Abstract
Cryptococcus neoformans is encapsulated yeast that predominately infects immunocompromised individuals. Liver disease is an under-recognized predisposition for cryptococcal disease. We report two nonalcoholic, nondiabetic, and human immunodeficiency virus - negative cirrhotic patients, with spontaneous cryptococcal peritonitis. Cryptococcus infection was diagnosed by culture of ascitic fluid and peripheral blood in both. We treated the first patient with amphotericin-B, but he expired. The second patient with earlier diagnosis, survived to discharge with fluconazole treatment. We suggest a high clinical suspicion for Cryptococcus as a possible etiology of spontaneous peritonitis in cirrhotic patients.
Keywords: Cirrhosis, Cryptococcus neoformans, India ink staining, spontaneous bacterial peritonitis, spontaneous cryptococcal peritonitis
Introduction
Cryptococcosis is the third most commonly occurring invasive fungal infection, after candidiasis and aspergillosis. Among human immunodeficiency negative (HIV) - negative patients, liver cirrhosis is an important risk factor for cryptococcal disease, with multiple case reports describing this association. However, liver disease as a predilection for cryptococcal disease is often under-appreciated, with resultant delay in diagnosis, and poor outcome.
We describe two patients with decompensated cirrhosis, and spontaneous fungal peritonitis due to Cryptococcus infection. Both patients were HIV negative, without any history of alcohol use, diabetes mellitus, or use of immunosuppressive drugs.
Case Reports
Case 1
A 65-year-old male was admitted to the hospital with complaints of progressive jaundice, weakness, and increasing ascites since 1 month. He was diagnosed as chronic hepatitis B-related cirrhosis, with virus-related flare leading to hepatic decompensation. His Child-Pugh-Turcotte (CTP) and model of end stage liver disease (MELD) scores were 10 and 37, respectively.
His laboratory parameters at admission revealed, total white cell-count (total leukocyte count [TLC]): 11.8 × 103/mm3, with 78% neutrophils, international normalized ratio (INR): 2.1, blood urea nitrogen: 169 mg/dl, creatinine: 3.8 mg/dl, total bilirubin: 39.7 mg/dl (direct: 20.7), alanine aminotransferase (ALT): 44 U/L, alkaline phosphatase (ALP): 151 U/L, albumin: 3 g/dl, and ammonia: 180 μmol/L. His chest radiograph did not show any infiltrates.
The patient was treated empirically with meropenem, but he deteriorated clinically, and was shifted to the intensive care unit (ICU) with worsening encephalopathy. Abdominal paracentesis revealed slightly turbid ascitic fluid, albumin level of 0.8 g/L, cell-count of 900/mm3, with predominantly lymphocytes, and a negative Gram-stain. Potassium hydroxide and India ink preparation of the ascitic fluid showed encapsulated budding yeast cells, with morphology suggestive of Cryptococcus neoformans [Figure 1]. Creamy white colonies were grown in culture [Figure 2], which were identified as C. neoformans by Biomerieux is a USA based diagnostic manufacturer of VITEK COMPACT SYSTEM- an automated system utilizing growth based technology, it accommodate colorimetric reagent cards (for yeast and yeast like organisms) that are incubated and interpreted automatically in yeast card; further susceptibility testing was also done in the antibiotic sensitivity card by the Vitek 2 Compact System. The isolate was sensitive to fluconazole, flucytosine, amphotericin B and voriconazole.
Figure 1.

India ink staining demonstrating the capsule of Cryptococcus
Figure 2.

Cryptococcus colonies on blood agar medium
The patient was treated with amphotericin-B (AmBisome 50 mg/day) along with cefoperazone-sulbactam. We repeated ascitic fluid paracentesis was repeated twice, at 3 days intervals-similar cytological findings were found without any decrease in the ascitic fluid cell-count. The ascitic fluid cultures and peripheral blood cultures repeatedly grew C. neoformans, despite amphotericin-B treatment. Bacterial cultures were negative. The urine cultures grew Candida tropicalis that persisted despite catheter change.
