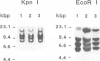Abstract
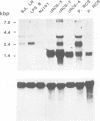
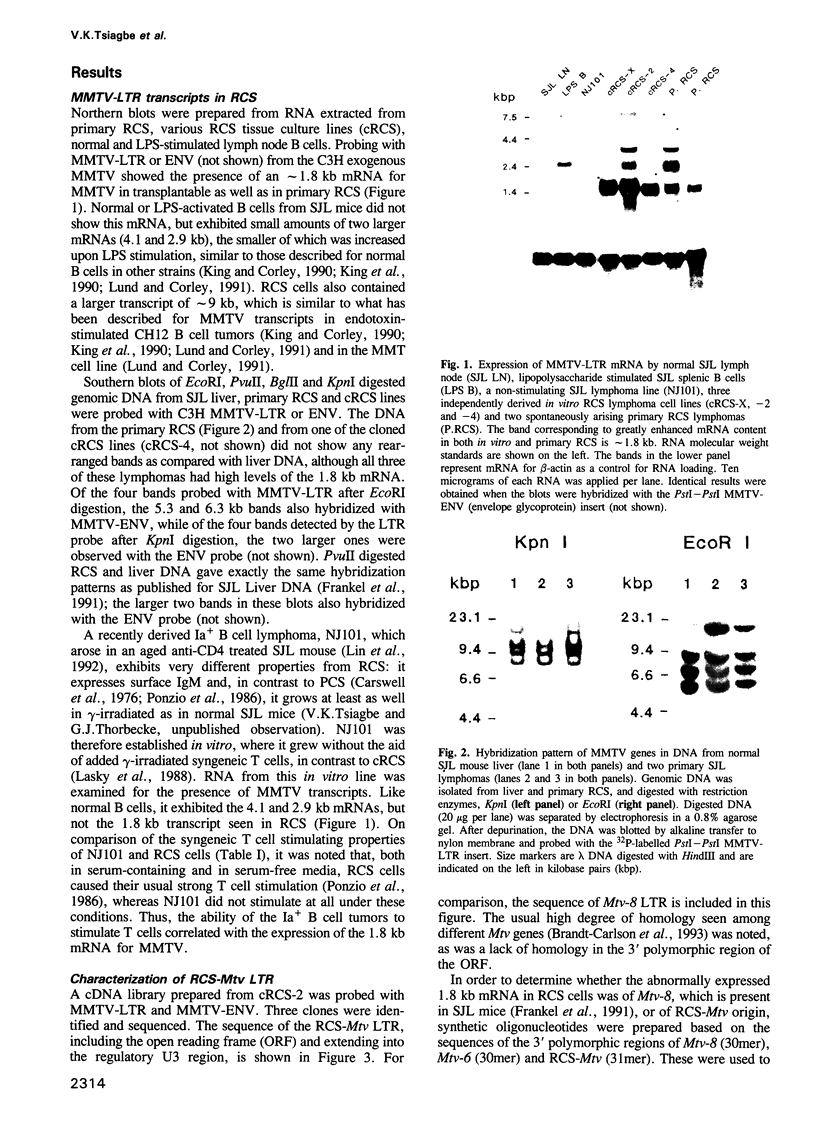
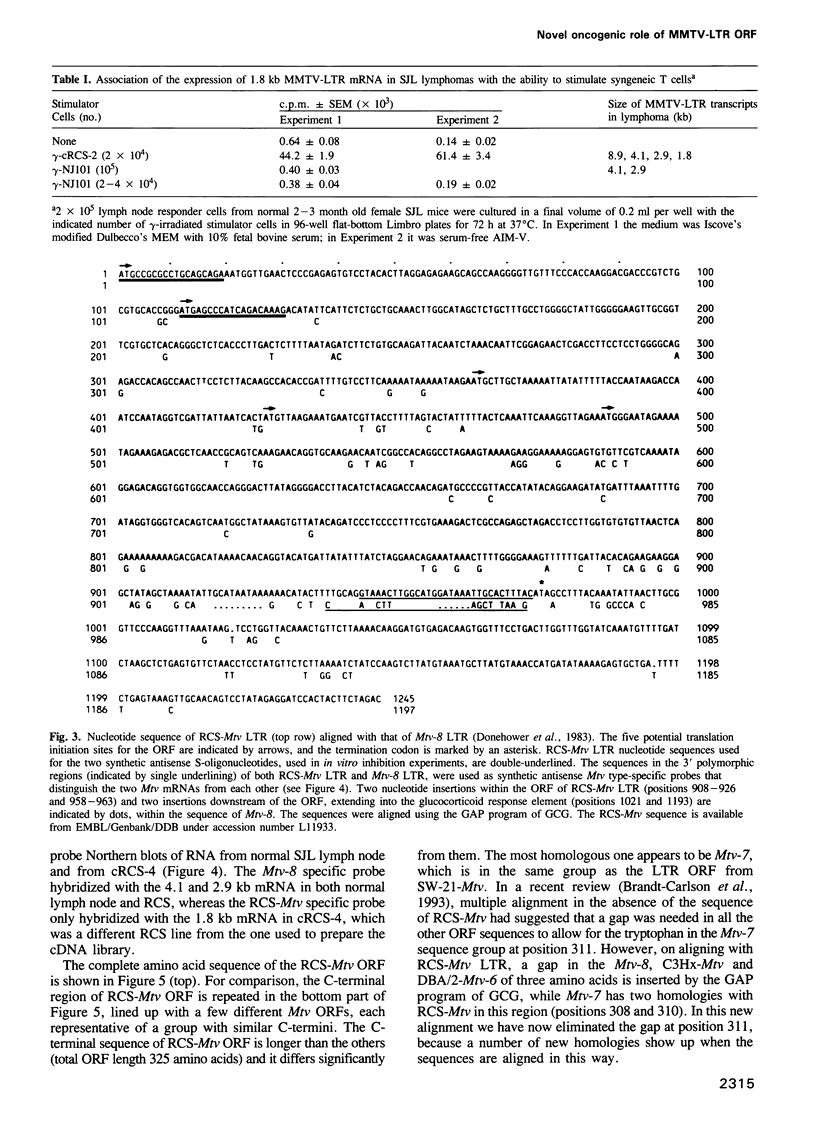
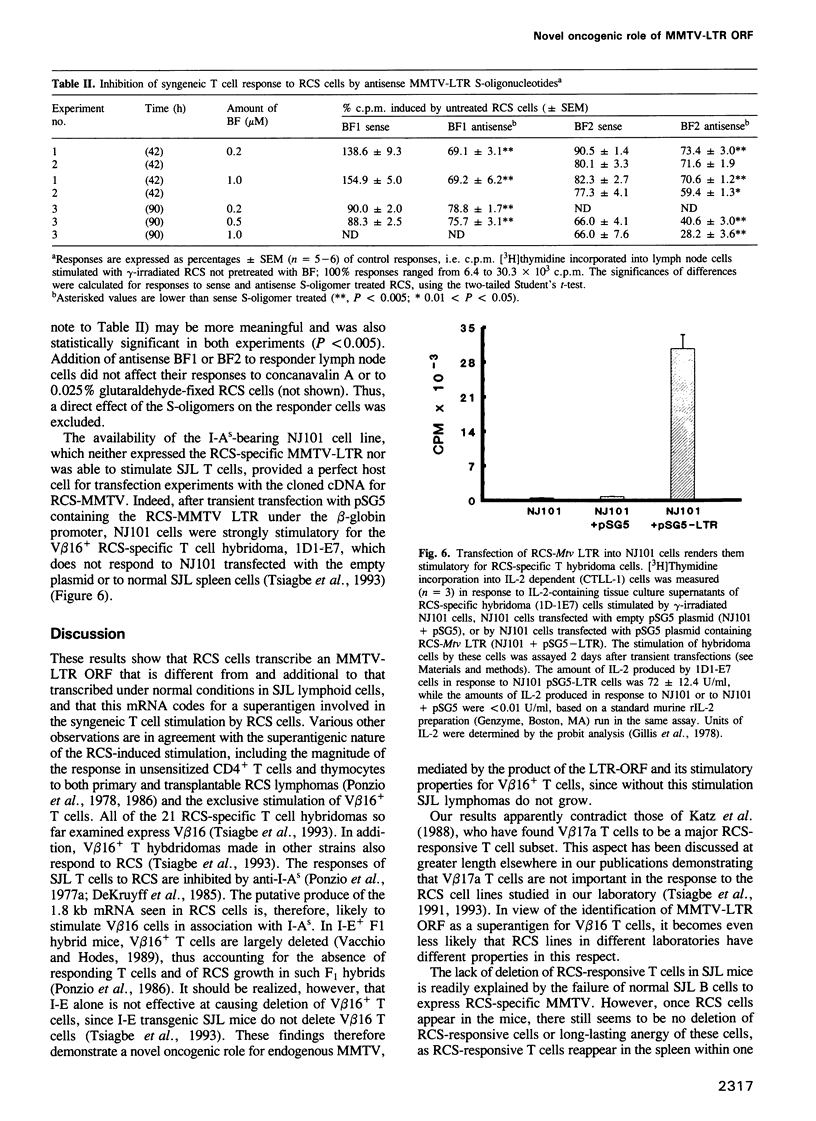
The MHC class II I-A(s) positive B cell lymphomas reticulum cell sarcoma (RCS) that arise in > 90% of SJL mice by the age of 12 months have superantigen-like stimulating properties. In the present study, therefore, RCS cell lines were examined for abnormal expression of endogenous mouse mammary tumor virus (MMTV) proviruses. Extraordinarily high expression of a 1.8 kb mRNA hybridizing with the long terminal repeat (LTR) of MMTV was found in both primary lymphomas and in vitro RCS lines, but not in an SJL B cell lymphoma, NJ101, that does not stimulate syngeneic T cells, or in LPS activated SJL B cells. A cDNA was cloned from cRCS-2 and sequenced. A 31mer oligonucleotide probe, prepared based on the unique C-terminal sequence of this RCS-Mtv LTR, detected the 1.8 kb mRNA in all RCS lymphomas, while a similar probe for the C-terminal sequence of Mtv-8 LTR hybridized with the larger mRNA present in normal B cells and in NJ101. Preincubation with 19mer antisense S-oligonucleotides, prepared based on the sequences of the first two potential translation initiation sites common to both Mtv-8 and the RCS-Mtv LTR, significantly reduced the ability of RCS cells to stimulate syngeneic T cells. Moreover, transfection of NJ101 cells with the cloned RCS-MMTV cDNA conferred V beta 16 T cell stimulating properties on to these cells. It is concluded that expression of the product of this MMTV-LTR mRNA provides RCS with the strong T cell stimulating properties that it needs for its growth. These results thus identify a novel oncogenic property of MMTV-LTR.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Beutner U., Frankel W. N., Cote M. S., Coffin J. M., Huber B. T. Mls-1 is encoded by the long terminal repeat open reading frame of the mouse mammary tumor provirus Mtv-7. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5432–5436. doi: 10.1073/pnas.89.12.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt-Carlson C., Butel J. S., Wheeler D. Phylogenetic and structural analyses of MMTV LTR ORF sequences of exogenous and endogenous origins. Virology. 1993 Mar;193(1):171–185. doi: 10.1006/viro.1993.1113. