Abstract
Infantile hemangiomas (IH) are common vascular tumours. IH have a characteristic natural course. They proliferate rapidly during the early infantile period followed by a period of gradual regression over several years. Most of the uncomplicated IH undergo spontaneous involution, with a small proportion of cases requiring intervention. These are children with IH in life-threatening locations, local complications like haemorrhage, ulceration and necrosis and functional or cosmetic disfigurements. Systemic corticosteroids have been the first line of treatment for many years. Recently, non-selective beta-blockers, such as oral propranalol and topical timolol, have emerged as promising and safer therapies. Other treatment options include interferon α and vincristine which are reserved for life-threatening haemangiomas that are unresponsive to conventional therapy. This review mainly focuses on the current trends and evidence-based approach in the management of IH.
KEYWORDS: Infantile hemangioma, steroids, propranolol, timolol, pulsed dye laser
INTRODUCTION
Infantile hemangiomas (IH) are the most common benign vascular tumours of infancy. IH occur in 1-4% of the Caucasian infants and are less common in the African and Asian races.[1,2] The various risk factors include female gender, prematurity, low birth weight, multiple pregnancies, advanced maternal age and in vitro fertilization.[2] IH most commonly affect the head and neck region. Morphologically, hemangiomas are classified into superficial, deep and mixed types. Superficial hemangiomas, when fully formed, are characterized by bright red vascular plaques or nodules. Deep haemangiomas manifest as partially compressible, subcutaneous, bluish vascular swellings. Mixed haemangiomas have both superficial and deep components. Based on their distribution, IH can also be classified into localized, segmental, indeterminate and multifocal.[3,4] Localized hemangiomas are spatially confined while in segmental IH, there are clusters of lesions confined to a developmental segment or a large anatomic territory.[5] In multifocal hemangiomas, the infants have 5 or more non-contiguous lesions. They are often associated with systemic involvement, especially the liver. The other sites of involvement include the central nervous system, lungs, kidneys and eyes.[6]
Unlike vascular malformations, IH have a characteristic natural course; a rapid proliferative phase in infancy which is followed by a gradual involutional phase over the next several years of life. Chang et al. in their study on growth characteristics of IH in 433 patients observed that in most IH growth occurred before 5 months of age. However, segmental haemangiomas showed a trend toward higher growth rate after 6 months of age. Deeper IH exhibited a 1-month delay in onset and also showed sustained growth when compared to superficial IH.[3] This study highlighted that the initial few weeks up to five months is the crucial period in IH growth. Complications such as ulceration, distortion of vital structures and skin disfigurement are also expected to occur during this critical period. Hence, the treating dermatologists and paediatricians should be aware of these facts which will be useful in the clinical decision making.[3]
Treatment indications
The treatment of IH depends on the following factors: Type of hemangioma, stage of the lesion, location and extent, number and distribution of the lesion (segmental/non-segmental), associated systemic involvement, presence or absence of ulceration and psychosocial distress of the parents or child. In general, any function threatening (ocular, ear, nasal tip, lip, large disfiguring facial lesion and genitalia involvement) or life threatening (airway lesion) IH, which constitute about 10-20% of the cases, need intervention [Table 1].[7] The remaining majority of the cases need only active non-intervention, which consists of education about the natural course, treatment options and anticipatory guidance.[4,7]
Table 1.
Treatment indications in infantile haemangiomas

Systemic steroids and propranolol are the two main drugs in the management of IH.
Pre-treatment evaluation
Most cases of IH can be diagnosed clinically, but imaging studies (MRI, Doppler ultrasound) or tissue biopsy may be required in doubtful cases. Additional investigations may be required depending upon the site(s) and extent of involvement.
Segmental hemangiomas may be associated with visceral hemangiomatosis, with the location of visceral lesions often correlating with the site of cutaneous involvement. Infants with large segmental hemangiomas on the face are at risk for PHACES syndrome (Posterior fossa anomalies, Haemangiomas, Arterial anomalies, Coarctation of aorta and Cardiac defects, Eye abnormalities, Sternal clefting and Supra-umbilical raphe) and should undergo a thorough ophthalmological, cardiac and neurological evaluation. Magnetic resonance imaging (MRI) with angiography of the head and neck region is usually indicated in such infants.[8,9,10] Similar to PHACES syndrome, segmental hemangiomas in the perineal region may be associated with malformations of the urogenital or anogenital systems; these include SACRAL (spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localisation) and PELVIS (perineal haemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus and skin tag) syndrome. Haemangiomas on the lumbosacral region may be a marker of occult spinal dysraphism. A spinal MRI is indicated as a screening test for all cases with lumbosacral haemangiomas.[11] Laryngeal involvement can occur if haemangiomas are present on the cervicofacial, mandibular or the ‘beard’ distribution. Affected infants should be watched for signs and symptoms of airway obstruction (stridor, hoarseness) and referred for a laryngeal examination.[12] Infants with multiple cutaneous haemangiomas (diffuse neonatal haemangiomatosis) constitute another group which is at risk for visceral involvement, and it is generally recommended to screen the patient for hepatic involvement by abdominal ultrasound.[13,14] Raised levels of iodothyronine deiodinase have been demonstrated in large proliferative haemangiomas, which can lead to hypothyroidism. Till date, most of the cases of hypothyroidism have been noted in association with hepatic haemangiomatosis, though increased iodothyronine deiodinase activity has been detected in some large cutaneous lesions as well. Hence, a recommendation has been made to perform serial thyroid function tests in patients with hepatic haemangiomatosis or large cutaneous haemangiomas.[15,16]
Once a decision has been made to treat the haemangioma, pre-treatment investigations should be tailored to the chosen therapeutic modality. Before initiating propranolol therapy, the children need to be assessed for the following contraindications: bronchial asthma, heart failure, sinus bradycardia, hypoglycaemia, hypotension, heart block and known allergy to propranolol. The initial examination should include a thorough cardiopulmonary assessment including pulse rate, blood pressure and blood sugar. A baseline electrocardiogram (ECG) is usually recommended. Although echocardiography is not a routine investigation, it may be required to exclude functional and structural heart diseases.[7] The pre-treatment work-up before starting steroid therapy include ruling out active infection or primary immunodeficiency disease, (complete blood count with differential leucocyte count, serum biochemistry, chest x-ray and urine and stool microscopy), and a baseline anthropometric examination (height, weight) and blood pressure, which should be monitored serially.
