Abstract
The GABAB agonist baclofen has been widely researched clinically and preclinically as a treatment of alcohol use disorders (AUDs). However, the efficacy of baclofen remains uncertain. The clinically used racemic compound can be separated into separate enantiomers. These enantiomers have produced different profiles in behavioral assays, with the S- compound often being ineffective compared to the R- compound, or the S- compound antagonizing the effects of the R- compound. We have previously demonstrated that the R(+)-baclofen enantiomer decreases binge-like ethanol intake in the Drinking-in-the-Dark (DID) paradigm, whereas the S(-)-baclofen enantiomer increases ethanol intake. One area implicated in drug abuse is the nucleus accumbens shell (NACsh).The current study sought to define the role of the NACsh in the enantioselective effects of baclofen on binge-like ethanol consumption by directly microinjecting each enantiomer into the structure. Following bilateral cannulation of the NACsh, C57Bl/6J mice were given 5 days of access to ethanol or saccharin for 2 hours, 3 hours into the dark cycle. On Day 5 mice were given an injection of aCSF, 0.02 R(+)-, 0.04R(+)-, 0.08 S(-)-, or 0.16 S(-)-baclofen (μg/side dissolved in 200nl of aCSF). It was found that the R(+)-baclofen dose-dependently decreased ethanol consumption, whereas the high S(-)-baclofen dose increased ethanol consumption, compared to the aCSF group. Saccharin consumption was not affected. These results further confirm that GABAB receptors and the NACsh shell are integral in mediating ethanol intake. They also demonstrate that baclofen displays bidirectional, enantioselective effects which are important when considering therapeutic uses of the drug.
Keywords: Nucleus accumbens shell, baclofen, Drinking-in-the-Dark, ethanol, binge-like, GABAB receptor
1. Introduction
Dopaminergic and GABAergic projections to and from the nucleus accumbens shell (NACsh1) to areas including the ventral tegmental area (VTA) and ventral pallidus [1-4] have been implicated in addiction. Specifically, the NACsh has been implicated in stimulant reinforcement [5]. The “incentive arousal” view of dopamine response suggests that accumbal dopamine is not responsible for the behavioral reaction to the stimuli, but rather works as an amplifier signal for the stimuli – moderating whether or not the stimuli result in a behavioral response [6]. Bassareo et al. [7] showed that ethanol and a chocolate + sucrose tastant both elicit a dopaminergic response from the NACsh upon an animal's first experience with the reinforcers. However, upon second presentation of the reinforcers, although the chocolate + sucrose tastant no longer elicited a dopaminergic response, the ethanol reinforcer elicited an even greater dopaminergic response in the NACsh. Dopaminergic habituation to a tastant and maladaptive dopaminergic responses to drugs of abuse have not been associated with the nucleus accumbens core (NACcore) or prefrontal cortex (PFC)[8-10], making the NACsh a particularly important region for eliciting a response to a drugs of abuse [6].
Ethanol interacts with GABAergic neurons of the NACsh. 80% of ethanol reactive cells in the NACsh are GABAergic; a higher percentage than the NACcore and PFC [11]. However, to date, few studies have attempted to alter ethanol intake in preclinical models of consumption by manipulation of NACsh GABAergic function. Rewal et al. [12] and Nie et al. [13] demonstrated that viral knock-down of extrasynaptic GABAA receptor subunits in the NACsh, but not NACcore, reduced ethanol intake without affecting saccharin intake. While these results suggest a role for GABAA receptors, GABAB receptors have not been investigated, even though they comprise and entire third of the GABA receptors of the NAC [14].
Baclofen, a GABAB receptor agonist used to treat severe forms of epilepsy, has been repeatedly shown to alter ethanol consumption. Clinically, baclofen has been used to effectively treat symptoms of alcohol use disorders (AUDs), such as craving and anxiety, in European populations [15]. However, the results in U.S. populations have not been as conclusive [16]. These conflicting results may be due to different alcoholic populations, with European studies often focusing on a population of severe AUD sufferers. A U.S. study examining hospital inpatients at a high risk for alcohol withdrawal syndrome did find baclofen to be effective at reducing these symptoms [17].
