Abstract
Background:
The efficacy and safety of using combination chemotherapy with cetuximab as first-line treatment in patients with K-ras wild-type colorectal cancers has been well established. In general, weekly cetuximab was given with biweekly chemotherapy FOLFOX-4 or FOLFIRI, synchronizing them would be appealed to both patients and health care professionals.
Materials and Methods:
This Phase II, prospective study investigated the efficacy and safety of using biweekly cetuximab 500 mg/m2 with chemotherapy FOLFOX-4 or FOLFIRI as first-line treatment for Chinese patients with K-ras wild-type metastatic colorectal cancer. The study endpoints included overall objective response (OR), progression-free survival (PFS), overall survival (OS) and safety.
Results:
Total 15 Chinese patients (male: 10 [67%]; median age: 60 [range 41-80]) were enrolled. Patients received median 12 cycles (range 2-12) of chemotherapy + cetuximab (FOLFOX-4 + cetuximab: 9 [60%]; FOLFIRI + cetuximab: 6 [40%]). Six patients (40%) with non-progressive disease after 12 cycles of chemotherapy + cetuximab carried on maintenance cetuximab. Median duration of follow-up (FU) was 23.7 months. The OR was 40% (complete response: 0%; partial response: 40%) for a disease control rate of 87%. Median PFS and OS were 7.8 months and 17.9 months respectively. For maintenance cetuximab phase, median PFS since the start of maintenance cetuximab was 6.8 months and median OS was 17.0 months. The only grade 3-4 toxicities were neutropenia (26.7%) in chemotherapy phase and acneiform rashes (16.7%) in maintenance phase.
Conclusions:
Biweekly cetuximab with combination chemotherapy was effective and safe as weekly dose. Further studies are warranted for the role of maintenance cetuximab.
Keywords: Cetuximab, chemotherapy, colorectal cancer, maintenance, metastasis
Introduction
Colorectal cancer is the third commonest cancer world-wide. Nearly 40% of patients had metastatic disease at presentation.[1] K-ras oncogene mutational status has been found to be a predictor of response to cetuximab therapy in metastatic colorectal cancer (mCRC) and reported to be present in 35-40% of colorectal cancers.[2,3] The activity of epidermal growth factor receptor (EGFR) targeting monoclonal antibody cetuximab is limited to patients whose tumors do not harbor mutation at codons 12 and 13 of the K-ras gene.[4,5,6,7] This observation was confirmed in two large clinical studies: Phase III CRYSTAL study (Cetuximab combined with irinotecan in first-line therapy for mCRC)[8] and large-scale Phase II OPUS study (Oxaliplatin and Cetuximab in First-Line treatment of mCRC).[9]
The recent CRYSTAL study updates revealed that the addition of cetuximab to FOLFIRI in patients with K-ras wild-type disease resulted in significant improvement in progression-free survival (PFS), overall survival (OS) and objective response (OR) compared with FOLFIRI alone.[10] The large Phase II OPUS study also demonstrated that the addition of cetuximab to FOLFOX-4 significantly improved PFS and OR in patients with K-ras wild-type tumors.[9]
More recently, the pooled analysis of CRYSTAL and OPUS study published in 2012 consolidated the previous finding that the addition of cetuximab to first-line chemotherapy in patients with K-ras wild-type tumors led to a significant increase in OR, reduction in risk of disease progression and risk of death compared with chemotherapy alone.[11] However, conflicting results was revealed in the Phase III Medical Research Council (MRC) COIN study which confirmed that adding cetuximab to FOLFOX-4 improved response rate but not PFS or even OS in patients with K-ras wild-type tumors.[12]
Nevertheless, cetuximab has been approved in a number of countries for clinical use in patients with K-ras wild-type mCRC. The typical dose is a weekly schedule while most chemotherapy regimens for mCRC are usually given biweekly or every 3 weeks.[13,14] The notion of synchronizing the administration of cetuximab and concomitant chemotherapy would definitely simplify treatment administration and reduce its impact on patients’ convenience. Pharmacokinetics and pharmacodynamic studies have provided evidence that biweekly administration of cetuximab is not different from weekly schedule.[15,16,17]
The aim of our study is to investigate the feasibility, efficacy and safety of using biweekly cetuximab with oxaliplatin-based chemotherapy or irinotecan-based chemotherapy as first-line treatment for Chinese patients with K-ras wild-type mCRC.