Corticosteroids
Systemic corticosteroids (prednisolone) have been the mainstay of treatment for IH, for several decades. The mechanism of action of steroids is not entirely clear, though it is postulated to have an inhibitory effect on the production of vascular endothelial growth factor A (VEGF-A) by stem cells in haemangiomas.[17,18] Steroids are most effective in the early proliferative phase. The usual recommended dose is 2-4 mg/kg/day, which should be continued until cessation of growth or shrinkage of haemangioma followed by gradual tapering.[19] Oral steroids at a dose of 2-3 mg/kg/day result in 75% response, >3 mg/kg/day show 94% response but with greater side effects while a lesser dose of <2 mg/kg/day results in poor response and a rebound phenomenon in 70% of the cases.[19] Bennett et al. in their meta-analysis on the efficacy of corticosteroids for IH included 184 children in a 10-case series. Statistical analysis revealed that a mean prednisone dose of 2.9 mg/kg (range, 1-4.5 mg/kg/day) was used over a mean period of 1.8 months (range, 0.5-5.4 months) before the dose was tapered. The overall response rate was 84% (range, 60-100%) and the mean rebound rate was 36% (range, 0-65%).[19] In a recent study on 20 children with IH, the lesions responded to oral prednisolone at a mean dose of 3.1 mg/kg/day given for a mean duration of 9.16 weeks (range, 3-15 weeks). The mean total duration of treatment including the tapering dose used was 29.9 weeks (range, 15-44 weeks). Overall, 90% of the cases showed >25% improvement with no case of rebound phenomenon during their follow up period of 6 months to 1 year.[20]
High dose pulse corticosteroids (methyl prednisolone 30 mg/kg/day infused over 1 hour daily for 3 days) may be useful in IH which need immediate therapeutic response (e.g. large peri-ocular IH with complete visual axis occlusion or large airway haemangioma with respiratory difficulty). Usually three or more monthly doses are required followed by shorter oral steroid regimen. Pope et al. in their randomized controlled trial of oral versus high dose pulse steroids in 20 children with problematic IH concluded that oral corticosteroids offer more clinical and biological benefit than pulsed steroids. However, patients in the oral steroid group had a higher frequency of side effects such as hypertension (18% vs. 13%), abnormal cortisol levels (78% vs. 60%) and growth retardation.[21] Figure 1a and b show good response (of hemangioma on the scalp) to steroids in one of our cases. The child also developed Cushingoid face.
Figure 1.
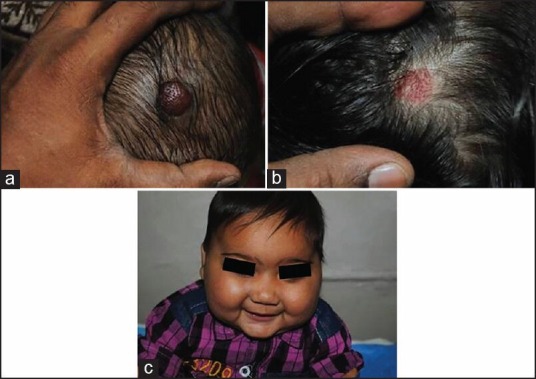
(a-c) Serial photographs of haemangioma involving the scalp responded to steroid for 3 months. (a) baseline, (b) 3 months of treatment, c: showing cushingoid face
Long term steroid use is associated with many side effects. Boon et al., in their study of complications of systemic corticosteroid therapy for haemangiomas, identified cushingoid facies in 44 of 62 children which began 1 to 2 months after starting at a dose of 2-3 mg/kg/day.[22] Although it was not statistically significant the authors observed that the cushingoid facies was higher in children who received therapy for more than 6 months. The cushingoid facies resolved spontaneously during the final few months of tapering steroids. Twenty-two (35%) children slipped off their growth curve for weight during the treatment, and 91% of these children returned to their baseline within 2 weeks to 16 months (mean, 7 months; median, 4 months) after treatment. They also observed a transient decrease in weight gain in 26 children (42%). Of these, 23 children returned to their pre-treatment weight curve within 2 years after therapy (mean, 7.4 months; median, 6 months). Three children remained at a lower percentile for a longer period of time. Children who received treatment for more than 6 months or who received the therapy during the first 3 months of life were found to be more likely to have this transient slowdown of gain in height. Personality changes such as irritability, depressed mood, euphoria, insomnia and restlessness was reported in 29% of their cases, appeared within 2 weeks of therapy and resolved immediately after stopping the drug. The other side effects reported were gastric irritation (21%, n = 13), oral and/or perineal candidiasis (6%, n = 4) and steroid myopathy (n = 1). The overall risk of bacterial infection was not increased. Serious complications such as aseptic necrosis of femoral head, osteoporosis and cataracts were not seen in any patient.[22] George et al., in a retrospective review, found that 16 (73%) of 22 children treated with systemic steroids had irritability, fussiness or insomnia. Hypertension was observed in 10 patients (45%), while abnormal morning cortisol levels were found in 13 of the 15 patients (87%) tested. In addition, low-dose corticotropin stimulation test results were abnormal in 2 of the 3 infants tested.[23] In a prospective study on 16 infants with haemangiomas treated with prednisolone at doses 2-3 mg/kg/day for 4 weeks followed by a tapering period (mean duration of steroid therapy 7.2 months), only one case (6%) was found to have adrenal insufficiency (on corticotropin stimulation test) after therapy completion, which normalised 3 months later. The authors concluded that there is a low risk of adrenal insufficiency following completion of steroid therapy in infants with haemangiomas. The authors commented that measuring morning serum cortisol levels, which is less specific than corticotropin stimulation test for assessing the HPA axis suppression, and measuring the cortisol levels while the patient is still on corticosteroid therapy could explain the higher incidence of adrenal insufficiency reported in the earlier studies.[24]
Propranolol
Propranolol is a non-selective -β adrenergic receptor blocker that recently has emerged as a good therapeutic option in IH. In 2008, Leaute-Labreze et al. reported regression of a facial IH in a child who was treated with propranolol for obstructive hypertrophic cardiomyopathy.[25] Ever since there has been a series of publications describing its therapeutic efficacy and side effects.[26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] Currently, it is widely used in IH, particularly for complicated and ulcerated IH, and appears to be the best therapeutic modality.[31]
Oral propranolol has three different pharmacological effects: Early, intermediate and long-term effects. The early effect of a visible change in colour and considerable softening of the lesion occurs within 1-3 days, after the initiation of therapy. This is related to the β2 inhibitory effect of propranolol that decreases the release of the vasodilator transmitters such as nitric oxide. The resulting vasoconstriction of the feeding capillaries is responsible for the early changes in haemangiomas. In view of its immediate clinical effect, propranolol is nowadays considered as the promising therapy in IH with ulceration or haemangiomas involving vital structures such as eyes, airways, genitalia etc.
