Abstract
Background:
Use of noninvasive ventilation (NIV) outside guideline recommendations is common. We audited use of NIV in our tertiary care critical care unit (CCU) to evaluate appropriateness of use and patient outcomes when used outside level I recommendations.
Materials and Methods:
Prospective observational study of all patients requiring NIV. Clinical parameters and arterial blood gases were recorded at initiation of NIV and 2 h later (or earlier if clinically warranted). NIV titration and decision to intubate were left to the discretion of treating intensivist. Patients were categorized into two groups: Group 1: Those with level I indications for use of NIV and group 2: All other levels of indications. Patients were followed until hospital discharge.
Results:
From January 2010 to June 2010, 1120 patients were admitted to the CCU. Of these 106 patients required NIV support with 40.6% (n = 43/106) being in group 1 and 59.4% (n = 63/106) in group 2. Of these 35.8% patients (38/106) failed NIV and required endotracheal intubation. NIV failure rates (41.27% vs. 27.91%; P = 0.02) and mortality (30.6% vs. 18.6%; P = 0.03) were significantly higher in group 2 patients. In a logistic regression analysis Acute Physiology and Chronic Health Evaluation (APACHE) II score (P = 0.02), time on NIV before intubation (P = 0.001) and baseline PaCO2 levels (P = 0.01) were strongly associated with mortality.
Conclusion:
Noninvasive ventilation failure and mortality rates were significantly higher when used outside level I recommendations. APACHE II score, baseline PaCO2 and duration on NIV prior to intubation were predictors of increased mortality.
Keywords: Acute respiratory failure, invasive ventilation, Indian Society of Critical Care Medicine recommendations, noninvasive ventilation, NIV failure
Introduction
Significant variability exists in the practice patterns of use of noninvasive ventilation (NIV) across the world. The Indian Society of Critical Care Medicine (ISCCM) has formulated evidence-based guidelines for the appropriate use of NIV in acute respiratory failure (ARF).[1] As per this guideline the level I indications for NIV use are acute exacerbation of chronic obstructive pulmonary disease (COPD), cardiogenic pulmonary edema and acute hypoxic respiratory failure in transplant and immunocompromized patients. Despite this, NIV is generally used for indications beyond level I, in an attempt to minimize cost, complications of endotracheal intubation (ETI) and mechanical ventilation. In this study, we audited the use of NIV in our tertiary care multidisciplinary critical care unit (CCU) and evaluated the appropriateness of its use and impact on patient outcomes.
Materials and Methods
Study center
Single center, 70-bed CCU of a tertiary care Indian hospital. This CCU is managed by intensivists and has 24-h in-house physician cover under the supervision of intensivists.
Study type
Prospective and observational study.
Study period
From January 2010 to June 2010.
Study design
All patients admitted to the CCU were screened for ARF defined as moderate to severe dyspnea with use of accessory muscles, moderate to severe acidosis and/or hypercapnia and respiratory rate >25 breaths/min. The decision to initiate NIV was left to the discretion of the treating intensivist. Those diagnosed to have ARF had their clinical parameters and arterial blood gases (ABG) results recorded at baseline and after 2 h of NIV initiation or earlier if clinically warranted. The clinical decision to continue with NIV or to perform ETI was also left to the discretion of the treating intensivist. Patients who were intubated emergently in a periarrest situation were classified as crash intubations, while those who were stable and intubated in a planned manner were classified as elective intubation. Mode of NIV used was standardized to bi-level pressure support in all patients. Exclusion criteria were inability to protect the airways (Glasgow coma scale ≤8 with impaired cough or swallowing), hemodynamic instability (uncontrolled arrhythmia, need for very high doses of inotropes/vasopressors or recent myocardial infarction), inability to use the interface (facial abnormalities, facial burns, facial trauma, facial anomaly), severe gastrointestinal (GI) symptoms (vomiting, obstructed bowel, recent GI surgery), life-threatening hypoxemia, copious secretions and those who were extubated from invasive ventilation during this hospitalization.
Machines used for NIV were either portable noninvasive ventilator (BiPAP Synchrony™, Respironics®, USA and BiPAP VISION®, Respironics Inc®, USA) or conventional ventilators (ESPRIT, Respironics®, USA and Puritan Bennett 840™, USA). Oronasal mask (Inter System Services®, India) was used as an interface in all patients.
