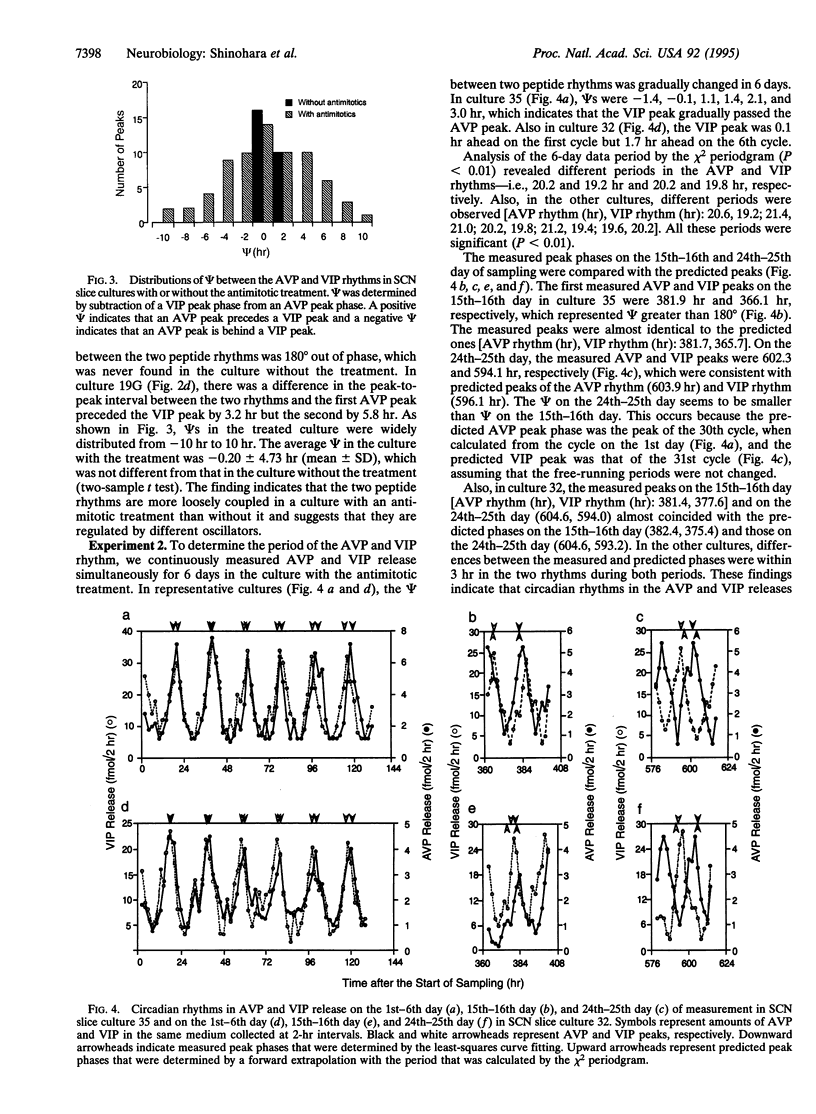
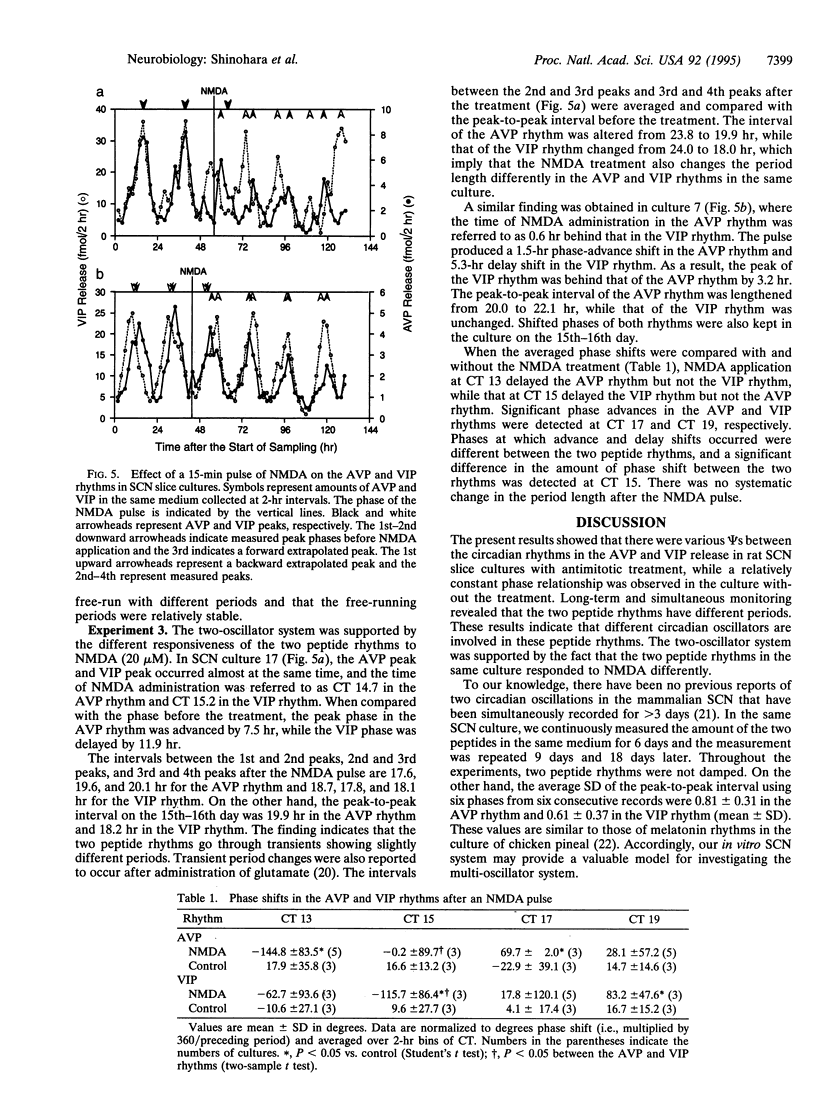
In the rat suprachiasmatic nucleus slice culture, circadian rhythms in the release of arginine vasopressin and vasoactive intestinal polypeptide were measured simultaneously and longitudinally. The phase relationship between the two peptide rhythms was relatively constant in the culture without a treatment of antimitotic drugs but became diverse by an introduction of antimitotics, which is generally used to reduce the number of glial cells. By monitoring the two rhythms continuously for 6 days, different periods were detected in culture with the antimitotic treatment. Furthermore, N-methyl-D-aspartate shifted the phase of the two peptide rhythms in the same culture differently. These results indicate that the arginine vasopressin and vasoactive intestinal polypeptide release are under control of different circadian oscillators.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cagampang F. R., Inouye S. T. Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain Res. 1994 Mar 7;639(1):175–179. doi: 10.1016/0006-8993(94)91780-9. [DOI] [PubMed] [Google Scholar]
- Cagampang F. R., Yang J., Nakayama Y., Fukuhara C., Inouye S. T. Circadian variation of arginine-vasopressin messenger RNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1994 Jul;24(1-4):179–184. doi: 10.1016/0169-328x(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Colwell C. S., Menaker M. NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. J Biol Rhythms. 1992 Summer;7(2):125–136. doi: 10.1177/074873049200700204. [DOI] [PubMed] [Google Scholar]
- Earnest D. J., Sladek C. D. Circadian vasopressin release from perifused rat suprachiasmatic explants in vitro: effects of acute stimulation. Brain Res. 1987 Oct 6;422(2):398–402. doi: 10.1016/0006-8993(87)90952-8. [DOI] [PubMed] [Google Scholar]
- Fukuhara C., Nishiwaki T., Cagampang F. R., Inouye S. T. Emergence of VIP rhythmicity following somatostatin depletion in the rat suprachiasmatic nucleus. Brain Res. 1994 May 9;645(1-2):343–346. doi: 10.1016/0006-8993(94)91671-3. [DOI] [PubMed] [Google Scholar]
