Abstract
Background and Aims:
Scrub typhus, a zoonotic rickettsial infection, is an important reason for intensive care unit (ICU) admission in the Indian subcontinent. We describe the clinical profile, organ dysfunction, and predictors of mortality of severe scrub typhus infection.
Materials and Methods:
Retrospective study of patients admitted with scrub typhus infection to a tertiary care university affiliated teaching hospital in India during a 21-month period.
Results:
The cohort (n = 116) aged 40.0 ± 15.2 years (mean ± SD), presented 8.5 ± 4.4 days after symptom onset. Common symptoms included fever (100%), breathlessness (68.5%), and altered mental status (25.5%). Forty-seven (41.6%) patients had an eschar. Admission APACHE-II score was 19.6 ± 8.2. Ninety-one (85.2%) patients had dysfunction of 3 or more organ systems. Respiratory (96.6%) and hematological (86.2%) dysfunction were frequent. Mechanical ventilation was required in 102 (87.9%) patients, of whom 14 (12.1%) were solely managed with non-invasive ventilation. Thirteen patients (11.2%) required dialysis. Duration of hospital stay was 10.7 ± 9.7 days. Actual hospital mortality (24.1%) was less than predicted APACHE-II mortality (36%; 95% Confidence interval 32-41). APACHE-II score and duration of fever were independently associated with mortality on logistic regression analysis.
Conclusions:
In this cohort of severe scrub typhus infection with multi-organ dysfunction, survival was good despite high severity of illness scores. APACHE-II score and duration of fever independently predicted mortality.
Keywords: Intensive care, organ dysfunction, outcome, rickettsia, ventilation
Introduction
Scrub typhus, an acute febrile illness (AFI) caused by the rickettsia Orientia tsutsugamushi, is a zoonotic disease with protean clinical manifestations.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] Once a dreaded disease in the pre-antibiotic era, reports suggest that scrub typhus continues to be a public health problem in South Asian and Western Pacific regions.[14,16,17,18,19,20,21] More recent publications have highlighted that scrub typhus is an important cause of acute undifferentiated febrile illness in India[18] leading to organ dysfunction and death in some patients. However, studies describing its severe manifestations have been restricted to short reports. In a recent, large study of 208 patients with scrub typhus from Korea,[22] the factors associated with severe complications from scrub typhus was evaluated in a case-control study in which the authors compared patients with severe scrub typhus with those with mild disease. They observed that age, absence of eschar, white blood cell (WBC) count, and albumin levels to be associated with severe complications.[22] In this study, although organ involvement was presented in terms of pneumonia, shock, meningoencephalitis, renal failure, and myocarditis, there was no formal assessment of severity of illness or characterization of organ dysfunction using organ dysfunction scores.[23,24] Further, respiratory involvement was presented as pneumonia without information on ventilatory requirements and duration of ventilation. This study was thus undertaken to characterize the nature and extent of organ dysfunction in patients admitted with severe scrub typhus infection to the intensive care unit (ICU) and to evaluate its impact on mortality.
Materials and Methods
All patients admitted to the medical ICU or high dependency unit (HDU) of a tertiary care teaching hospital in South India with an AFI with organ dysfunction over a 21-month period (August 2008-April 2010) were considered. Patients were identified from the ICU database and data was obtained from patient case records and electronic database. The study was approved by the Institutional Review Board (IRB).