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Mathis D., Cone R. E., Jones P. P., Ponzio N. M., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. VIII. Prominent role of RCS cell I-A antigens in the stimulation of syngeneic T cells. Immunogenetics. 1983;18(4):399–413. doi: 10.1007/BF00372472. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Lerman S. P., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. II. Fate of labeled tumor cells in normal and irradiated syngeneic mice. Cell Immunol. 1976 Apr;23(1):39–52. doi: 10.1016/0008-8749(76)90170-2. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Choi Y., Marrack P., Kappler J. W. Structural analysis of a mouse mammary tumor virus superantigen. J Exp Med. 1992 Mar 1;175(3):847–852. doi: 10.1084/jem.175.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff R. H., Brown P. H., Thorbecke G. J., Ponzio N. M. Characterization of SJL T cell clones responsive to syngeneic lymphoma (RCS): RCS-specific clones are stimulated by activated B cells. J Immunol. 1985 Nov;135(5):3581–3586. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gollob K. J., Palmer E. Divergent viral superantigens delete V beta 5+ T lymphocytes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5138–5141. doi: 10.1073/pnas.89.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T., Ponzio N. M., Nagler C., Vilcek J., Coico R. F., Thorbecke G. J. Ia-restricted interaction of normal lymphoid cells and SJL lymphoma (reticulum cell sarcoma) leading to lymphokine production. III. Relative roles of reticulum cell sarcoma and normal lymphoid cells in lymphokine production. J Natl Cancer Inst. 1984 Feb;72(2):321–331. [PubMed] [Google Scholar]
- Held W., Shakhov A. N., Waanders G., Scarpellino L., Luethy R., Kraehenbuhl J. P., MacDonald H. R., Acha-Orbea H. An exogenous mouse mammary tumor virus with properties of Mls-1a (Mtv-7). J Exp Med. 1992 Jun 1;175(6):1623–1633. doi: 10.1084/jem.175.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Katz I. R., Chapman-Alexander J., Jacobson E. B., Lerman S. P., Thorbecke G. J. Growth of SJL/J-derived transplantable reticulum cell sarcoma as related to its ability to induce T-cell proliferation in the host. III. Studies on thymectomized and congenitally athymic SJL mice. Cell Immunol. 1981 Nov 15;65(1):84–92. doi: 10.1016/0008-8749(81)90054-x. [DOI] [PubMed] [Google Scholar]