Materials and Methods
This study was a prospective, single-arm, open-label Phase II trial conducted in a single institution to investigate the efficacy and safety of biweekly cetuximab and chemotherapy as first-line treatment in patients with K-ras wild-type mCRC.
Eligible patients must have metastatic disease with K-ras wild-type status of tumour tissue and confirmed histologically adenocarcinoma of the colon or rectum.
Patients’ inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteria
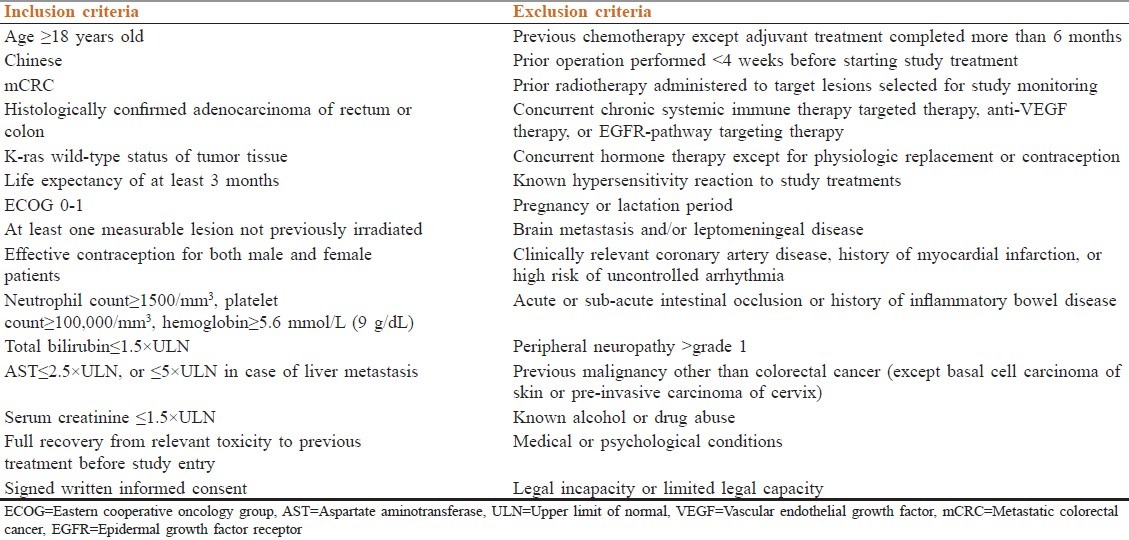
The study protocol was approved by the institutional review board of the treating institution and conducted in accordance with the Declaration of Helsinki 2000 as well as the Declaration of Istanbul 2009. All patients provided written informed consent before enrollment into the study.
Procedures
Baseline assessments include medical history, physical examination, vital signs, blood taking for hematology with complete blood counts, including differential leucocyte count, clinical biochemistry with liver and renal function tests, magnesium level, tumor marker carcinoembryonic antigen and computed tomography (CT) scan of the abdomen and pelvis.
If confirmed eligible after baseline assessment, patients then received chemotherapy either FOLFOX-4[13] or FOLFIRI[14] at preference of patients and physicians combined with biweekly cetuximab. Cetuximab 500 mg/m2 was given intravenously over 120 min at the first dose, followed by 60-min infusion in subsequent treatments every 2 weeks. Pre-medication diphenhydramide 50 mg was given 30 min before infusion of cetuximab. 1 h after completion of cetuximab, chemotherapy using FOLFOX-4 (oxaliplatin 85 mg/m2 on day 1, folinic acid 200 mg/m2 on day 1-2 and 5-fluorouracil 400 mg/m2 bolus followed by 22-h continuous infusion of 600 mg/m2 on day 1-2) or FOLFIRI (irinotecan 180 mg/m2 on day 1, folinic acid 200 mg/m2 on day 1-2 and 5-fluorouracil 400 mg/m2 bolus followed by 22-h continuous infusion of 600 mg/m2 day 1-2) was given. Patients who had no radiological evidence of progression or unacceptable toxicity were treated for a maximum of 12 cycles of chemotherapy. Then they had an option to continue maintenance cetuximab with the same dose and schedule until further progressive disease (PD) or unacceptable toxicity developed.