Baclofen has also produced inconsistent results preclinically. For example, baclofen decreased operant responding for ethanol [18, 19], lowered the breakpoint for ethanol in alcohol preferring rats [20], and decreased intake of ethanol [21, 22] when administered systemically. Baclofen also decreased binge-like ethanol consumption when microinjected directly into the anterior, but not posterior, VTA [23]. Conversely, baclofen has also been demonstrated to increase ethanol consumption following acute and chronic systemic injections [24-26].
The baclofen that is used clinically is a racemic compound that breaks down into absolute configurations of R- and S- and positive (+) and negative (-) molecular rotations. We have observed enantioselective effects of baclofen on the binge-like ethanol consumption produced by Drinking-in-the-Dark (DID); R(+)-baclofen reduced drinking whereas S(-)-baclofen increased drinking (Kasten et al., submitted). Enantioselective effects of baclofen have also been demonstrated in other behavioral paradigms, with R-baclofen often being more behaviorally active. Olpe et al. [27] quantified the effects of R-, S-, and racemic baclofen on reflexes and electro-shock seizures. S- was completely ineffective, even at the highest dose, whereas R- was equally effective as the racemic compound. Paredes and Agmo [28] demonstrated that S(-)-baclofen was ineffective at inhibiting sexual behavior, whereas R-baclofen was twice as effective as the racemic compound. S-baclofen has been demonstrated to depress the trigeminal nucleus oralis response, but at a dose 20 times larger than the least effective R-dose [29]. In the periphery, S-baclofen reduced the response to R-baclofen [29]. While Olpe et al. [27] suggest that S-baclofen may play a role in potentiating the action of R-baclofen without being active itself, Fromm et al. [29] show that S-baclofen is not only active at higher doses, but that it also may inhibit the actions of R- in certain cases. Collectively, these studies show that the behavioral effects of baclofen are enantiomer dependent, and highlight the importance of considering enantioselective aspects of drug effects when accounting for the therapeutic use of the drug [30].
A second aspect of considering therapeutic potential is choosing an appropriate model of behavior to test the drug [29]. One of many such models is DID, which has been used extensively in our lab to test drug treatments via systemic and microinjection approaches [23, 31-33]. Developed by Rhodes et al. [34, 35], DID achieves high levels of binge-like drinking by introducing ethanol three hours into the dark cycle with a two hour access period. In a relatively short period of time, B6 mice reliably ingest enough ethanol to reach pharmacologically relevant BECs of over 1mg/ml without introducing food or water restrictions. As such, DID presents a simple model that can be used to screen potential AUD treatments that target various receptor systems, including the GABA, endocannabinoid, glutamate, and dopamine receptor systems [23, 32, 36].
We hypothesize that microinjection of R(+)-baclofen into the NACsh will reduce ethanol intake, but that microinjection of S(-)-baclofen will increase ethanol intake, in the binge-like DID paradigm. Further, we hypothesize that microinjection of baclofen into the NACsh will not affect saccharin intake, as the NACsh no longer releases dopamine in response to a second exposure to a saccharin reinforcer [7].
2. Method
2.1 General Design
In brief, male B6 mice underwent surgery for bilateral cannulation of the NACsh. Following at least 48 hours of recovery, mice began a five-day DID procedure where 20% ethanol or 0.2% saccharin was available for two hours, three hours into the dark period each day. On Day 5, animals received one of five possible microinjection doses; 0.02 or 0.04μg R(+)-baclofen, 0.08 or 0.16μg S(-)-baclofen, or artificial cerebrospinal fluid (aCSF) (Fig. 1).
Figure 1.