Institutional Ethics Committee approval was obtained for conducting this study.
Data collection
In addition to standard demographic data, data pertaining to reason for CCU admission, reason for NIV use, time spent on NIV before intubation, length of CCU and hospital stay, and mortality were collected. ABG values prior to the initiation of NIV, within 2 h of NIV initiation or earlier (if clinically warranted) were recorded. Data on whether the decision to intubate the patient electively or whether patients had crash intubation was also recorded. The disease severity was calculated using only Acute Physiology and Chronic Health Evaluation (APACHE) II scores. Patients were then grouped into two groups. Group 1 consisted of those who had a level I indications for use of NIV as per the NIV guidelines laid down by ISCCM[1] and group 2 consisted of all other patients with level II, III indications for NIV use and patients in whom NIV was used for indications outside the guidelines. In those patients who required ETI the time to intubation was also noted and categorized as appropriate if done within 2 h of initiating NIV or delayed if done after 2 h of initialization. All patients were followed until discharge from hospital. Primary outcome evaluated was NIV failure leading to ETI and secondary outcome was all-cause mortality.
Statistical analysis
Descriptive statistics was used to describe age, gender, APACHE II score, group 1 versus group 2, intubation and mortality. Continuous variables were expressed as mean ± standard deviation (SD) and compared between groups using independent sample t-test. Qualitative and categorical variables expressed as number and percentages were compared with Chi-square test or Kruskal-Wallis test. P <0.05 was considered as statistically significant. Statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 17.0 (Chicago, IL, USA).
Results
Noninvasive ventilation practices
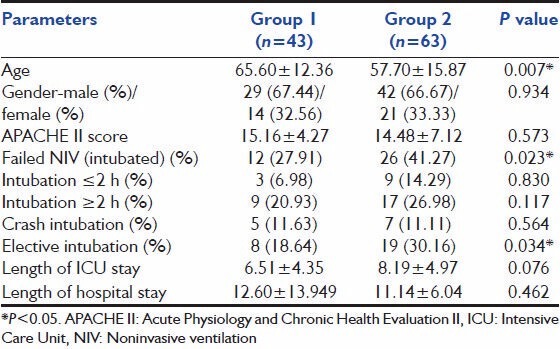
A total of 1120 patients was admitted to the CCU during the study period. Of this 106 patients were diagnosed to have ARF and received NIV therapy. There were 71 (67%) men and 35 (33%) women with a mean age of 60.91 years (SD ± 15.00) and a mean APACHE II score of 14.75 (SD ± 6.11). Of these 40.6% (n = 43) were group 1 patients, with level I indication for NIV use, while 59.4% (n = 63) were group 2 patients (nonlevel I indications). Both groups were matched in their demographics, in all parameters other than age, where group 2 had younger patients (65.60 ± 12.36 vs. 57.70 ± 15.87, P = 0.007) [Table 1].
Table 1.
Demographics of patients
Noninvasive ventilation failure
Failure of NIV was defined as the need for ETI and was seen in 35.8% patients (n = 38). Significantly more patients in group 2 failed NIV compared to group 1 (41.3% vs. 27.9%; P = 0.02) [Figure 1]. Of all the intubations only 31.6% (n = 12) were done within 2 h (appropriate) while 68.4% (n = 26) were done after 2 h (delayed). Emergent intubation was done in 31.6% (12 patients), while 68.4% (26 patients) were intubated electively. There was no difference between group 1 and group 2 in the number of emergent intubations [Table 1].
Figure 1.
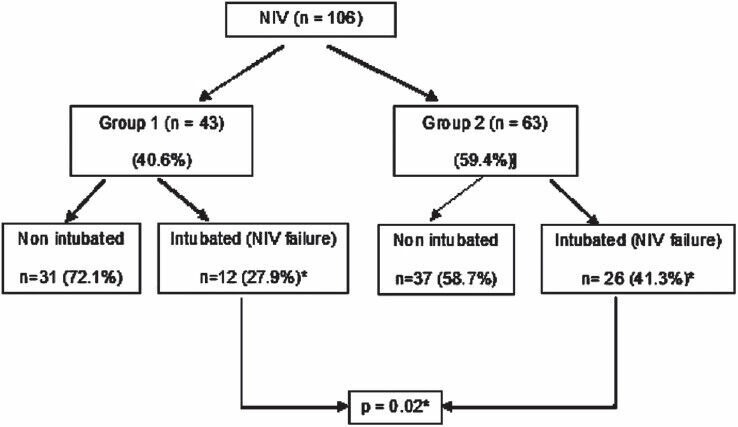
Primary outcome – noninvasive ventilation failure
Mortality
The overall all mortality in our study population was 25.5% (n = 27). Mortality was 18.6% (n = 8) in group 1 patients, while group 2 had a mortality of 30.2% (n = 19) (P = 0.034) [Figure 2].