- Katz J. D., Ohnishi K., Lebow L. T., Bonavida B. The SJL/J T cell response to both spontaneous and transplantable syngeneic reticulum cell sarcoma is mediated predominantly by the V beta 17a+ T cell clonotype. J Exp Med. 1988 Nov 1;168(5):1553–1562. doi: 10.1084/jem.168.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. B., Corley R. B. Lipopolysaccharide and dexamethasone induce mouse mammary tumor proviral gene expression and differentiation in B lymphocytes through distinct regulatory pathways. Mol Cell Biol. 1990 Aug;10(8):4211–4220. doi: 10.1128/mcb.10.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. B., Lund F. E., White D. A., Sharma S., Corley R. B. Molecular events in B lymphocyte differentiation. Inducible expression of the endogenous mouse mammary tumor proviral gene, Mtv-9. J Immunol. 1990 Apr 15;144(8):3218–3227. [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky J. L., Ponzio N. M., Thorbecke G. J. Characterization and growth factor requirements of SJL lymphomas. I. Development of a B cell growth factor-dependent in vitro cell line, cRCS-X. J Immunol. 1988 Jan 15;140(2):679–687. [PubMed] [Google Scholar]
- Lasky J. L., Thorbecke G. J. Characterization and growth factor requirements of SJL lymphomas. II. Interleukin 5 dependence of the in vitro cell line, cRCS-X, and influence of other cytokines. Eur J Immunol. 1989 Feb;19(2):365–371. doi: 10.1002/eji.1830190222. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lerch H. P., Frank R., Collins J. Cloning, sequencing and expression of the L-2-hydroxyisocaproate dehydrogenase-encoding gene of Lactobacillus confusus in Escherichia coli. Gene. 1989 Nov 30;83(2):263–270. doi: 10.1016/0378-1119(89)90112-1. [DOI] [PubMed] [Google Scholar]
- Lerman S. P., Carswell E. A., Chapman J., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. III. Promotion of tumor growth in irradiated mice by normal lymphoid cells. Cell Immunol. 1976 Apr;23(1):53–67. doi: 10.1016/0008-8749(76)90171-4. [DOI] [PubMed] [Google Scholar]
- Lin T. Z., Fernandes H., Yauch R., Ponzio N. M., Raveche E. IL-10 production in a CD5+ B cell lymphoma arising in a CD4 monoclonal antibody-treated SJL mouse. Clin Immunol Immunopathol. 1992 Oct;65(1):10–22. doi: 10.1016/0090-1229(92)90242-g. [DOI] [PubMed] [Google Scholar]
- Lopez D. M., Charyulu V., Paul R. D. B cell subsets in spleens of BALB/c mice: identification and isolation of MMTV-expressing and MMTV-responding subpopulations. J Immunol. 1985 Jan;134(1):603–607. [PubMed] [Google Scholar]