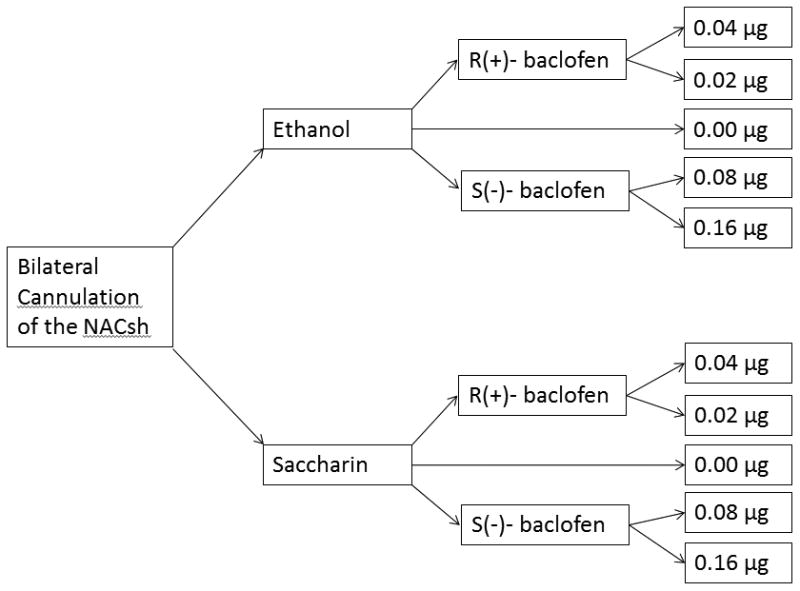
Study design.
2.2 Subjects
Male B6 mice were bred in-house. Breeders for our colony were obtained from Jackson Laboratory (Bar Harbor, ME) and replaced every few months by new breeder pairs purchased from Jackson Laboratory. A total of 118 animals were used in this study. One aCSF control group (dose = 0.0 μg) was used for each reinforcer (ethanol or saccharin) to reduce the number of animals associated with this project (see Fig. 1).
Animals received food at all times and water ad lib apart from during implementation of DID. Lights were kept on a reverse light-dark schedule with lights off at 8am. Animals were group housed until the time of surgery, after which time they were individually housed. Animals were at least 58 days of age at the time of surgery, and at least 60 days of age at the time DID was initiated. Procedures were approved by the IUPUI School of Science Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (The National Academic Press, 2003).
2.3 Drugs
Ethanol (190 proof) was obtained from Pharmco, Inc (Brookfield, CT). The 20% v/v ethanol solution was prepared with tap water. Saccharin (≥99%) was obtained from Sigma Aldrich (St. Louis, MO) and was dissolved in tap water to make a 0.2% saccharin solution. R(+)- and S(-)-baclofen was obtained from Sigma Aldrich (St. Louis, MO). Baclofen was dissolved in aCSF (Sigma Aldrich, St. Louis, MO) to doses of 0.02 and 0.04 μg for R(+)-baclofen, and 0.08 and 0.16 μg for S(-)-baclofen. Unadulterated aCSF was used as the 0.0 μg control group. Drug was delivered in a volume of 200 nl per side at a flow rate of 382 nl/min.
Drug doses were chosen with the goal of finding the highest dose that did not affect ethanol intake, as well as the lowest dose that did cause an effect on ethanol intake for each enantiomer. Initial R(+)-baclofen doses for this study were based on those previously used by our lab [23,47]. Pilot doses of 0.01, 0.02, and 0.04 μg of R(+)-baclofen suggested that 0.04 μg/side would be the lowest dose to reduce ethanol intake, whereas 0.02 μg/side would be the highest dose that would not affect intake. S(-)-baclofen doses were expected to be higher than R(+)-baclofen doses, because S(-)- is the less potent enantiomer. Piloting appropriate doses for S(-)-baclofen began by using a high dose of 0.32 μg/side. This dose appeared to incapacitate the animals, and was therefore cut in half to 0.16 μg/side. The 0.16 μg/side appeared to potentially increase drinking in the pilot group, and was kept as one of the two final doses. To choose the second final dose, 0.08 μg/side and 0.24 μg/side doses were piloted. The 0.24 μg/side did not appear to increase ethanol intake more than the 0.16 μg/side dose, therefore the 0.08 μg/side dose was chosen because it appeared to be the highest dose that would not affect intake. All pilot groups had an n=3. Mice that were used to pilot successful doses were included in the final analyses.
2.4 Surgery
The NACsh was targeted (coordinates of ML: ±0.63, AP: 1.18, DV: 2.0) by bilateral cannulation. Surgical procedures followed those currently outlined in our approved IACUC protocol. Detailed surgical procedures are described in our previous publications [23, 32, 33].