All patients with AFI had a full work up for the cause of fever that included three smears for malarial parasites, serology for dengue, leptospirosis, scrub typhus, enteric fever and retroviral infections, blood, urine, and sputum or endotracheal cultures, and a computed tomography of the brain and cerebrospinal fluid analysis (CSF), if clinically indicated. Scrub typhus serology was tested in batches for IgM antibodies to O. tsutsugamushi using commercial enzyme-linked immunosorbent assay (ELISA) kits (Panbio Ltd, Brisbane, Australia). The kit uses a specific 56 kDa recombinant antigen of O. tsutsugamushi and has a sensitivity and specificity of above 90% when compared with the recommended gold standard tests, namely the immunofluorescent antibody (IFA) and indirect immunoperoxidase tests.[25] An individual was considered scrub typhus seropositive if a value of ≥16 units was obtained. After 18th August 2009, we switched over to the Scrub Typhus Detect IgM ELISA (InBios International Inc., Seattle, USA) test, as the former was unavailable where an optical density of ≥ 0.5 was considered as diagnostic of scrub typhus. A diagnosis of scrub typhus was made when a patient with an AFI had an eschar and a positive IgM ELISA for scrub typhus and other causes of fever excluded.[18,26] Patients with AFI with organ-dysfunction were treated with empiric antibiotics (usually pipericillin-tazobactam along with enteral doxycycline and intravenous azithromycin) pending culture and serology reports. In those with the typical eschar and multiorgan dysfunction suggestive of scrub typhus (acute respiratory distress syndrome with or without shock, renal failure, elevated transaminase levels, leukocytosis, and thrombocytopenia), broad-spectrum antibiotics were stopped early and only doxycycline and azithromycin continued if the IgM ELISA was positive and the cultures were negative. Intravenous azithromycin was added to the regimen as intravenous doxycycline is not available in our country and concerns regarding the absorption of enteric administered doxycycline in critically ill patients.
Organ support was provided to patients based on the nature and extent of organ dysfunction. Respiratory support was provided as non-invasive ventilation (NIV) or invasive mechanical ventilation (IMV) based on standard criteria for their use. Cardiovascular support included vasoactive therapy for patients in shock and renal replacement therapy for those with acute kidney injury needing dialysis support.
Severity of illness scores (Acute Physiology and Chronic Health Evaluation II, APACHE-II) and Sequential Organ Failure Assessment (SOFA) scores) were calculated at admission.[23,24] The presence of organ dysfunction during the stay in ICU/HDU was also assessed. Organ dysfunction was assessed using SOFA scores. Organ dysfunction was said to be present if the organ specific SOFA score was ≥1 and organ failure if SOFA score was ≥3.[27] The main outcome of interest was in-hospital mortality. Other outcomes included need and duration of ventilation, type of ventilatory support (invasive or non-invasive), need for dialysis and duration of ICU and hospital stay.
Statistical aspects
Statistical analysis was done using SPSS Version 16. Descriptive statistics were obtained for all study variables. All categorical variables were compared using Chi-square and Fisher's exact test and continuous variables using Student's t-test. All data were expressed as mean (±SD). Bivariate as well as multivariate logistic regression analyses were performed to find the predictors of mortality and expressed as odds ratio (OR) with 95% confidence intervals (CI). For all statistical analysis, P < 0.05 was considered statistically significant.
Results
During the study period, 2263 patients were admitted to the medical critical care unit; 116 (5.1%) were diagnosed as scrub typhus based on presentation as AFI, a positive IgM ELISA to scrub typhus, the exclusion of other diagnoses and a therapeutic response to doxycycline.
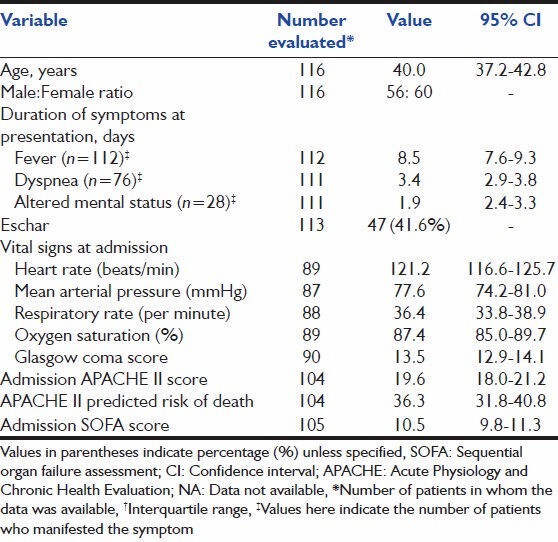
Baseline characteristics are summarized in Table 1. Fever was present in all patients. Dyspnea and altered mental status were present in 68.5% and 25.2% of patients, respectively. Eschar was present in 47 patients (41.6%). The admission APACHE-II and SOFA scores of 19.6 ± 8.2 and 10.5 ± 3.9 suggested a cohort of sick patients.
Table 1.