In the proliferative phase of IH there is an increased expression of pro-angiogenic factors in particular the vascular endothelial growth factors (VEGF) and basic fibroblast growth factors (bFGF). The intermediate effects of propranolol are due to a downregulation of both VEGF and bFGF resulting in inhibition of proangiogenic cascade and angiogenesis. The long term effect of propranolol is due to apoptosis resulting in regression of haemangiomas.[41] This may be the reason for its use in the post proliferative phase.[41]
Ideally, propranolol should be initiated immediately once the decision is taken. Although there is no published evidence that early start of propranolol therapy results in less deforming residual skin changes, it is always better to start the treatment as early as possible before rapid proliferation of the tumour. A recent meta-analysis (including 1264 children in 41 studies) showed that oral propranolol was initiated at a mean age of 6.6 months (range from 3 days to 10 years).[27] In a single-centre prospective study of 174 children, propranolol was administered at a mean age of 4.8 months (0.9-29 months).[31] In an another recent review of 28 studies on IH, propranolol was initiated during infancy at a mean age of 4.5 months (range, 27 days-12 months). In 11 cases, propranolol was started after 12 months of age.[26]
In the recent consensus conference report on haemangioma, a target dose of 1-3 mg/kg has been recommended.[7] However, many researchers advocate a dose of 2 mg/kg/day. Hermans et al. in their large prospective study used a higher dose of 3 mg/kg/day in patients with haemangiomas causing airway obstruction or associated with repeated severe bleeding due to ulceration.[31] Few authors have also used a maximum dose of 4 mg/kg/day.[14] It is usually administered at a low starting dose of 0.5 to 1 mg/kg/day which is gradually escalated to the optimal dose over a period of days to weeks.[7,27]
The duration of therapy depends on the morphological type of haemangioma, extent of involvement and the treatment indications. Hogeling et al., based on their randomized controlled trial (RCT), suggest a treatment course of at least 6 months which could be modified according to the morphological subtypes, for example deep and mixed IH may continue to proliferate up to 1 year of age and hence, treatment may be extended up to this period.[35] The mean duration of treatment varies from 6 to 10 months. In a recent study on the propranolol treatment in complicated IH, the authors have used the drug for a mean duration of 10.7 months. In the deep and mixed type haemangiomas, they continued the treatment until the age of 12-16 months, in ulcerated lesions up to 9-12 months and in deep peri-orbital and airway lesions up to 15-18 months of age.[31] Marqueling et al. in their meta analysis of 1264 children in 41 studies found that the propranolol was administered for an average duration of 6.4 months (range, 1 weeks to 15 months).[27]
Oral propranolol is known to be effective during the rapid proliferative phase but there are several reports which have shown involution where propranolol was initiated at the end of growth phase or later.[27,31,32,36] Hogeling et al., in their trial of 40 children with IH, included a combination of growing, plateaued and involuting lesions. The authors noted a steady decrease in percentage volume for all weeks in the propranolol group compared to placebo.[35] This suggests that propranolol can be used at all stages of IH. Schupp et al.[32] and Zvulunov et al.[36] reported good clinical improvement with propranolol for IH beyond proliferative phase. However, Holmes et al. did not observe any improvement in children with IH in whom the propranolol was started after 9 months of age.[39] Hence, large prospective studies are needed to confirm the efficacy of propranolol in older age group of children.
A meta-analysis shows that the overall response rate is 98% (range, 82-100%).[27] Similar efficacy has also been observed in a large prospective study.[31] The treatment response in most of the published studies is based on the visual changes in the colour, volume and assessment of serial photographs. Only few studies categorized the response in terms of percentage reduction. Price et al. defined haemangioma clearance of 75% or more by correlating percentage of decrease in volume, cosmetically acceptable result by the physician and/or parent and a lack of need for further treatment. Based on these criteria they report >75% clearance in 81% (48/59) of the cases over a mean period of 7.9 months.[42] Talaat et al. reported 75% clearance in 75% and >50% clearance in 94% of their 80 cases with IH in 5-8 months (mean 6.53 ± 0.75).[40]
With propranolol therapy, the therapeutic efficacy is observed immediately. Sans et al. used propranolol at a dose of 2-3 mg/kg/day in 32 children with severe IH and noticed immediate changes in colour (within 24 hours) and softening in all the cases. Airway obstructive symptoms and haemodynamic compromise regressed within 48 hours.[29] Hermans et al. also reported improvement of respiratory symptoms in airway haemangioma within hours of treatment initiation with propranolol.[31] Propranolol also seems to be a promising drug for ulcerated haemangiomas. Painful ulceration in haemangioma has been reported to heal completely within 2 months. In a recent study of 20 children with ulcerated IH, oral propranolol significantly decreased the duration of ulceration compared with the control group (8.7 vs. 22.4 weeks, P < 0.01).[43] All these published observations suggest that oral propranolol can be considered as the best therapeutic option in cases with airway and other complicated IH. Figures 2 and 3 show near complete response to propranolol in two of our cases.
Figure 2.
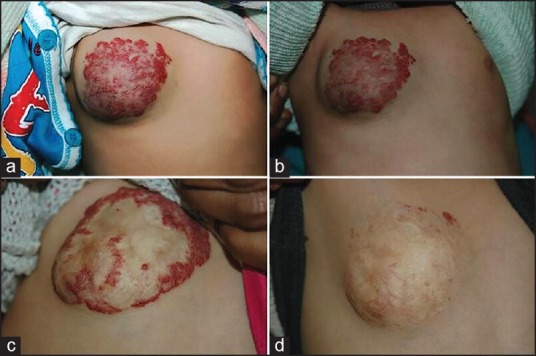
(a-d) Serial photographs of haemangioma on the right pectoral region responded to 30 months of propranolol. a: baseline; b: 1 month after treatment; c: 2 years of treatment; d: 2.5 years of treatment (Courtesy of Somesh Gupta)
Figure 3.
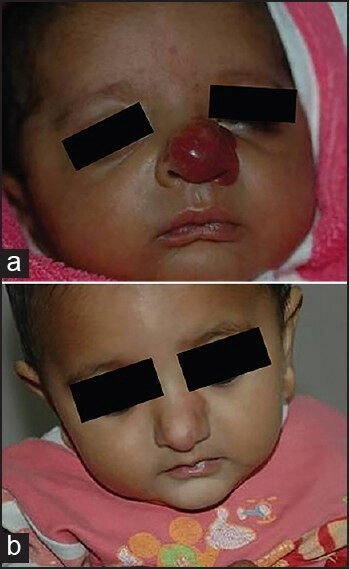
(a and b) Photographs of haemangioma on the nose showing an excellent response to propranolol for 10 months. a: baseline; b: 10 months of treatment (courtesy of Somesh Gupta)
Rebound or regrowth of haemangioma, defined as increase in size, change in colour or both, can occur after discontinuation of treatment. Studies showed that stopping propranolol before the age of 1 year increases the risk of rebound growth. In deeper haemangiomas, the proliferative phase could be longer and hence stopping the treatment early may be a risk factor for rebound growth.[42] In a recent retrospective analysis, the authors found that 2 of the 68 (3%) children in whom propranolol was started at 3 months and discontinued at 10 months, had relapsed.[42] In an another study, 2 of 32 cases had relapsed after stopping the drug before 1 year of age.[28] Menezes et al. in their meta-analysis found that 14 of 64 children who had treatment for a mean period of 5.1 months had a relapse.[26] Table 2 shows the summary of propranolol treatment in infantile hemangioma. In these cases, propranolol was stopped at a mean age of 7.2 months.[26] Relapse after cessation of propranolol after 1 year of age has also been reported.[42] In a recent meta-analysis, it has been reported in 17% of children who received propranolol for an average of 6.4 months.[27] Relapsed haemangiomas have been effectively re-treated with propranolol.[42] They can also be treated with adjunctive systemic steroids.[44]
Table 2.