Physical examinations, vital signs, hematology and biochemistry were performed and monitored at baseline and before each chemotherapy or treatment cycle.
Tumor response was evaluated by CT every four cycles (i.e., 8 weeks) until PD. Tumor assessment was classified as complete response (CR), partial response (PR), stable disease or PD according to Response Evaluation Criteria in Solid Tumors version 1.0.[18] Primary study endpoint was PFS. Secondary endpoints were overall OR, disease control rate (DCR) and OS. Toxicity was evaluated according to National Cancer Institute Common Terminology Criteria version 3.0.
Statistical analysis
All data were analyzed with Statistical Package for Social Sciences version 19 by IBM. PFS and OS were analyzed by the Kaplan-Meier method and stratified log-rank test.
Results
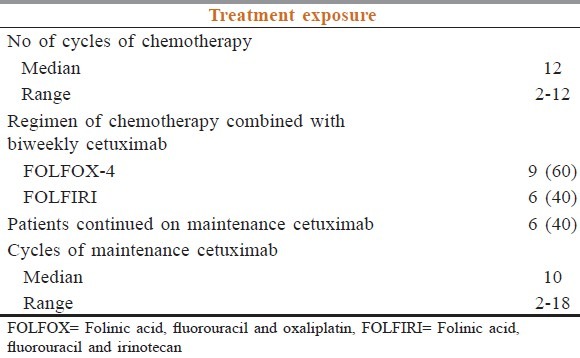
Between December 2009 and August 2010, a total of 15 Chinese patients were recruited into the study. 10 were male (67%) and the median age was 60 years old (range 41-80) [Table 2]. The distribution of metastatic sites was: Liver (12; 80%), distant lymph nodes (7; 47%), peritoneum (6; 40%), lung (5; 33%), spleen (1; 7%) and bone (1; 7%). Patients received a median number of 12 cycles (range 2-12) of chemotherapy with cetuximab. Nine patients (60%) received FOLFOX-4 with cetuximab and six patients (40%) received FOLFIRI with cetuximab regimen. Six patients (40%) continued maintenance cetuximab after completion of 12 cycles of chemotherapy with cetuximab. The median number of cycles of maintenance cetuximab was 10 (range from 2 to 18) [Table 3].
Table 2.
Patient's demographics
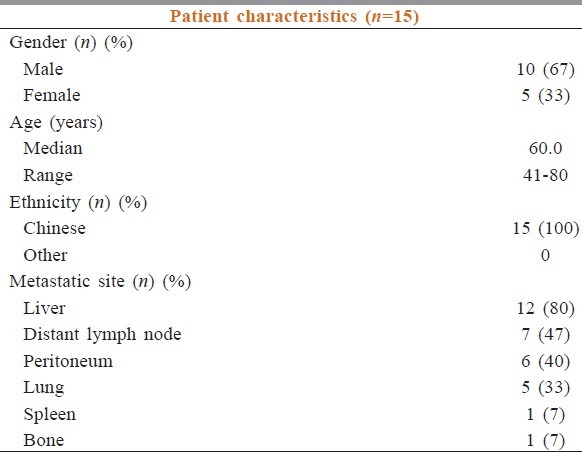
Table 3.
Summary of the number of cycles of chemotherapy and cetuximab
Duration of median follow-up was 23.7 months. OR was 40%. No patient had CR and six had PR. DCR was 87%. Median PFS counting from starting date of chemotherapy was 7.8 months with 1-year PFS rate 23.3% [Figure 1]. Median OS was 17.9 months and 1-year OS was 60% [Figure 2].
Figure 1.
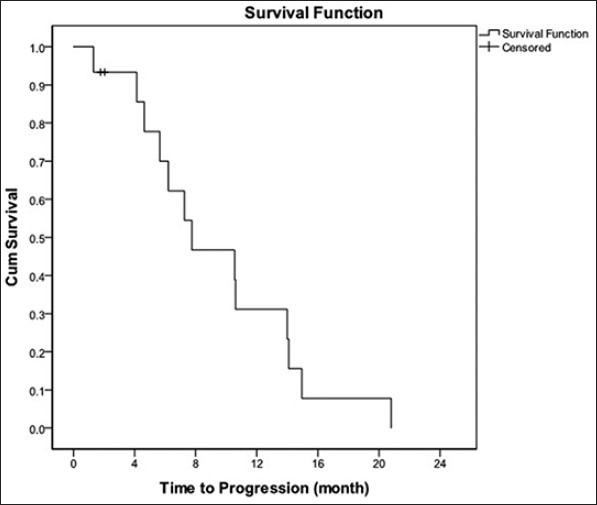
Progression - free survival curve, median PFS: 7.8 months; 1 - year PFS rate: 23.3%
Figure 2.