Baseline characteristics of patients with severe scrub typhus admitted to the ICU and included in the study
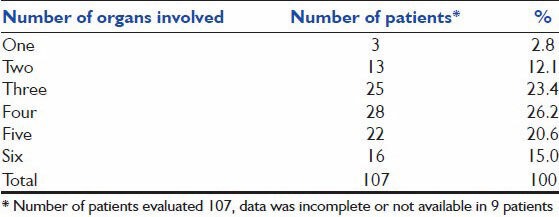
Data on organ dysfunction at hospitalization was available in 107 patients [Table 2]. The median frequency of organ dysfunction was 4. Respiratory organ dysfunction predominated and was present in 112 patients (96.6%). Cardiovascular dysfunction was present in 61.7% and renal and hepatic dysfunction in 63.8% or patients. Sixteen patients (15%) had evidence of dysfunction of all six organs during the course of hospitalization.
Table 2a.
Frequency and distribution of organ system dysfunction during ICU stay
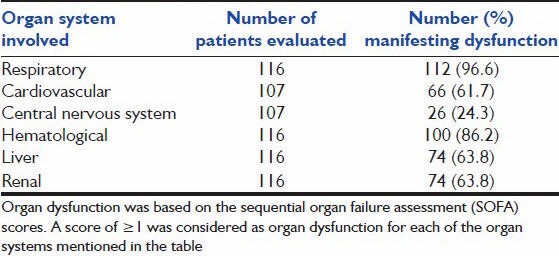
Table 2b.
Categorization based on number of organ systems involved
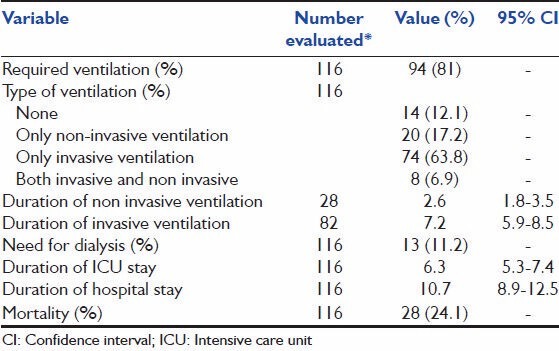
Ventilatory support was required in 87.9% of patients. Twenty patients (17.2%) were managed solely on NIV without the need for IMV; 8 other patients (6.9%) progressed from needing NIV to requiring IMV, and 74 patients (63.8%) were only invasively ventilated [Table 3]. The duration of ventilation was 2.6 days (95% CI, 1.8-3.5 days) for those needing NIV and 7.2 (95% CI, 5.9-8.5 days) in those needing IMV. Dialysis was required in 13 patients (11.2%). The duration of ICU and hospital stay were 6.3 (95% CI, 5.3-7.4) and 10.7 (95% CI, 8.9-12.5) days respectively. Hospital mortality was 24.1%.
Table 3.
Main outcomes
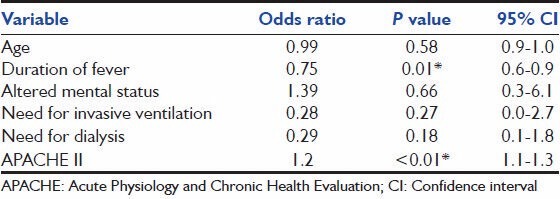
On univariate analysis [Table 4], age, APACHE-II score, duration of fever, cardiovascular, neurologic or renal dysfunction, and need for IMV were associated with mortality. However, on multivariate logistic regression analysis, only APACHE-II score and duration of fever were independently associated with mortality [Table 5].
Table 4.