Summary of propranolol treatment in infantile hemangioma

Propranolol-resistant IH have also been described as an absence of treatment response either in the proliferative phase or during the post-proliferative phase, after at least 4 weeks of oral propranolol at dosage of ≥2 mg/kg/day.[44] It is reported to occur at a frequency of 0.9% (10/1130). Of the 10 such cases reported from France, 8 had haemangioma on the face. Five children were in the proliferative phase (≤4.2 months) and showed continued growth. The remaining 5 (≥8 months) children, who did not show any decrease in the IH size, were in the post-proliferative phase.[44] This phenomenon needs to be investigated in a large scale studies in order to understand the different factors which influence the treatment outcome in children with IH.
Propranolol, being a beta blocker, has a negative inotropic and chronotropic effect on the heart. The effect on the pulse rate and BP can be seen around 2 hours after the oral dose. The most serious side effect is hypoglycaemia especially in infants < 3 months of age with poor feeding. The other possible side effects include pulmonary symptoms, sleep disturbances, somnolence, cold extremities and gastrointestinal (GI) complaints. These adverse reactions are not serious, reversible and dose-dependent.[7] In 174 patients treated for IH from a single centre, 108 children (62.1%) had 1 or more adverse reactions. The two most common problems were cold extremities (63, 36.2%) and nocturnal restlessness (39, 22.5%). Twenty-eight children (16.1%) were less active during the day. Lower blood pressure was observed in 6 cases (3.4%). Sixteen patients (3.2%) had pulmonary symptoms, out of which 9 needed treatment. In 12 children (6.9%), GI complaints were recorded.[31] In another meta-analysis of 39 studies in 1189 children, 371 cases had adverse reactions. The most common adverse event was sleep changes, which included insomnia, nightmares, night restlessness, fatigue and sleep disturbance in 136 cases followed by acrocyanosis in 61 children. There were 41 reports of GI problem like diarrhoea and gastroesophageal reflux. Respiratory adverse events were seen in 35 patients. Hypotension and bradycardia were seen in 39 and 8 cases, respectively. Hypoglycaemia was reported only in 4 cases.[27] The other side effects reported in the literature include irritability, profuse sweating and temporary hypotonia.[27]
Steroids versus propranolol
There are only a few studies, retrospective and open RCTs which have compared the efficacy of these two drugs. In a recent multi-centric retrospective analysis of propranolol versus corticosteroids for IH, 110 children were studied. Sixty eight children received propranolol at a dose of 2 mg/kg/day for a mean period of 7.9 months (range, 3.5-14 months) and 42 received prednisolone at a dose of 4 mg/kg/day for a mean period of 5.2 months (range, 2.5-8.0 months). On comparing the outcome parameters, 56 of 68 (82%) on propranolol achieved clearance of 75% or more compared to only 12 of 42 (29%) patients in the steroid group (P < 0.01). Only 1 child in the propranolol group had side effect in contrast to all the cases in the steroid group (P < 0.01). Other outcome parameters such as the number of cases with ulceration [4 (6%) vs. 11 (26%); P < 0.01] and the number of cases who required surgical referral [8 (12%) vs. 12 (29%); P = 0.03] were also significantly lower in the propranolol group than in the steroid group.[42] In another retrospective study, 12 selected pairs of infants were compared. The mean duration of treatment in the steroid and propranolol groups was 12.7 months (range, 5-28 months) and 10.6 months (range, 7-13 months), respectively. Investigator rating after 1, 2 and 6 months of treatment suggested that the response to propranolol is significantly better than steroids. Using VAF, the mean improvement after 6 months of treatment in both groups was 44.8% and 78.7%, respectively (P < 0.001).[45] In an open RCT from India, the authors have compared the efficacy of oral propranolol versus steroids versus both in 30 children with IH. The children were treated for a minimum period of 3 months. Group A received propranolol (2-3 mg/kg/day), group B received prednisolone (1-4 mg/kg/day) and group C was treated with both. Children in groups A and C had a significantly earlier response (mean initial response time in group A - 4.1 days; Group C - 4.7 days vs. Group B - 9.8 days; P < 0.05). Flattening of the haemangioma lesion was also sooner and more significant in group A and C than in group B (P < 0.05). The mean reduction in the size was significant in group A and C at 3, 6 and 12 months while in group B it was significant only at 6 months. Groups B and C had higher number of complications.[46] From these reports, oral propranolol appears to be a better drug than steroids. However, prospective blinded RCTs will provide better understanding of its efficacy and safety.
Other systemic drugs
Other treatment options like interferon α, vincristine and cyclophosphamide are reserved for life-threatening haemangiomas which are unresponsive to conventional therapy.[47]
TOPICAL THERAPY
Timolol
Timolol is a non-selective β blocker which is used for increased intra-ocular pressure. In 2010, Guo and Ni first reported the clinical response to timolol 0.5% solution in a 4-month-old infant who had a thin haemangioma plaque on the upper eyelid.[48] Subsequently, several pilot studies and case series have shown its clinical efficacy.[49,50,51,52] It has been mostly used for localized, non-ulcerated superficial IHs. Chan et al. found timolol to be more effective for lesions with a mean diameter of <11.3 mm (i.e. 100 mm3 in volume) as compared to larger lesions.[53] It is to be applied two times a day. One drop of timolol maleate (0.5%) contains 0.25 mg of the drug.[53] It is recommended to apply 1 drop of the gel two times a day. Some authors recommend 3-4 times application.[52] Timolol does not penetrate deeply and hence it is not useful in deep haemangiomas.[52] Treatment is more effective in the proliferative phase than in the involution phase, and also, plaques respond better than nodules.[53]
Recently, the safety and efficacy of topical timolol has been studied in a blinded, randomized, placebo-controlled trial. The medications (topical timolol maleate gel 0.5% and placebo) were applied (1 drop) twice daily for 24 weeks. The response was periodically assessed. The authors observed that there was a significant improvement in absolute volume reduction, proportional growth and clinical appearance after 12-16 weeks of timolol therapy compared to the placebo group. No significant side effects were reported. The authors suggested that 2 drops per day application of topical timolol maleate 0.5 % gel is safe and effective therapy for IH which does not require systemic medications.[53]
Propranolol
Topical propranolol 1% ointment has been used successfully in the treatment of superficial IHs, especially as an adjuvant therapy during the wait-and-watch period. Kunzi-Rapp et al. in their series of 45 cases of superficial haemangiomas, used propranolol 1% ointment locally twice daily.[54] Of the 65 haemangiomas treated, 59% showed regression and 26% had cessation of growth. In 15% of the cases, no response was seen. Concomitant use of propranolol ointment with pulsed dye laser (PDL) in two of their cases of ulcerated haemangioma led to healing of ulcers in 3 and 6 weeks, respectively. In a retrospective chart review by Xu et al., 16 (57%) out of 28 haemangiomas demonstrated good response, 9 (33%) showed partial response and 3 (10%) had no response.[55] No side-effects have been reported so far.