The patient developed lactic acidosis, and deteriorating renal function with anuria. The next-of-kin refused further interventions, and he died after 2 weeks of hospitalization. Postmortem liver biopsy showed cirrhosis, neutrophilic cholangiolitis, and cellular and canalicular cholestasis. No fungal elements were seen in the liver biopsy specimen.
Case 2
A 57-year-old male, with hepatitis C virus-related cirrhosis was admitted to the ICU with complaints of fever, pain abdomen, and increasing ascites. His CTP and MELD scores were 11 and 17, respectively.
His laboratory parameters at admission revealed, TLC: 7.85 × 103 cells/mm3, with 76% neutrophils, INR: 1.9, creatinine: 0.7 mg/dl, total bilirubin: 7.6 mg/dl (direct: 4.0 mg/dl), ALT: 55 U/L, ALP: 87 U/L, albumin: 1.6 g/dl, and ammonia: 120 μmol/l. On computed tomographic study, cirrhotic liver with ascites, and small right pleural effusion with underlying consolidation was seen.
Abdominal paracentesis revealed slightly turbid ascitic fluid, cell-count of 800/mm3, with predominantly lymphocytes, and albumin levels of 0.2 g/L. The patient was empirically treated with Ceftriaxone 1 g twice daily. Two days later, blood and ascitic fluid culture grew C. neoformans, with similar sensitivity as in the first case [Figure 3]. There was no bacterial growth on peripheral blood culture. Repeat ascitic fluid sample was sent after 2 days, which again grew the same fungal species. The patient was treated with intravenous fluconazole 200 mg once daily, and Levofloxacin 500 mg once daily for 7 days. The patient gradually improved and was discharged.
Figure 3.
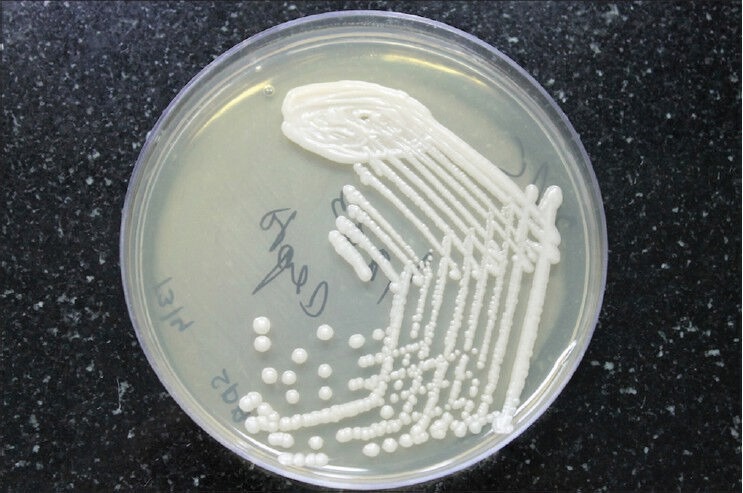
Cryptococcus colonies on Sabroud's dextrose agar medium
Discussion
Risk factors for disseminated cryptococcal disease are HIV infection, liver cirrhosis, lymphoid malignancies, corticosteroid treatment, and continuous ambulatory peritoneal dialysis (CAPD).[1] Cryptococcal peritonitis is considered uncommon and is seen mainly in patients with advanced cirrhosis, and those on CAPD. In older series, peritonitis accounted for < 5% of all cryptococcosis cases in HIV-negative patients, although these are probably underestimates.[2] Leukocyte functional impairment, complement dysfunction, and decreased opsonin activity are believed to predispose cirrhotic patients to cryptococcosis.
When should we suspect fungal peritonitis? The symptoms and signs of cryptococcal peritonitis are nonspecific. However, negative bacterial cultures, prolonged course of infection, recent antibiotic use, and history of previous ascitic fluid sampling may be useful clues. The ascitic fluid can be mildly turbid or even bloody. Most reports describe a modest ascitic fluid cell-count with lymphocytic predominance, although neutrophilic dominance is also reported. While ascitic fluid lymphocytosis may reflect previous antibiotic use, persistent high cell-count despite appropriate antibiotics may be an important pointer to cryptococcal etiology. Our first patient did not show any reduction in his ascitic fluid cell-count, and the second showed a delayed response.