Bivariate analysis of predictors of survival in severe scrub typhus infection
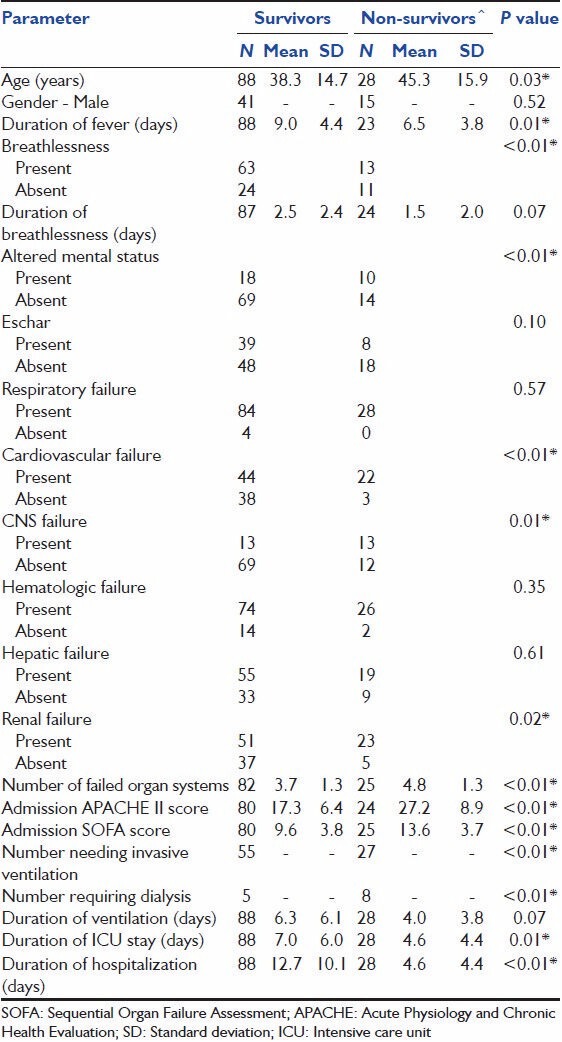
Table 5.
Multivariate analysis of predictors of survival
Discussion
Scrub typhus is a common cause of organ dysfunction and failure treated in the ICUs of the Indian subcontinent. During our study, scrub typhus infection accounted for 5.1% of the total admissions in our unit. Given the relative importance of this disease in this setting, we set out to characterize the type and extent of organ dysfunction in severe scrub typhus infection and assess the course and outcome of such patients admitted to the ICU.
Pulmonary dysfunction and failure were the most common complications of scrub typhus infection observed in our study. Previous studies have reported an incidence of 11-29%.[7,9,12,17] for acute respiratory distress syndrome (ARDS) in patients admitted for scrub typhus. In our study, 85 patients (73.3%) fulfilled criteria for the diagnosis of ARDS, the higher incidence probably a reflection of the inclusion of only the sicker ICU patients in the cohort. Some studies[1,7,11,28] have reported the development of non-cardiac pulmonary edema and interstitial pneumonia in scrub typhus, and postulated that it is primarily driven by a vasculitic process that occurs in some individuals. Patients with respiratory failure, in our study, were managed with either IMV or NIV with 17.2% managed only on NIV. The duration of ventilation was significantly lower (P = 0.001) in those who were managed solely with NIV or those who were initially managed with NIV than in those who were managed on IMV [Table 3]. Although this suggests that NIV is associated with significantly shorter ventilation duration, this might also be a reflection of sicker patients needing IMV right from admission. Indeed the admission APACHE-II scores were significantly higher (P = 0.007) in those who needed IMV (21.32 ± 8.6) when compared with those requiring NIV (16.63 ± 7.24). The mortality of 24% in our cohort of patients is consistent with other published cohorts of scrub typhus patients with ARDS of 22-45%.[7,9] The actual mortality in our study was lower than the predicted APACHE-II mortality.
Shock was also common in our cohort of patients. Cardiovascular dysfunction was evident in 61.7% of patients and was associated with increased risk of death (OR 6.1, 95% CI 1.6-33.6) with 1/3rd of patients with cardiovascular dysfunction succumbing to the illness. In contrast, a study from Korea[22] reported shock in only 7.2% of patients with severe scrub typhus infection. Our mortality in those with cardiovascular dysfunction is similar to the reported mortality of about 50% in patients with other etiologies of septic shock.[29,30]
Thrombocytopenia was frequent with 86% having levels of <150,000/cmm and 47% with levels <50,000/cmm. This is in contrast to an early study[22] that reported platelet counts of <100,000/cmm in only 31.5% of patients with severe scrub typhus infection and in 21% in non-severe scrub typhus infection.
Renal failure was associated with mortality in our study with 13 patients requiring dialysis. However, on multivariate analysis, dialysis did not appear to impact survival in our patients although 61.5% of patients requiring dialysis succumbed to the illness.