Imiquimod
Imiquimod, an immune response modifier with anti-angiogenic and pro-apoptotic properties, has been used in the treatment of superficial IH. Imiquimod 5% cream is applied on alternate days at bed time and left for 8 hours. It is washed with mild soap the next morning. The duration of therapy is about 4 months. Severe inflammatory reactions may occur with application of imiquimod. Qiu et al. studied the efficacy and safety of imiquimod in their retrospective comparative study of imiquimod versus timolol in superficial proliferative IH. They found that the efficacy of imiquimod was comparable with timolol. However, inflammatory changes were seen in 13 of 20 (65%) cases in the imiquimod group while none of cases in the timolol group had side effects.[56]
Topical steroids
Potent steroids have been used to treat flat or minimally raised vascular plaques of IH particularly at sites prone to ulcerations and disfigurement. Described adverse effects include localised atrophy, hypopigmentation, hypertrichosis and infections. Garzon et al. retrospectively studied the clinical effects and safety of ultrapotent topical corticosteroids (clobetasol propionate 0.05% in 27 infants, betamethasone dipropionate 0.05% in 4 and halobetasol propionate in 3 cases) in 34 children. The treatment was given for a period ranging from 4 weeks to 21 weeks. They found that 35% showed good response, 38% partial response and 27% no response. The authors found good safety profile for lesions at all sites, including the peri-ocular region.[57] Following the successful reports of topical timolol, topical steroids are less often prescribed in the current practice.
Other modalities
Pulsed Dye Laser (PDL)
PDL has been used successfully for the treatment of ulcerated IH, which reduces the pain and promotes healing.[58] It is also used to remove the residual telangiectasia. However, the use of PDL in uncomplicated IH is controversial. There are non-randomized studies which claim that PDL is better than the wait-and-watch policy.[59,60] In a large prospective RCT by Batta et al., 121 children with uncomplicated early haemangiomas were assigned to PDL (585 nm) treatment or observation.[61] At the end of 1 year, there was no significant differences between the 2 groups (60 in PDL group, 61 in observation group) in terms of complete clearance or residual features. The adverse effects were significantly more in the PDL group which led the authors to conclude that PDL is no better than observation in uncomplicated superficial IH.[61] In another recent prospective RCT on 22 infants, the intervention group was treated with PDL (595 nm). The authors did not observe any difference in echo depth or surface area at 1 year between the intervention (n = 11) and observation groups (n = 11). However, the cosmetic outcome was significantly better in the PDL group.[62] In a comparative study between traditional PDL (585 nm) and long PDL (LPDL; 595 nm), the authors found that a similar number of infants achieved complete clearance or showed minimal residual signs at 1 year of age (14/26, 54% in PDL group vs. 17/26, 65% in LPDL group; P = 0.4), although the infants in LPDL group suffered significantly less side-effects like hypo- or hyperpigmentation, textural changes and the period of maximal proliferation was also significantly shortened (106 days in LPDL versus 177 days in PDL group).[63]
There is no consensus on the optimal settings of PDL and selection of type of haemangiomas suitable for treatment with laser. Currently, the use of PDL is confined to the treatment of ulceration and post-involution erythema and telangiectasias.[64]
Other lasers
Frequency-doubled Nd:YAG laser was found to be less effective than PDL in a retrospective study on 50 infants with superficial IH.[65] In another study, the use of sequential 595-nm PDL followed by 1064-nm Nd:YAG laser led to excellent response in 18 (72%) out of 25 infants with IH on skin and mucous membrane in the head and neck region.[66] Good results with intralesional therapy using KTP laser have been described in treating voluminous haemangiomas.[67]
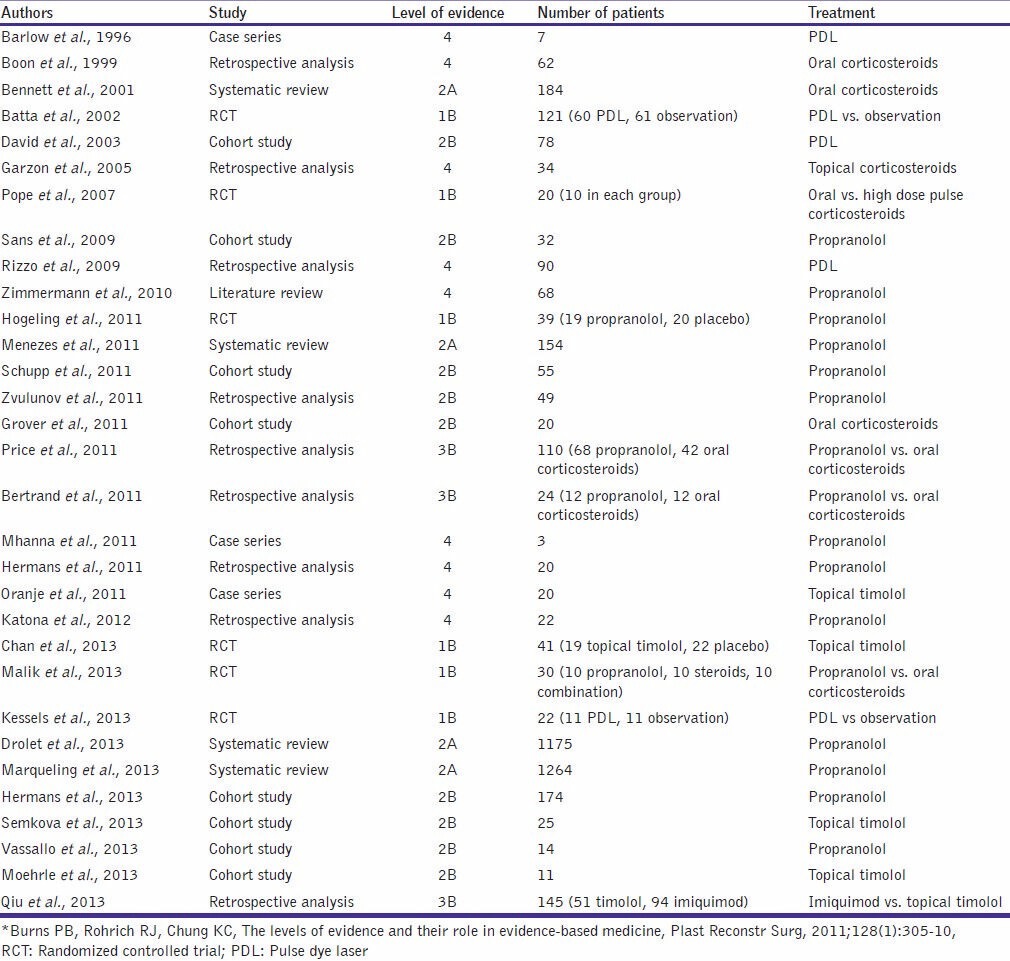
The level of evidence with respect to studies using various treatment modalities is given in Table 3.