- Gillette M. U., Reppert S. M. The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res Bull. 1987 Jul;19(1):135–139. doi: 10.1016/0361-9230(87)90176-6. [DOI] [PubMed] [Google Scholar]
- Glazer R., Gozes I. Diurnal oscillation in vasoactive intestinal peptide gene expression independent of environmental light entraining. Brain Res. 1994 Apr 25;644(1):164–167. doi: 10.1016/0006-8993(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Hiroshige T., Honma K., Watanabe K. Ontogeny of the circadian rhythm of plasma corticosterone in blind infantile rats. J Physiol. 1982 Apr;325:493–506. doi: 10.1113/jphysiol.1982.sp014164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K., Honma S., Hiroshige T. Response curve, free-running period, and activity time in circadian locomotor rhythm of rats. Jpn J Physiol. 1985;35(4):643–658. doi: 10.2170/jjphysiol.35.643. [DOI] [PubMed] [Google Scholar]
- Ito C., Wakamori M., Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am J Physiol. 1991 Feb;260(2 Pt 1):C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Morin A., Denoroy L., Jouvet M. Daily variations in concentration of vasoactive intestinal polypeptide immunoreactivity in discrete brain areas of the rat. Brain Res. 1991 Jan 4;538(1):136–140. doi: 10.1016/0006-8993(91)90387-b. [DOI] [PubMed] [Google Scholar]
- Murakami N., Takamure M., Takahashi K., Utunomiya K., Kuroda H., Etoh T. Long-term cultured neurons from rat suprachiasmatic nucleus retain the capacity for circadian oscillation of vasopressin release. Brain Res. 1991 Apr 5;545(1-2):347–350. doi: 10.1016/0006-8993(91)91312-o. [DOI] [PubMed] [Google Scholar]
- Prosser R. A., Edgar D. M., Heller H. C., Miller J. D. A possible glial role in the mammalian circadian clock. Brain Res. 1994 Apr 18;643(1-2):296–301. doi: 10.1016/0006-8993(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Robertson L. M., Takahashi J. S. Circadian clock in cell culture: I. Oscillation of melatonin release from dissociated chick pineal cells in flow-through microcarrier culture. J Neurosci. 1988 Jan;8(1):12–21. doi: 10.1523/JNEUROSCI.08-01-00012.