2.5 DID Procedures
Following surgery, animals had at least 48 hours of recovery before initiation of DID. During the recovery time, the cannula stylets were removed daily to prevent obstruction. Although daily restraint was necessary to remove the stylets, timed restraint to habituate the animal to the microinjection procedure began on Day 3 of DID. Stylet removal and restraint occurred immediately prior to the bottles-on time to acclimate animals to any restraint stress associated with the microinjection procedure.
DID procedures were slightly modified from that of Rhodes et al. [34, 35] and Moore et al. [31]. Three hours into the dark cycle animals received two hours of access to either a 20% ethanol solution or a 0.2% saccharin solution in 10mL ball-bearing sipper tubes for five days, with access to only water for the remaining 22 hours. The saccharin group was included as a control group for drug effects on alternative reinforcers. Fluid leak was monitored by recording fluid levels of bottles placed on empty cages on the animal rack. Fluid levels were recorded to the level of 0.05 mls.
Restraint took place on Day 3 and mock microinjections took place on Day 4. The mock microinjection procedure consisted of removing the stylets and inserting mock microinjectors into the cannula. The mock injectors reached 1mm into the brain beyond the end of the cannula. They were held in place for one minute to mimic the microinjection procedure. Drug was administered via microinjection on Day 5. R(+)-baclofen was microinjected with the hypothesis that it would reduce ethanol consumption. S(-)-baclofen was microinjected with the hypothesis that it would increase ethanol consumption. On Days 1-3, fluid volumes were recorded immediately before and after fluid access. On Days 4 and 5, fluid volumes were recorded immediately before and after fluid access, as well as every 20 minutes during fluid access to capture the time course of the drug effects on Day 5.
2.6 Intra-NACsh Microinjections
Details on construction of guide cannulae, stylets, and microinjection tubing as well as the microinjection procedure are detailed in our previous publications [23, 32, 33].
2.7 Blood Ethanol Concentrations
Immediately following ethanol access on day 5, retro-orbital sinus blood samples were collected using 50 μL heparinized microcapillary tubes. Samples were immediately placed in the centrifuge and plasma and decanted. The samples were stored at −80°C until time of assay, at which point they were analyzed for ethanol content using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
2.8 Histological verification of Cannulae Placement
Immediately following the end of drinking on Day 5, animals were euthanized by cervical dislocation and brains were extracted and flash-frozen using methylbutane (−25°C). Brains were stored in the −20°C freezer until they were sliced using a cryostat, thaw-mounted on slides, thionin stained, and cover slipped. Placement of cannulae was inspected and verified by two different experimenters that were blind to the dose of baclofen given and amount of reinforcer consumed. A representative bilateral hit of the NACsh is shown in Fig. 2.
Figure 2.
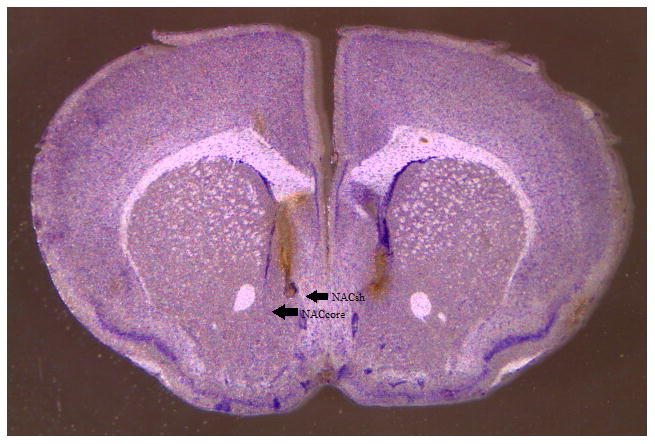
Histological representation of accurate cannulae placement in the nucleus accumbens shell.
2.9 Statistical Analysis
Statistical analyses only included animals that made it through all five days of drinking and that had histologically verified bilateral hits of the NACsh. Final sample sizes were N = 44 (84.62% hit rate) for the ethanol analyses and N = 42 (85.71% hit rate) for the saccharin analyses. Ethanol and saccharin results were analyzed separately. Within the ethanol and saccharin groups, R(+)- and S(-)-baclofen were analyzed together because they shared a control group. Significance level for all overall analyses was set at p < .05, with all post-hoc tests corrected for multiple comparisons.