Table 3.
Level of evidence: Therapeutic modalities*
Congenital haemangiomas
These are fully formed haemangiomas at birth. There are two sub-types: Rapidly involuting congenital haemangiomas (RICH) and non-involuting congenital haemangiomas (NICH). In both the forms, the proliferative phase occurs in utero. Unlike IH, these are GLUT 1 negative tumours. They manifest as erythematous to violaceous large hemispherical tumours and a peripheral halo of pallor. The surface may show telangiectasia. RICH completely involutes by 12-18 months of age [Figure 4a and 4b]. NICH lesions do not involute over time [Figure 4c and 4d] and hence may require surgical excision or embolization.
Figure 4.
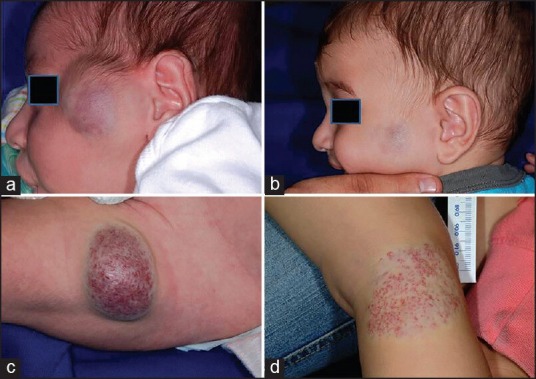
(a and b) Congenital haemangioma, (a) RICH at 1 month, (b) after resolution at 7 months of age, (c and d) NICH (courtesy of Anthony J Mancini)
CONCLUSION
IH are the most common benign vascular tumours. They are a cause of parental discomfort and anxiety and need to be carefully assessed from the treatment point of view. Most IH are uncomplicated and can be managed by active non-intervention alone. However, the recent encouraging reports of topical timolol in haemangiomas suggest that it can be advised even for uncomplicated localized lesions to accelerate the involution process. Systemic steroids have been considered the therapeutic mainstay for complicated haemangiomas for several decades. Recently, there have been several studies describing the efficacy and safety of propranolol in treating infantile haemangiomas. Though comparative studies between steroids and propranolol are few, a good therapeutic efficacy, even in the post-proliferation phase and a better side-effect profile has tilted the balance in favour of propranolol. Only future prospective trials involving a larger number of patients and a longer follow-up period will tell whether propranolol fulfils its therapeutic promise.
Q1. The maximum period of growth of infantile haemangiomas occurs at the following period:
First week after birth
Birth to 5 months
After 6 months
Birth to 1 year
Q2. PHACES syndrome is associated with facial segmental haemangioma and includes all except:
Posterior fossa abnormalities
Coarctation of the aorta
Sternal abnormalities
Oesophageal stenosis
Q3. The following infantile haemangiomas require treatment:
Ocular haemangiomas
Ulcerating haemangiomas
Nasal tip haemangiomas
All of the above
Q4. The first line of treatment of infantile haemangiomas is:
Systemic corticosteroids
Topical corticosteroids
Oral propranolol
Vincristine
Q5. Adverse effects of propranolol include all except:
Tachycardia
Hypoglycaemia
Bronchospasm
Hyperkalaemia
Answers online at www.jcasonline.com
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Li J, Chen X, Zhao S, Hu X, Chen C, Ouyang F, et al. Demographic and clinical characteristics and risk factors for infantile hemangioma: A Chinese case-control study. Arch Dermatol. 2011;147:1049–56. doi: 10.1001/archdermatol.2011.122. [DOI] [PubMed] [Google Scholar]
- 2.Kilcline C, Frieden IJ. Infantile hemangiomas: How common are they. A systematic review of the medical literature? Pediatr Dermatol. 2008;25:168–73. doi: 10.1111/j.1525-1470.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, et al. Hemangioma Investigator Group. Growth characteristics of infantile hemangiomas: Implications for management. Pediatrics. 2008;122:360–7. doi: 10.1542/peds.2007-2767. [DOI] [PubMed] [Google Scholar]
- 4.Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, et al. Prospective study of infantile hemangiomas: Clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–7. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 5.Metry DW, Hawrot A, Altman C, Frieden IJ. Association of solitary, segmental hemangiomas of the skin with visceral hemangiomatosis. Arch Dermatol. 2004;140:591–6. doi: 10.1001/archderm.140.5.591. [DOI] [PubMed] [Google Scholar]
- 6.Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: Clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567–76. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 7.Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics. 2013;131:128–40. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya JJ, Luo CB, Alvarez H, Rodesch G, Pongpech S, Lasjaunias PL. PHACES syndrome: A review of eight previously unreported cases with late arterial occlusions. Neuroradiology. 2004;46:227–33. doi: 10.1007/s00234-002-0902-z. [DOI] [PubMed] [Google Scholar]
- 9.Burrows PE, Robertson RL, Mulliken JB, Beardsley DS, Chaloupka JC, Ezekowitz RA, et al. Cerebral vasculopathy and neurologic sequelae in infants with cervicofacial hemangioma: Report of eight patients. Radiology. 1998;207:601–7. doi: 10.1148/radiology.207.3.9609880. [DOI] [PubMed] [Google Scholar]
- 10.Drolet BA, Dohil M, Golomb MR, Wells R, Murowski L, Tamburro J, et al. Early stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE association. Pediatrics. 2006;117:959–64. doi: 10.1542/peds.2005-1683. [DOI] [PubMed] [Google Scholar]
- 11.Orlow SJ, Isakoff MS, Blei F. Increased risk of symptomatic hemangiomas of the airway in association with cutaneous hemangiomas in a “beard” distribution. J Pediatr. 1997;131:643–6. doi: 10.1016/s0022-3476(97)70079-9. [DOI] [PubMed] [Google Scholar]