Although altered mental status is commonly described in severe scrub typhus infection,[2,3,15] several factors other than aseptic meningitis such as organ dysfunction (renal failure, hepatic dysfunction, hypercarbia, etc.) may contribute to the altered mental status. The assessment of mental status in our cohort of patients was also compounded by the fact that some patients were referred having been intubated in another hospital and been on sedation. Although altered mental status was associated with increased risk of death on univariate analysis, this was not significant on multivariate analysis.
Several factors were associated with mortality on univariate analysis. However, only the duration of fever and severity of illness at presentation (APACHE-II score) were associated with mortality on multivariate analysis. When the APACHE-II score was replaced with the SOFA score, the SOFA score was again significantly associated (P = 0.005) with mortality on multivariate analysis.
The association between severity of illness scores (APACHE-II and SOFA) and outcome in scrub typhus is not surprising, given that these scores have been validated in other ICU settings that include sepsis of varied etiology. The association between duration of fever and mortality merits discussion. The duration of fever prior to presentation to our referral hospital was significantly (P = 0.007) different between survivors (6.5 ± 3.8 days) and non-survivors (9.0 ± 4.4 days). Given that severe scrub typhus presents as an AFI with organ dysfunction, it is important that there is a high degree of suspicion for scrub typhus. Conventionally, fever with multiorgan dysfunction is treated with broad-spectrum antibiotics, whilst the treatment of choice for scrub typhus infection is doxycycline. The delay in recognizing the syndrome and initiating early therapy with doxycycline may affect outcome as suggested in our study. Indeed, given the common occurrence of scrub typhus in our setting, empiric antibiotic therapy for AFI with multiorgan dysfunction in our hospital includes doxycycline. The presence of an eschar supports the clinical diagnosis of scrub typhus and obviates the need for the addition of other broad-spectrum antibiotics unless bacterial super-infection is suspected.
Conclusion
Scrub typhus is a common cause of ICU admission in South India. Patients typically present with significant physiological derangement of multiple organ systems. Despite the high prevalence of multiorgan dysfunction and the severity of illness as evidenced by high APACHE-II scores on admission, scrub typhus patients had fewer than predicted mortality. This should encourage physicians and families to treat these patients aggressively, while reserving a great deal of optimism for improvement.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. Scrub typhus: Clinical, pathologic, and imaging findings. Radiographics. 2007;27:161–72. doi: 10.1148/rg.271065074. [DOI] [PubMed] [Google Scholar]
- 2.Chen PH, Hung KH, Cheng SJ, Hsu KN. Scrub typhus-associated acute disseminated encephalomyelitis. Acta Neurol Taiwan. 2006;15:251–4. [PubMed] [Google Scholar]
- 3.Kim DE, Lee SH, Park KI, Chang KH, Roh JK. Scrub typhus encephalomyelitis with prominent focal neurologic signs. Arch Neurol. 2000;57:1770–2. doi: 10.1001/archneur.57.12.1770. [DOI] [PubMed] [Google Scholar]
- 4.Premaratna R, Chandrasena TG, Dassayake AS, Loftis AD, Dasch GA, de Silva HJ. Acute hearing loss due to scrub typhus: A forgotten complication of a reemerging disease. Clin Infect Dis. 2006;42:e6–8. doi: 10.1086/498747. [DOI] [PubMed] [Google Scholar]
- 5.Aronoff DM, Watt G. Prevalence of relative bradycardia in Orientia tsutsugamushi infection. Am J Trop Med Hyg. 2003;68:477–9. [PubMed] [Google Scholar]
- 6.Kim DM, Kang DW, Kim JO, Chung JH, Kim HL, Park CY, et al. Acute renal failure due to acute tubular necrosis caused by direct invasion of Orientia tsutsugamushi. J Clin Microbiol. 2008;46:1548–50. doi: 10.1128/JCM.01040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CC, Liu SF, Liu JW, Chung YH, Su MC, Lin MC. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76:1148–52. [PubMed] [Google Scholar]