- Lund F. E., Corley R. B. Regulated expression of mouse mammary tumor proviral genes in cells of the B lineage. J Exp Med. 1991 Dec 1;174(6):1439–1450. doi: 10.1084/jem.174.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina I. J., Cannon N. A., Hyman R., Huber B. T. Macrophages and T cells do not express Mlsa determinants. J Immunol. 1989 Jul 1;143(1):39–44. [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R. Experimental models of lymphoproliferative disease. The mouse as a model for human non-Hodgkin's lymphomas and related leukemias. Am J Pathol. 1983 Nov;113(2):237–265. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio N. M., Alonso A., Alisauskas R. M. Dependence of lymphoma growth on 'reversed immunological surveillance'. Pathol Immunopathol Res. 1987;6(1):1–21. doi: 10.1159/000157038. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., Brown P. H., Thorbecke G. J. Host-tumor interactions in the SJL lymphoma model. Int Rev Immunol. 1986 Jul;1(3-4):273–301. doi: 10.3109/08830188609056610. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., Chapman-Alexander J., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice VI. Characterization of lymphoid cells that proliferate in response to RCS cells. Cell Immunol. 1978 Nov;41(1):157–171. doi: 10.1016/s0008-8749(78)80035-5. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., David C. S., Shreffler D. C., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. V. Nature of reticulum cell sarcoma surface antigen which induces proliferation of normal SJL/J T cells. J Exp Med. 1977 Jul 1;146(1):132–145. doi: 10.1084/jem.146.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio N. M., Hayama T., Nagler C., Katz I. R., Hoffmann M. K., Gilbert K., Vilcek J., Thorbecke G. J. Ia-restricted interaction of normal lymphoid cells and SJL lymphoma (reticulum cell sarcoma) leading to lymphokine production. II. Rapid production of antibody-enhancing factor, interleukin 2, and immune interferon. J Natl Cancer Inst. 1984 Feb;72(2):311–320. [PubMed] [Google Scholar]
- Pullen A. M., Choi Y., Kushnir E., Kappler J., Marrack P. The open reading frames in the 3' long terminal repeats of several mouse mammary tumor virus integrants encode V beta 3-specific superantigens. J Exp Med. 1992 Jan 1;175(1):41–47. doi: 10.1084/jem.175.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler R., Rich M. A. Pathogenesis of reticulum cell sarcoma in mice. J Natl Cancer Inst. 1968 Jul;41(1):125–143. [PubMed] [Google Scholar]