- 12.Drolet BA, Chamlin SL, Garzon MC, Adams D, Baselga E, Haggstrom AN, et al. Prospective study of spinal anomalies in children with infantile hemangiomas of the lumbosacral skin. J Pediatr. 2010;157:789–94. doi: 10.1016/j.jpeds.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 13.Dickie B, Dasgupta R, Nair R, Alonso MH, Ryckman FC, Tiao GM, et al. Spectrum of hepatic hemangiomas: Management and outcome. J Pediatr Surg. 2009;44:125–33. doi: 10.1016/j.jpedsurg.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Kulungowski AM, Alomari AI, Chawla A, Christison-Lagay ER, Fishman SJ. Lessons from a liver hemangioma registry: Subtype classification. J Pediatr Surg. 2012;47:165–70. doi: 10.1016/j.jpedsurg.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–9. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 16.Konrad D, Ellis G, Perlman K. Spontaneous regression of severe acquired infantile hypothyroidism associated with multiple liver hemangiomas. Pediatrics. 2003;112:1424–6. doi: 10.1542/peds.112.6.1424. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–9. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–13. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett ML, Fleischer AB, Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: An evidence-based evaluation. Arch Dermatol. 2001;137:1208–13. doi: 10.1001/archderm.137.9.1208. [DOI] [PubMed] [Google Scholar]
- 20.Grover C, Kedar A, Arora P, Lal B. Efficacy of oral prednisolone use in the treatment of infantile hemangiomas in Indian children. Pediatr Dermatol. 2011;28:502–6. doi: 10.1111/j.1525-1470.2011.01491.x. [DOI] [PubMed] [Google Scholar]
- 21.Pope E, Krafchik BR, Macarthur C, Stempak D, Stephens D, Weinstein M, et al. Oral versus high-dose pulse corticosteroids for problematic infantile hemangiomas: A randomized, controlled trial. Pediatrics. 2007;119:e1239–47. doi: 10.1542/peds.2006-2962. [DOI] [PubMed] [Google Scholar]
- 22.Boon LM, MacDonald DM, Mulliken JB. Complications of systemic corticosteroid therapy for problematic hemangioma. Plast Reconstr Surg. 1999;104:1616–23. doi: 10.1097/00006534-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 23.George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Arch Dermatol. 2004;140:963–9. doi: 10.1001/archderm.140.8.963. [DOI] [PubMed] [Google Scholar]
- 24.Lomenick JP, Reifschneider KL, Lucky AW, Adams D, Azizkhan RG, Woo JG, et al. Prevalence of adrenal insufficiency following systemic glucocorticoid therapy in infants with hemangiomas. Arch Dermatol. 2009;145:262–6. doi: 10.1001/archdermatol.2008.572. [DOI] [PubMed] [Google Scholar]
- 25.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo J-B, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 26.Menezes MD, McCarter R, Greene EA, Bauman NM. Status of propranolol for treatment of infantile hemangioma and description of a randomized clinical trial. Ann Otol Rhinol Laryngol. 2011;120:686–95. doi: 10.1177/000348941112001010. [DOI] [PubMed] [Google Scholar]
- 27.Marqueling AL, Oza V, Frieden IJ, Puttgen KB. Propranolol and infantile hemangiomas four years later: A systematic review. Pediatr Dermatol. 2013;30:182–91. doi: 10.1111/pde.12089. [DOI] [PubMed] [Google Scholar]
- 28.Mhanna A, Franklin WH, Mancini AJ. Hepatic infantile hemangiomas treated with oral propranolol--a case series. Pediatr Dermatol. 2011;28:39–45. doi: 10.1111/j.1525-1470.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 29.Sans V, de la Roque ED, Berge J, Grenier N, Boralevi F, Mazereeuw-Hautier J, et al. Propranolol for severe infantile hemangiomas: Follow-up report. Pediatrics. 2009;124:e423–31. doi: 10.1542/peds.2008-3458. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann AP, Wiegand S, Werner JA, Eivazi B. Propranolol therapy for infantile haemangiomas: Review of the literature. Int J Pediatr Otorhinolaryngol. 2010;74:338–42. doi: 10.1016/j.ijporl.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Hermans DJ, Bauland CG, Zweegers J, van Beynum IM, van der Vleuten CJ. Propranolol in a case series of 174 patients with complicated infantile haemangioma: Indications, safety and future directions. Br J Dermatol. 2013;168:837–43. doi: 10.1111/bjd.12189. [DOI] [PubMed] [Google Scholar]
- 32.Schupp CJ, Kleber JB, Günther P, Holland-Cunz S. Propranolol therapy in 55 infants with infantile hemangioma: Dosage, duration, adverse effects, and outcome. Pediatr Dermatol. 2011;28:640–4. doi: 10.1111/j.1525-1470.2011.01569.x. [DOI] [PubMed] [Google Scholar]
- 33.Katona G, Csákányi Z, Gács E, Szalai Z, Ráth G, Gerlinger I. Propranolol for infantile haemangioma: Striking effect in the first weeks. Int J Pediatr Otorhinolaryngol. 2012;76:1746–50. doi: 10.1016/j.ijporl.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Vassallo P, Forte R, Di Mezza A, Magli A. Treatment of infantile capillary hemangioma of the eyelid with systemic propranolol. Am J Ophthalmol. 2013;155:165–70.e2. doi: 10.1016/j.ajo.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:e259–66. doi: 10.1542/peds.2010-0029. [DOI] [PubMed] [Google Scholar]
- 36.Zvulunov A, McCuaig C, Frieden IJ, Mancini AJ, Puttgen KB, Dohil M, et al. Oral propranolol therapy for infantile hemangiomas beyond the proliferation phase: A multicenter retrospective study. Pediatr Dermatol. 2011;28:94–8. doi: 10.1111/j.1525-1470.2010.01379.x. [DOI] [PubMed] [Google Scholar]
- 37.O'Loughlin A, O'Donnell BF, Watson R. Mature infantile haemangiomas role for propranolol. J Eur Acad Dermatol Venereol. 2011;25:1363–4. doi: 10.1111/j.1468-3083.2010.03915.x. [DOI] [PubMed] [Google Scholar]
- 38.Aletaha M, Salour H, Bagheri A, Raffati N, Amouhashemi N. Successful treatment of orbital hemangioma with propranolol in a 5-year-old girl. Orbit. 2012;31:18–20. doi: 10.3109/01676830.2011.604899. [DOI] [PubMed] [Google Scholar]
- 39.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthetic Surg. 2011;64:445–51. doi: 10.1016/j.bjps.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Talaat AA, Elbasiouny MS, Elgendy DS, Elwakil TF. Propranolol treatment of infantile hemangioma: Clinical and radiologic evaluations. J Pediatr Surg. 2012;47:707–14. doi: 10.1016/j.jpedsurg.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 41.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–74. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 42.Price CJ, Lattouf C, Baum B, McLeod M, Schachner LA, Duarte AM, et al. Propranolol vs corticosteroids for infantile hemangiomas: A multicenter retrospective analysis. Arch Dermatol. 2011;147:1371–6. doi: 10.1001/archdermatol.2011.203. [DOI] [PubMed] [Google Scholar]