- 8.Young PC, Hae CC, Lee KH, Hoon CJ. Tsutsugamushi infection-associated acute rhabdomyolysis and acute renal failure. Korean J Intern Med. 2003;18:248–50. doi: 10.3904/kjim.2003.18.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsay RW, Chang FY. Acute respiratory distress syndrome in scrub typhus. QJM. 2002;95:126–8. doi: 10.1093/qjmed/95.2.126. [DOI] [PubMed] [Google Scholar]
- 10.Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31:240–4. [PubMed] [Google Scholar]
- 11.Song SW, Kim KT, Ku YM, Park SH, Kim YS, Lee DG, et al. Clinical role of interstitial pneumonia in patients with scrub typhus: A possible marker of disease severity. J Korean Med Sci. 2004;19:668–73. doi: 10.3346/jkms.2004.19.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichimura K, Uchida Y, Arai K, Nakazawa K, Sasaki J, Kobayashi K, et al. Afebrile scrub typhus (Tsutsugamushi disease) with acute respiratory distress syndrome. Intern Med. 2002;41:667–70. doi: 10.2169/internalmedicine.41.667. [DOI] [PubMed] [Google Scholar]
- 13.Hu ML, Liu JW, Wu KL, Lu SN, Chiou SS, Kuo CH, et al. Short report: Abnormal liver function in scrub typhus. Am J Trop Med Hyg. 2005;73:667–8. [PubMed] [Google Scholar]
- 14.Mahajan SK, Rolain JM, Kanga A, Raoult D. Scrub typhus involving central nervous system, India, 2004-2006. Emerg Infect Dis. 2010;16:1641–3. doi: 10.3201/eid1610.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayen JJ, Pond HS, et al. Scrub typhus in Assam and Burma: A clinical study of 616 cases. Medicine (Baltimore) 1946;25:155–214. doi: 10.1097/00005792-194605000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, Ryu SY, et al. Rapid increase of scrub typhus, South Korea, 2001-2006. Emerg Infect Dis. 2009;15:1127–9. doi: 10.3201/eid1507.080399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, Abraham OC, et al. Scrub typhus: An unrecognized threat in South India-clinical profile and predictors of mortality. Trop Doct. 2010;40:129–33. doi: 10.1258/td.2010.090452. [DOI] [PubMed] [Google Scholar]
- 18.Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, et al. Acute undifferentiated febrile illness in adult hospitalized patients: The disease spectrum and diagnostic predictors-an experience from a tertiary care hospital in South India. Trop Doct. 2010;40:230–4. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 19.Kuo CC, Huang JL, Ko CY, Lee PF, Wang HC. Spatial analysis of scrub typhus infection and its association with environmental and socioeconomic factors in Taiwan. Acta Trop. 2011;120:52–8. doi: 10.1016/j.actatropica.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Lee E, Rhee HC. Cost-benefit analysis of the tsutsugamushi disease prevention program in South Korea. Jpn J Infect Dis. 2012;65:222–7. doi: 10.7883/yoken.65.222. [DOI] [PubMed] [Google Scholar]
- 21.Faa AG, McBride WJ, Garstone G, Thompson RE, Holt P. Scrub typhus in the Torres Strait islands of north Queensland, Australia. Emerg Infect Dis. 2003;9:480–2. doi: 10.3201/eid0904.020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10:108. doi: 10.1186/1471-2334-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 24.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 25.Coleman RE, Sangkasuwan V, Suwanabun N, Eamsila C, Mungviriya S, Devine P, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. 2002;67:497–503. doi: 10.4269/ajtmh.2002.67.497. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, He S, Wang S, Yu H, Li X, Zhang D, et al. Comparison of a rapid diagnostic test and microimmunofluorescence assay for detecting antibody to Orientia tsutsugamushi in scrub typhus patients in China. Asian Pac J Trop Med. 2011;4:666–8. doi: 10.1016/S1995-7645(11)60169-7. [DOI] [PubMed] [Google Scholar]
- 27.Moreno R, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study Working Group on Sepsis related problems of the ESICM. Care Med. 1999;22:686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 28.Choi YH, Kim SJ, Lee JY, Pai HJ, Lee KY, Lee YS. Scrub typhus: Radiological and clinical findings. Clin Radiol. 2000;55:140–4. doi: 10.1053/crad.1999.0336. [DOI] [PubMed] [Google Scholar]
- 29.Marchick MR, Kline JA, Jones AE. The significance of non-sustained hypotension in emergency department patients with sepsis. Intensive Care Med. 2009;35:1261–4. doi: 10.1007/s00134-009-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettila V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31:1066–71. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]