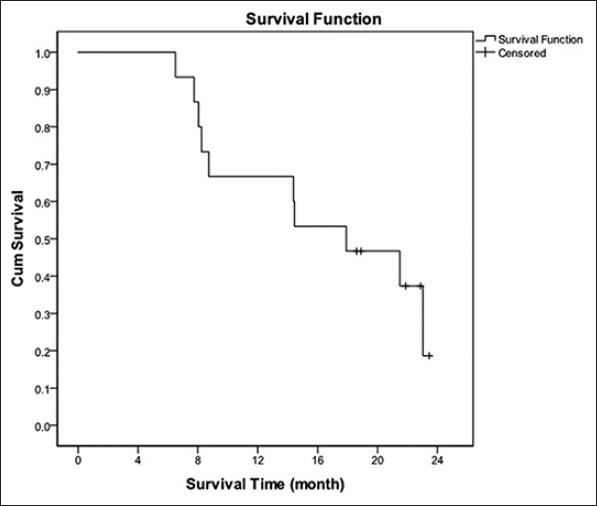
Overall survival curve, median survival: 17.9 months, 1 - year OS: 60%
The only grade 3-4 toxicity in these 15 patients during the chemotherapy phase was neutropenia (26.7%). Other grade 1-2 toxicities included acneiform rash (93.3%), hand-foot syndrome (73.3%), nausea and vomiting (66.7%), diarrhea (40.0%), peripheral neuropathy (40.0%), liver toxicity (20.0%), thrombocytopenia (13.3%) and infusion reaction (6.7%). Nine patients had their chemotherapy delayed due to neutropenia or thrombocytopenia. In a total of 148 cycles of chemotherapy with cetuximab, 16 cycles were delayed (10.8%) with an average of 7.4 days of delay in the total treatment period.
Six patients had stable disease after 12 cycles of chemotherapy and carried on the maintenance cetuximab. The median PFS (calculated from the start of maintenance therapy until progression or death from any cause) was 6.8 months and the median OS was 17.0 months. The best treatment response achieved before disease progression was stable disease in these six patients. The only grade 3-4 toxicities detected during maintenance treatment was acneiform rash (one patient, 16.7%). There was no infusion reaction or hypomagnesaemia.
There were two serious adverse events (SAE). One patient had perforation of the tumor causing intra-abdominal abscess after two cycles of FOLFOX-4 with cetuximab. He had exploratory laparotomy and it was subsided after intensive intravenous antibiotics. Chemotherapy was stopped and permanently discontinued. Another patient, who had history of hypertension and diabetes mellitus, suffered from thrombosis of the left femoral artery after three cycles of FOLFIRI with cetuximab. Chemotherapy was stopped. He subsequently had femoral artery embolectomy and above-knee amputation. Both SAE were not related to the study medications.
Discussion
Monoclonal antibody against EGFR was first tested in animal studies in 1980s. After decades of numerous clinical trials, the first monoclonal antibody was approved in the treatment of mCRC. Examples of anti-EGFR monoclonal antibody include cetuximab and panitumumab, binding to the extra-cellular domain of EGFR, blocking the ligand binding-induced receptor dimerization and subsequent tyrosine kinase activation.[19] Cetuximab, in particular, is also able to elicit antibody-dependent cellular cytotoxicity against tumor cells.[20,21] K-ras is a guanosine 5’- triphosphate-binding protein which plays crucial in the downstream signaling cascade of various receptor tyrosine kinase including EGFR. K-ras activating mutation is found in about 40% of colorectal cancer, which leads to the constitutive activation of K-ras protein independent of the upstream regulation, thus conferring a poor response to anti-EGFR monoclonal antibody. Subsequent Phase II and Phase III clinical trials have confirmed the role and efficacy of anti-EGFR monoclonal antibody in K-ras wild-type mCRC.