- Sopchak L., King S. R., Miller D. A., Gabra N., Thrush G. R., Lerman S. P. Progression of transplanted SJL/J lymphomas attributed to a single aggressive H-2Ds-negative lymphoma. Cancer Res. 1989 Feb 1;49(3):665–671. [PubMed] [Google Scholar]
- Stavnezer J., Lasky J. L., Ponzio N. M., Scheid M. P., Thorbecke G. J. Reticulum cell sarcomas of SJL mice have rearranged immunoglobulin heavy and light chain genes. Eur J Immunol. 1989 Jun;19(6):1063–1069. doi: 10.1002/eji.1830190616. [DOI] [PubMed] [Google Scholar]
- Tax A., Ewert D., Manson L. A. An antigen cross-reactive with gp52 of mammary tumor virus is expressed on a B cell subpopulation of mice. J Immunol. 1983 May;130(5):2368–2371. [PubMed] [Google Scholar]
- Theunissen H. J., Paardekooper M., Maduro L. J., Michalides R. J., Nusse R. Phorbol ester-inducible T-cell-specific expression of variant mouse mammary tumor virus long terminal repeats. J Virol. 1989 Aug;63(8):3466–3471. doi: 10.1128/jvi.63.8.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiagbe V. K., Asakawa J., Ponzio N. M., Thorbecke G. J. Paraproteins and primary lymphoma in SJL mice. II. Primary lymphomas do not produce paraprotein. Cell Immunol. 1990 Sep;129(2):503–512. doi: 10.1016/0008-8749(90)90224-f. [DOI] [PubMed] [Google Scholar]
- Tsiagbe V. K., Nicknam M. H., Fattah D., Thorbecke G. J. IL-5 responsive subsets among normal and lymphomatous murine B cells. Ann N Y Acad Sci. 1992 May 4;651:270–273. doi: 10.1111/j.1749-6632.1992.tb24624.x. [DOI] [PubMed] [Google Scholar]
- Tsiagbe V. K., Rabinowitz J. L., Thorbecke G. J. I-E expression does not by itself influence growth of or T cell unresponsiveness to SJL lymphomas. Cell Immunol. 1991 Sep;136(2):329–339. doi: 10.1016/0008-8749(91)90356-g. [DOI] [PubMed] [Google Scholar]
- Tsiagbe V. K., Thorbecke G. J. Paraproteins and primary lymphoma in SJL mice. I. Individuality of idiotypes on paraproteins. Cell Immunol. 1990 Sep;129(2):494–502. doi: 10.1016/0008-8749(90)90223-e. [DOI] [PubMed] [Google Scholar]
- Vacchio M. S., Hodes R. J. Selective decreases in T cell receptor V beta expression. Decreased expression of specific V beta families is associated with expression of multiple MHC and non-MHC gene products. J Exp Med. 1989 Oct 1;170(4):1335–1346. doi: 10.1084/jem.170.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- White J., Blackman M., Bill J., Kappler J., Marrack P., Gold D. P., Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989 Sep 15;143(6):1822–1825. [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]