Figure 2.
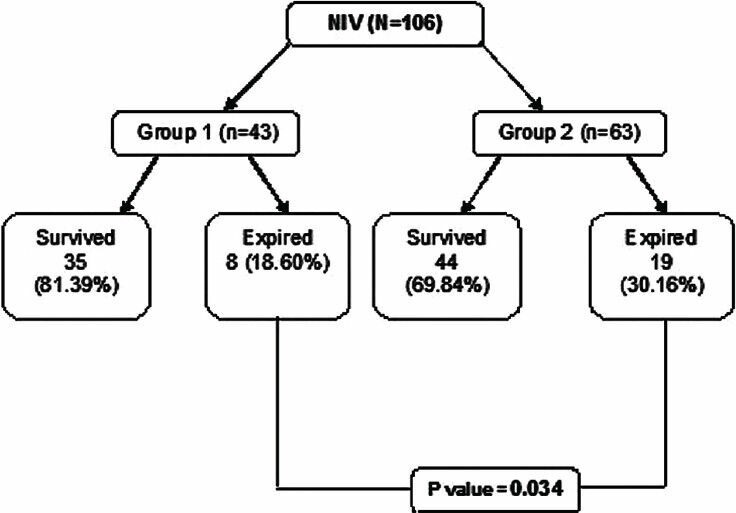
Secondary outcome – mortality
The mean duration of intensive care unit stay in this study was 7.5 days (SD ± 4.78), with those in group 1 having 6.5 days (SD ± 4.4) and those in group 2 having 8.2 days (SD ± 5.0), which was not statistically significant [Table 1]. The mean duration of hospital stay in this study was 11.7 days (SD ± 10.0), with those in group 1 having 12.6 days (SD ± 13.9) and those in group 2 having 11.1 days (SD ± 6.0) (P = 0.023) [Table 1].
Logistic regression analysis was done with age, sex, APACHE II, group of patient, time on NIV prior to intubation, baseline pH, PaCO2, and PaO2 in the model with mortality as an outcome. We found that APACHE II score (P = 0.02), time on NIV before intubation (P = 0.001) and baseline PaCO2 had strong association with mortality (P = 0.02).
Discussion
In the resource-poor countries, where facilities for intensive care are sparse and expensive,[2,3] NIV offers an attractive and cheaper option for ventilator support. ISCCM had published evidence-based guidelines, as early as 2006, for appropriate use of NIV in ARF in the Indian scenario.[1] Despite the availability of these guidelines use of NIV has increased over the years, even outside the prescribed recommendations.[4] Routine audit of practice would help us introspect and implement best practices. We, therefore, audited use of NIV in our semi-closed 70 bed medical and surgical CCU.
In our study, majority (59.4%) of the subjects who required NIV were in group 2, with nonlevel I indications for NIV use [Table 2]. This was similar to two other Indian studies, published by the same group of authors looking at the of NIV use in North India.[5,6] However, another study from South India had discordant results and demonstrated that majority of patients placed on NIV in their institution had level I indications.[7]
Table 2.