- 43.Hermans DJ, van Beynum IM, Schultze Kool LJ, van de Kerkhof PC, Wijnen MH, van der Vleuten CJ. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: A study of 20 cases with matched historical controls. J Am Acad Dermatol. 2011;64:833–8. doi: 10.1016/j.jaad.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 44.Caussé S, Aubert H, Saint-Jean M, Puzenat E, Bursztejn AC, Eschard C, et al. Groupe de Recherche Clinique en Dermatologie Pédiatrique. Propranolol-resistant infantile haemangiomas. Br J Dermatol. 2013;169:125–9. doi: 10.1111/bjd.12417. [DOI] [PubMed] [Google Scholar]
- 45.Bertrand J, McCuaig C, Dubois J, Hatami A, Ondrejchak S, Powell J. Propranolol versus prednisone in the treatment of infantile hemangiomas: A retrospective comparative study. Pediatr Dermatol. 2011;28:649–54. doi: 10.1111/j.1525-1470.2011.01551.x. [DOI] [PubMed] [Google Scholar]
- 46.Malik MA, Menon P, Rao KL, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: A randomized controlled study. J Pediatr Surg. 2013;48:2453–9. doi: 10.1016/j.jpedsurg.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Miller T, Frieden IJ. Hemangiomas: New insights and classification. Pediatr Ann. 2005;34:179–87. doi: 10.3928/0090-4481-20050301-07. [DOI] [PubMed] [Google Scholar]
- 48.Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255–6. doi: 10.1001/archophthalmol.2009.370. [DOI] [PubMed] [Google Scholar]
- 49.Semkova K, Kazandjieva J. Topical timolol maleate for treatment of infantile haemangiomas: Preliminary results of a prospective study. Clin Exp Dermatol. 2013;38:143–6. doi: 10.1111/j.1365-2230.2012.04425.x. [DOI] [PubMed] [Google Scholar]
- 50.Calvo M, Garcia-Millán C, Villegas C, Fueyo-Casado A, Burón I. Topical timolol for infantile hemangioma of the eyelid. Int J Dermatol. 2013;52:603–4. doi: 10.1111/j.1365-4632.2011.05290.x. [DOI] [PubMed] [Google Scholar]
- 51.Moehrle M, Léauté-Labrèze C, Schmidt V, Röcken M, Poets CF, Goelz R. Topical timolol for small hemangiomas of infancy. Pediatr Dermatol. 2013;30:245–9. doi: 10.1111/j.1525-1470.2012.01723.x. [DOI] [PubMed] [Google Scholar]
- 52.Oranje AP, Janmohamed SR, Madern GC, de Laat PC. Treatment of small superficial haemangioma with timolol 0.5% ophthalmic solution: A series of 20 cases. Dermatology. 2011;223:330–4. doi: 10.1159/000334778. [DOI] [PubMed] [Google Scholar]
- 53.Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics. 2013;131:e1739–47. doi: 10.1542/peds.2012-3828. [DOI] [PubMed] [Google Scholar]
- 54.Xu G, Lv R, Zhao Z, Huo R. Topical propranolol for treatment of superficial infantile hemangiomas. J Am Acad Dermatol. 2012;67:1210–3. doi: 10.1016/j.jaad.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Kunzi-Rapp K. Topical propranolol therapy for infantile hemangiomas. Pediatr Dermatol. 2012;29:154–9. doi: 10.1111/j.1525-1470.2011.01615.x. [DOI] [PubMed] [Google Scholar]
- 56.Qiu Y, Ma G, Yang J, Hu X, Chen H, Jin Y, et al. Imiquimod 5% cream versus timolol 0.5% ophthalmic solution for treating superficial proliferating infantile haemangiomas: A retrospective study. Clin Exp Dermatol. 2013;38:845–50. doi: 10.1111/ced.12150. [DOI] [PubMed] [Google Scholar]
- 57.Garzon MC, Lucky AW, Hawrot A, Frieden IJ. Ultrapotent topical corticosteroid treatment of hemangiomas of infancy. J Am Acad Dermatol. 2005;52:281–6. doi: 10.1016/j.jaad.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 58.David LR, Malek MM, Argenta LC. Efficacy of pulse dye laser therapy for the treatment of ulcerated haemangiomas: A review of 78 patients. Br J Plast Surg. 2003;56:317–27. doi: 10.1016/s0007-1226(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 59.Rizzo C, Brightman L, Chapas AM, Hale EK, Cantatore-Francis JL, Bernstein LJ, et al. Outcomes of childhood hemangiomas treated with the pulsed-dye laser with dynamic cooling: A retrospective chart analysis. Dermatol Surg. 2009;35:1947–54. doi: 10.1111/j.1524-4725.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 60.Barlow RJ, Walker NP, Markey AC. Treatment of proliferative haemangiomas with the 585 nm pulsed dye laser. Br J Dermatol. 1996;134:700–4. [PubMed] [Google Scholar]
- 61.Batta K, Goodyear HM, Moss C, Williams HC, Hiller L, Waters R. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: Results of a 1-year analysis. Lancet. 2002;360:521–7. doi: 10.1016/S0140-6736(02)09741-6. [DOI] [PubMed] [Google Scholar]
- 62.Kessels JP, Hamers ET, Ostertag JU. Superficial hemangioma: Pulsed dye laser versus wait-and-see. Dermatol Surg. 2013;39:414–21. doi: 10.1111/dsu.12081. [DOI] [PubMed] [Google Scholar]
- 63.Kono T, Sakurai H, Groff WF, Chan HH, Takeuchi M, Yamaki T, et al. Comparison study of a traditional pulsed dye laser versus a long-pulsed dye laser in the treatment of early childhood hemangiomas. Lasers Surg Med. 2006;38:112–5. doi: 10.1002/lsm.20257. [DOI] [PubMed] [Google Scholar]
- 64.Frieden IJ. Which hemangiomas to treat--and how? Arch Dermatol. 1997;133:1593–5. [PubMed] [Google Scholar]
- 65.Raulin C, Greve B. Retrospective clinical comparison of hemangioma treatment by flashlamp-pumped (585 nm) and frequency-doubled Nd:YAG (532 nm) lasers. Lasers Surg Med. 2001;28:40–3. doi: 10.1002/1096-9101(2001)28:1<40::AID-LSM1014>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 66.Saafan AM, Salah MM. Using pulsed dual-wavelength 595 and 1064 nm is more effective in the management of hemangiomas. J Drugs Dermatol. 2010;9:310–4. [PubMed] [Google Scholar]
- 67.Burstein FD, Williams JK, Schwentker AR, Nahai F. Intralesional laser therapy treatment for hemangiomas: Technical evolution. J Craniofac Surg. 2006;17:756–60. doi: 10.1097/00001665-200607000-00029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