In suspected cryptococcal peritonitis, frequent abdominal paracentesis with bedside inoculations of fungal culture medium, India ink preparations, and serum and/or ascitic fluid cryptococcal antigen testing by latex agglutination or enzyme immunoassay may establish the diagnosis. Serum cryptococcal antigen titers are also a marker of response to therapy in disseminated cryptococcosis. However, no such data for serial ascitic fluid antigen titers exist, in cryptococcal peritonitis. Cryptococcus can be identified on cytology as yeast-like encapsulated organisms with Mucicarmine and periodic acid-Schiff positive capsule, which can be highlighted by use of India ink stain. A rapid diagnosis can be made with cytological smears of ascitic fluid, provided the suspicion for this infection is conveyed to the laboratory.[3] Species determination requires culture.[3,4] All patients with suspected or documented cryptococcosis should have blood and urine fungal cultures, bronchoalveolar lavage if pulmonary infiltrates are present, and a lumbar puncture for cerebrospinal fluid (CSF) examination in cases with altered mentation. Cryptococcus can be isolated from the blood in some cases of cryptococcal peritonitis. Similar to the present report, Jean et al., described fungemia in two cases with primary C. neoformans peritonitis.[5]
We did not find Cryptococcus organisms in the liver biopsy of the patient who succumbed, possibly because we did not use special stains to highlight the fungal elements. In a patient with postcardiac transplant HBV re-activation, cryptococci were demonstrated in dilated sinusoids in the liver biopsy, as groups of bright, rounded, colorless cells with a central nucleus, staining with Periodic acid-Schiff stain.[6] Cryptococcus can also directly infect the liver parenchyma, and there are reports of cryptococcal liver abscess,[7] necrotizing hepatitis,[8] and liver dysfunction in both immunocompromised and immunocompetent patients.[9,10,11]
Among both immunocompromised and immunocompetent patients, the most common clinical manifestation of cryptococcal infection is meningo-encephalitis.[2] CSF examination is often not possible due to coexisting coagulopathy in patients with decompensated cirrhosis, as in our patients. Altered mentation in cirrhotic patients with cryptococcal meningo-encephalitis may be attributed to hepatic encephalopathy, and imaging of the brain is usually normal. A high index of suspicion is needed in cirrhotic patients with poorly resolving or prolonged peritonitis for coexisting cryptococcal meningitis.
Prognosis for disseminated cryptococcosis and liver cirrhosis is poor. In a large review of 33 patients with cryptococcal peritonitis, Singh et al. have reported a high mortality rate of 81%.[12] Chuang et al. have reported that all 12 HIV-negative patients, with cirrhosis and disseminated cryptococcosis died.[13] Jean et al. have reported that liver cirrhosis was a strong independent predictor of 30 days mortality with disseminated cryptococcosis, with an adjusted hazard ratio of 16.3.[14] Our first patient died in spite of appropriate anti-fungal drugs.
The tropical climate of the Indian subcontinent offers a suitable environment for C. neoformans. There is widespread environmental prevalence of Cryptococcus species in India, and many reports of neurological infections in patients with and without HIV infection. However, there are only two previous reports of cryptococcal infection in patients with cirrhosis from this country, describing spontaneous cryptococcal peritonitis in two patients,[15] and pleural fluid infection in one patient with decompensated cirrhosis.[16] It is likely that cryptococcal peritonitis is an under-reported infection among patients with cirrhosis.