Case mix of the study
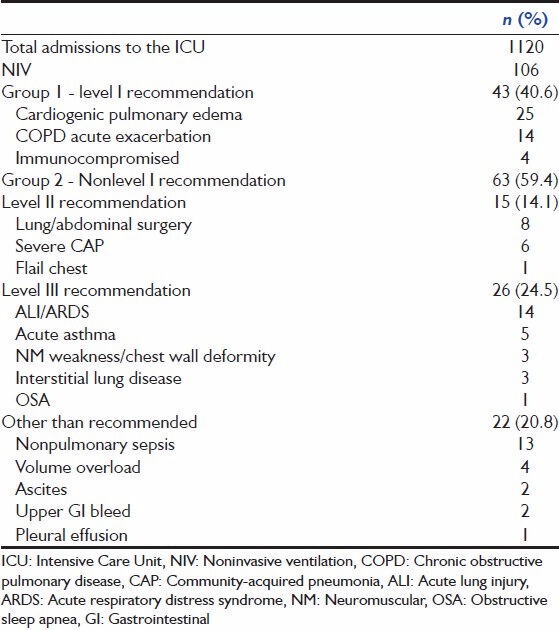
In our study, 35.8% (n = 38) failed NIV and required ETI. There is a wide variation in NIV failure rates across several studies.[5,6,7,8,9,10] It ranges from as high as 53.9%[5] to as low as 15%.[7] Our failure rates fall within this range. Failure rates of NIV therapy depend on the etiology and severity of ARF, with very well documented success rates in hypercapnia respiratory failure from COPD exacerbation and cardiogenic pulmonary edema (level I recommendation).[11,12,13,14,15] Another reason for this wide variation could be lack of objective criteria to define NIV failure and to initiate ETI. Only some studies laid down such criteria,[5,9,10] while others, including ours left it to the discretion of the treating intensivist or were retrospect data collection that could not control for selection bias.[4] This makes comparing rates of NIV failure between studies difficult.
Usage of NIV therapy outside of the level I indications has been shown not only to lead to higher rates of therapy failures, but also to worse outcomes.[13,16] In our study the overall mortality was 25.5% (n = 27), with significantly higher mortality in group 2 (18.6% vs. 30.2%, P = 0.034), showing consistency with other studies. There has been a consistent association reported between the delayed intubation and decreased survival.[10] In our study, only 31.6% (n = 12) of the intubations were done within 2 h (appropriate), while 68.4% (n = 26) were done after 2 h (delayed) (P = 0.023). One of the reasons for this could have been that we did not have objective criteria for initiating ETI. We could not explore whether the indications outside level I recommendations or the delay in recognition of NIV failure and termination of NIV was the reason for the increased mortality observed.
We hypothesize that when patients are placed on NIV outside the level I indications, it is possible that clinicians have a bias towards “observing” them longer on NIV leading to longer time on NIV before intubation. Failing to recognize NIV failure early and delaying intubation has been shown to worsen outcomes.[17]
As far as we are aware there are no other studies that have shown duration of first line NIV therapy to be a predictor of mortality. However, use of NIV in nonlevel I indications has been shown to have high incidences of failure.[12,13] Studies that looked at severity of illness scores as predictors of success of NIV therapy have shown conflicting results.[13,15,17,18] Predictors of mortality have been very variable with NIV failure being a common factor.[10,12]
Our study is one of the largest to look at NIV practices. It has its limitations. Even though, the study sample size is one of the largest it did not allow for assessment of specific conditions in the study population. This limited us in grouping our patients into only two groups. It will be difficult to assess, from our study if there are subgroups of patients in group 2 who would still benefit from NIV support. A study with a larger number of patients would be necessary to answer this question.
We did not set down strict criteria for the initiation, titration or termination of NIV support. This was left to the discretion of the treating intensivist. This could have altered our data on NIV failure rates. However, our intention was to capture the existing NIV practices and evaluate the outcomes. We hence did not want to protocolize the NIV management for the purpose of this study.
As stated earlier, we do not have enough power to assess whether the indications outside level I or the delay in NIV termination or both lead to increasing mortality. Last, ours is a single-center study and will not reflect NIV practices in other centers across India.
Despite these limitations, our study results have important clinical implications. First, we have clearly shown that NIV is predominantly initiated for indications outside level I recommendations. Second, our study is one of the few to demonstrate that indications outside level I recommendations lead to higher rates of NIV failure. Clinicians should factor this in prior to initiating NIV. Last, our study also shows that delay in NIV termination is associated with adverse outcomes. We suggest that clinicians have strict objective criteria for termination of NIV to avoid delays in NIV termination.