Drinking acquisition on Days 1-4 was assessed using a one-way analysis of variance (ANOVA) with day as the within-subject factor. Tukey's post-hoc tests were used when warranted. A one-way ANOVA was used to analyze total Day 4 drinking with treatment as the between-subjects factor to verify that total baseline drinking following mock microinjection did not vary between groups. Following this verification, treatment effects on total Day 5 drinking were assessed using a one-way ANOVA with treatment as the between-subjects factor. For the ethanol animals, a one-way ANOVA with treatment as the between-subjects factor was also used to assess dose effects on BEC following intake, and a bivariate Pearson's correlation was used to compare total intake to BEC. Independent samples t-tests were used for all necessary post-hoc tests on total 2hr consumption and BEC data. Post-hoc tests were corrected for multiple comparisons for each drug dose compared to the control.
To compare time course of intake on micro- versus mock-injection day, a two-way repeated measures ANOVA with Day and Time-Bin as within-subjects factors for each treatment group independently was used. Paired samples t-tests were used as post-hoc tests for repeated measures analyses. To assess for treatment-effects on time course of intake, a two-way repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor was used. Day 4 intake was assessed first to verify that there were no baseline differences in time course between each dose group. Following this verification, Day 5 data was assessed. One-way ANOVAs were used at each time-point with treatment as the between subjects factor as post-hoc tests for a significant two-way ANOVA. Tukey's post-hoc tests were used for any significant one-way ANOVA effect.
3. Results
3.1 Ethanol Consumption
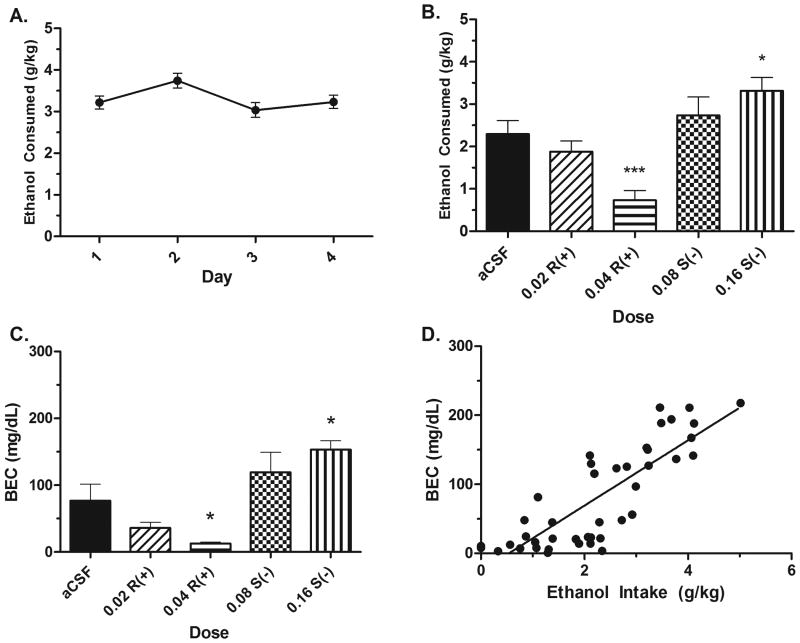
The ANOVA on Days 1-4 acquisition of drinking revealed a significant effect; F(3,175) = 3.21, p < .05 (Fig. 3A). Tukey's post-hoc revealed that Day 2 drinking was significantly higher than Day 3 drinking (p < .05). Total Day 4 drinking following the mock injection did not differ between groups; F(4,43) = .322, p > .05, data not shown. There were significant treatment effects on total Day 5 ethanol intake following the microinjection; F(4,43) = 10.14, p < .001. Independent samples t-tests corrected for multiple comparisons for each treatment to the aCSF group revealed that the 0.04 μg R(+)-baclofen group drank significantly less than the aCSF group, whereas the 0.16 μg S(-)-baclofen group drank significantly more than the aCSF group (p's < .05) (Fig. 3B). This pattern was replicated in the BEC data, with the 0.04 R(+)-baclofen group showing significantly lower BECs than the aCSF group, while the 0.16 S(-)-baclofen group showed significantly higher BECs immediately following ethanol intake (p's < .05) (Fig. 3C). BECs and total two hour ethanol consumption were strongly correlated; r(43) = 0.834, p < .001 (Fig. 3D).