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K., Honma S., Katsuno Y., Abe H., Honma K. Circadian rhythms in the release of vasoactive intestinal polypeptide and arginine-vasopressin in organotypic slice culture of rat suprachiasmatic nucleus. Neurosci Lett. 1994 Mar 28;170(1):183–186. doi: 10.1016/0304-3940(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Shinohara K., Tominaga K., Isobe Y., Inouye S. T. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993 Feb;13(2):793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T., Moore R. Y. Glutamate shifts the phase of the circadian neuronal firing rhythm in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1994 Aug 29;178(1):47–50. doi: 10.1016/0304-3940(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Sokolove P. G., Bushell W. N. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978 May 8;72(1):131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Tominaga K., Shinohara K., Otori Y., Fukuhara C., Inouye S. T. Circadian rhythms of vasopressin content in the suprachiasmatic nucleus of the rat. Neuroreport. 1992 Sep;3(9):809–812. doi: 10.1097/00001756-199209000-00022. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Koibuchi N., Ohtake H., Yamaoka S. Circadian rhythms of vasopressin release in primary cultures of rat suprachiasmatic nucleus. Brain Res. 1993 Oct 8;624(1-2):115–120. doi: 10.1016/0006-8993(93)90067-w. [DOI] [PubMed] [Google Scholar]
- Wray S., Castel M., Gainer H. Characterization of the suprachiasmatic nucleus in organotypic slice explant cultures. Microsc Res Tech. 1993 May 1;25(1):46–60. doi: 10.1002/jemt.1070250108. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kovács K., Lolait S. J. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology. 1993 Aug;133(2):585–590. doi: 10.1210/endo.133.2.8344200. [DOI] [PubMed] [Google Scholar]
- del Rio J. A., Heimrich B., Soriano E., Schwegler H., Frotscher M. Proliferation and differentiation of glial fibrillary acidic protein-immunoreactive glial cells in organotypic slice cultures of rat hippocampus. Neuroscience. 1991;43(2-3):335–347. doi: 10.1016/0306-4522(91)90298-3. [DOI] [PubMed] [Google Scholar]