Conclusions
Noninvasive ventilation is often used outside recommended indications and persisted with longer than recommended. Its use outside of the level I recommendations is associated with higher chances of failure and an increased likelihood of death. Those patients who deteriorate to require emergent intubation have the worst outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Chawla R, Khilnani GC, Suri JC, Ramakrishnan N, Mani RK, Prayag S, et al. Guidelines for noninvasive ventilation in acute respiratory failure. Indian J Crit Care Med. 2006;10:117–47. [Google Scholar]
- 2.Jayaram R, Ramakrishnan N. Cost of intensive care in India. Indian J Crit Care Med. 2008;12:55–61. doi: 10.4103/0972-5229.42558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan N. Critical economics of life and death: Intense+Expensive care=Intensive care? Indian J Crit Care Med. 2013;17:67–8. doi: 10.4103/0972-5229.114817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnell D, Timsit JF, Darmon M, Vesin A, Goldgran-Toledano D, Dumenil AS, et al. Noninvasive mechanical ventilation in acute respiratory failure: Trends in use and outcomes. Intensive Care Med. 2014;40:582–91. doi: 10.1007/s00134-014-3222-y. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Agarwal R, Aggarwal AN, Gupta D, Jindal SK. A survey of noninvasive ventilation practices in a respiratory ICU of North India. Respir Care. 2012;57:1145–53. doi: 10.4187/respcare.01541. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Gupta R, Aggarwal AN, Gupta D. Noninvasive positive pressure ventilation in acute respiratory failure due to COPD vs other causes: Effectiveness and predictors of failure in a respiratory ICU in North India. Int J Chron Obstruct Pulmon Dis. 2008;3:737–43. doi: 10.2147/copd.s3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George IA, John G, John P, Peter JV, Christopher S. An evaluation of the role of noninvasive positive pressure ventilation in the management of acute respiratory failure in a developing country. Indian J Med Sci. 2007;61:495–504. [PubMed] [Google Scholar]
- 8.Singh VK, Khanna P, Rao BK, Sharma SC, Gupta R. Outcome predictors for non-invasive positive pressure ventilation in acute respiratory failure. J Assoc Physicians India. 2006;54:361–5. [PubMed] [Google Scholar]
- 9.Agarwal R, Handa A, Aggarwal AN, Gupta D, Behera D. Outcomes of noninvasive ventilation in acute hypoxemic respiratory failure in a respiratory intensive care unit in north India. Respir Care. 2009;54:1679–87. [PubMed] [Google Scholar]
- 10.Carrillo A, Gonzalez-Diaz G, Ferrer M, Martinez-Quintana ME, Lopez-Martinez A, Llamas N, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–66. doi: 10.1007/s00134-012-2475-6. [DOI] [PubMed] [Google Scholar]
- 11.Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive-pressure ventilation in acute respiratory failure outside clinical trials: Experience at the Massachusetts General Hospital. Crit Care Med. 2008;36:441–7. doi: 10.1097/01.CCM.0000300084.67277.90. [DOI] [PubMed] [Google Scholar]
- 12.Phua J, Kong K, Lee KH, Shen L, Lim TK. Noninvasive ventilation in hypercapnic acute respiratory failure due to chronic obstructive pulmonary disease vs. other conditions: Effectiveness and predictors of failure. Intensive Care Med. 2005;31:533–9. doi: 10.1007/s00134-005-2582-8. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: A multi-center study. Intensive Care Med. 2001;27:1718–28. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 14.Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, et al. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–13. doi: 10.1164/ajrccm.161.3.9808143. [DOI] [PubMed] [Google Scholar]
- 15.Meduri GU, Turner RE, Abou-Shala N, Wunderink R, Tolley E. Noninvasive positive pressure ventilation via face mask. First-line intervention in patients with acute hypercapnic and hypoxemic respiratory failure. Chest. 1996;109:179–93. doi: 10.1378/chest.109.1.179. [DOI] [PubMed] [Google Scholar]
- 16.Domenighetti G, Gayer R, Gentilini R. Noninvasive pressure support ventilation in non-COPD patients with acute cardiogenic pulmonary edema and severe community-acquired pneumonia: Acute effects and outcome. Intensive Care Med. 2002;28:1226–32. doi: 10.1007/s00134-002-1373-8. [DOI] [PubMed] [Google Scholar]
- 17.Demoule A, Girou E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–65. doi: 10.1007/s00134-006-0324-1. [DOI] [PubMed] [Google Scholar]
- 18.Antón A, Güell R, Gómez J, Serrano J, Castellano A, Carrasco JL, et al. Predicting the result of noninvasive ventilation in severe acute exacerbations of patients with chronic airflow limitation. Chest. 2000;117:828–33. doi: 10.1378/chest.117.3.828. [DOI] [PubMed] [Google Scholar]