Cetuximab combined with oxaliplatin or irinotecan-based chemotherapy as first-line treatment for K-ras wild-type mCRC has been reported to improve OR, PFS and even OS in different studies,[8,9,10,11] although the recent MRC COIN study did not reproduce such benefits.[12] Cetuximab was generally given in weekly dose (400 mg/m2 as induction in the first dose followed by weekly dose with 250 mg/m2 ) while most chemotherapy regimens for colorectal cancers are given every 2 weeks. It would be reasonable and desirable to synchronize the administration of cetuximab and chemotherapy to improve treatment complexity. Pharmacokinetics and pharmacodynamic studies has proven that biweekly administration of cetuximab has no observable difference when given weekly.[15,16,17]
Our study demonstrated the feasibility, efficacy and safety of combination of biweekly cetuximab with chemotherapy was comparable with weekly cetuximab as first-line treatment for patients with K-ras wild type mCRC.
In our study, OR was 40% and DCR was 84%, which was similar to the histological data (around 81% as in CRYSTAL study). The median PFS and OS were also similar to those reported in CRYSTAL (median PFS: 9.9 months; median OS: 23.5 months)[10] and OPUS study (median PFS: 8.3 months; median OS: 22.8 months).[9]
Biweekly cetuximab with combination of oxaliplatin or irinotecan-based chemotherapy was well tolerated with just 13.3% patients suffering from grade ≥3 neutropenia. Other side effects included mild grade 1-2 gastrointestinal toxicities, hand-foot syndrome, acneiform rash. There were no severe adverse events related to the study medications occurred in our patients. The toxicity profile was in line with the reported incidence in those large scale studies: CRYSTAL,[10] OPUS[9] and COIN[12] study, reflecting that this biweekly regimen is safe when used in combination with chemotherapy.
In our trial, there was a subgroup of six patients having maintenance cetuximab after completion of 12 cycles of chemotherapy. The median number of cycles of maintenance cetuximab was 10 (range from 2 to 18). The median PFS was 6.8 months after the commencement of maintenance treatment and the median OS was 17.0 months. It was well tolerated with only one patient having acneiform rash. Despite the small sample size without any meaningful conclusion, use of maintenance cetuximab in patients with stable disease after first-line chemotherapy was demonstrated to be safe and it is worthwhile to be further elucidated in future studies.
There were several limitations in this study. First, patients enrolled in this study could choose either oxaliplatin or irinotecan-based chemotherapy. This made the efficacy and toxicity profile difficult for comparison. Second, the sample size was too small with a total of only 15 patients and just six patients carried on the maintenance cetuximab. A prospective randomized-controlled trial is definitely warranted to investigate if maintenance therapy with cetuximab offers longer PFS or not.
Conclusion
Biweekly cetuximab with oxaliplatin or irinotecan-based chemotherapy in Chinese patients with K-ras wild-type mCRC was safe and feasible and has similar efficacy to the weekly regimen.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lea IA, Jackson MA, Li X, Bailey S, Peddada SD, Dunnick JK. Genetic pathways and mutation profiles of human cancers: Site- and exposure-specific patterns. Carcinogenesis. 2007;28:1851–8. doi: 10.1093/carcin/bgm176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–27. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 4.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 5.Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 7.Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 9.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–46. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–9. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 11.Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–75. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 12.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 14.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 15.Tabernero J, Pfeiffer P, Cervantes A. Administration of cetuximab every 2 weeks in the treatment of metastatic colorectal cancer: An effective, more convenient alternative to weekly administration? Oncologist. 2008;13:113–9. doi: 10.1634/theoncologist.2007-0201. [DOI] [PubMed] [Google Scholar]
- 16.Delbaldo C, Pierga JY, Dieras V, Faivre S, Laurence V, Vedovato JC, et al. Pharmacokinetic profile of cetuximab (Erbitux) alone and in combination with irinotecan in patients with advanced EGFR-positive adenocarcinoma. Eur J Cancer. 2005;41:1739–45. doi: 10.1016/j.ejca.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Tabernero J, Ciardiello F, Rivera F, Rodriguez-Braun E, Ramos FJ, Martinelli E, et al. Cetuximab administered once every second week to patients with metastatic colorectal cancer: A two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol. 2010;21:1537–45. doi: 10.1093/annonc/mdp549. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98:1275–80. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781–7. doi: 10.1002/ijc.22370. [DOI] [PubMed] [Google Scholar]