Lymphocytic ascitic fluid also should suggest tuberculosis/fungal and metastatic malignancy and these should typically be evaluated for in patients with high cell-counts with lymphocytic predominance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Saif MW, Raj M. Cryptococcal peritonitis complicating hepatic failure: Case report and review of the literature. J Appl Res. 2006;6:43–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Yehia BR, Eberlein M, Sisson SD, Hager DN. Disseminated cryptococcosis with meningitis, peritonitis, and cryptococcemia in a HIV-negative patient with cirrhosis: A case report. Cases J. 2009;2:170. doi: 10.1186/1757-1626-2-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Joshi D, Gangane N. Cryptococcus peritonitis a rare manifestation in HIV positive host: A case report. Diagn Cytopathol. 2011;39:365–7. doi: 10.1002/dc.21445. [DOI] [PubMed] [Google Scholar]
- 4.Han L, Husain M, Fairfax MR, Al-Abbadi MA. Cytological diagnosis of cryptococcal peritonitis on peritoneal fluid. Diagn Cytopathol. 2007;35:670–1. doi: 10.1002/dc.20703. [DOI] [PubMed] [Google Scholar]
- 5.Jean SS, Wang JL, Wang JT, Fang CT, Chen YC, Chang SC. Cryptococcus neoformans peritonitis in two patients with liver cirrhosis. J Formos Med Assoc. 2005;104:39–42. [PubMed] [Google Scholar]
- 6.Utili R, Tripodi MF, Ragone E, Casillo R, Pasquale G, De Santo L, et al. Hepatic cryptococcosis in a heart transplant recipient. Transpl Infect Dis. 2004;6:33–6. doi: 10.1111/j.1399-3062.2004.00041.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu PY, Yang Y, Shi ZY. Cryptococcal liver abscess: A case report of successful treatment with amphotericin-B and literature review. Jpn J Infect Dis. 2009;62:59–60. [PubMed] [Google Scholar]
- 8.Méndez-Sánchez N, Pichardo-Bahena R, Castrejón HV, Berron-Pérez RD, Hernández-Bautista VM, Soto-Ramírez L, et al. Necrotizing hepatitis and intrahepatic cholestasis associated to Cryptococcus sp. and Pneumocystis carinii. Ann Hepatol. 2006;5:289–90. [PubMed] [Google Scholar]
- 9.Lin JI, Kabir MA, Tseng HC, Hillman N, Moezzi J, Gopalswamy N. Hepatobiliary dysfunction as the initial manifestation of disseminated cryptococcosis. J Clin Gastroenterol. 1999;28:273–5. doi: 10.1097/00004836-199904000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Gollan JL, Davidson GP, Anderson K, White TA, Kimber CL. Visceral cryptococcosis without central nervous system or pulmonary involvement: Presentation as hepatitis. Med J Aust. 1972;1:469–71. doi: 10.5694/j.1326-5377.1972.tb46877.x. [DOI] [PubMed] [Google Scholar]
- 11.Procknow JJ, Benfield JR, Rippon JW, Diener CF, Archer FL. Cryptococcal hepatitis presenting as a surgical emergency. First isolation of Cryptococcus neoformans from point source in Chicago. JAMA. 1965;191:269–74. doi: 10.1001/jama.191.4.269. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Husain S, De Vera M, Gayowski T, Cacciarelli TV. Cryptococcus neoformans infection in patients with cirrhosis, including liver transplant candidates. Medicine (Baltimore) 2004;83:188–92. doi: 10.1097/01.md.0000126760.45299.69. [DOI] [PubMed] [Google Scholar]
- 13.Chuang YM, Ho YC, Chang HT, Yu CJ, Yang PC, Hsueh PR. Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis. 2008;27:307–10. doi: 10.1007/s10096-007-0430-1. [DOI] [PubMed] [Google Scholar]
- 14.Jean SS, Fang CT, Shau WY, Chen YC, Chang SC, Hsueh PR, et al. Cryptococcaemia: Clinical features and prognostic factors. QJM. 2002;95:511–8. doi: 10.1093/qjmed/95.8.511. [DOI] [PubMed] [Google Scholar]
- 15.Cleophas V, George V, Mathew M, Samal SC, Chandy GM. Spontaneous fungal peritonitis in patients with hepatitis B virus-related liver disease. J Clin Gastroenterol. 2000;31:77–9. doi: 10.1097/00004836-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Mutreja D, Malhotra R, Dutta U. Cryptococcus in pleural fluid cytology in a patient with hepatitis B virus-associated chronic liver disease. J Cytol. 2011;28:145–6. doi: 10.4103/0970-9371.83480. [DOI] [PMC free article] [PubMed] [Google Scholar]