Figure 3.
Microinjection of R(+)- and S(-)-baclofen into the NACsh bidirectionally affects ethanol consumption. A) Acquisition of ethanol drinking. B) Total two hour ethanol consumption is reduced by a 0.04 R(+)-baclofen dose, but increased by a 0.16 S(-)- baclofen dose compared to an aCSF microinjection. C) This bidirectional effect is also represented in the BECs immediately following intake on Day 5. D) BECs were strongly and significantly correlated with total ethanol intake. Asterisk (*) indicates significantly different than the aCSF group at p < .05. Asterisk (***) indicates significantly different than the aCSF group at p < .001. n's = 8-9 per group.
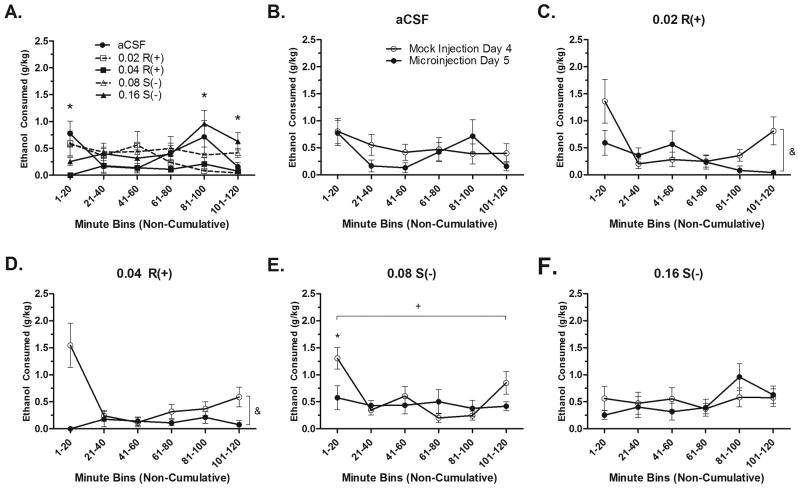
The time course of ethanol intake was then statistically compared. For both analyses, Greenhouse-Geisser statistics are reported because Mauchly's Test for Sphericity was significant (p's < .05). A treatment*time bin repeated measures ANOVA on Day 4 time course intake verified that there were no baseline differences between dose groups; F(11.38, 110.90) = 1.169; p > .05, data not shown. The time course of ethanol intake following micro-injection on Day 5 was significantly different between treatment groups; F(16.12, 153.18) = 1.931, p < .05. One-way ANOVAs at each time-point revealed that differences between treatment existed at the 1-20, 81-100, and 101-120 minute time bins (p's < .05). Tukey's post-hoc tests revealed that at 1-20 minutes, the 0.04 μg R(+)- group drank significantly less than the aCSF group (p < .05). At 81-100 minutes, the 0.16 μg S(-)- group drank significantly more than the 0.02 and 0.04 μg R(+)-groups (p's < .05). At the 101-120 minute time point, the 0.16 μg S(-)- group drank significantly more than the aCSF, 0.02, and 0.04 μg R(+)- groups (p's < .05) (Fig. 4A).
Figure 4.
Time-course of ethanol intakes. A) Following the microinjection, the time course of ethanol intake was significantly different between dose groups. Asterisk (*) indicates a significant post-hoc ANOVA at that time point, p < .05. Time course of ethanol intake following the mock and microinjections for the aCSF (n=8) (B), R(+)- 0.02 (n=9) (C), R(+)- 0.04 (n=9) (D), S(-)- 0.08 (n=9) (E), and S(-)- 0.16 (n=9) (F) groups. Asterisk (*) indicates a significant difference between the mock and microinjection intake at that time point, p < .05. Plus sign (+) indicates a main effect of time, p < .05. Ampersand (&) indicates a main effect of day, p < .05.
Repeated measures ANOVAs comparing the time course of drinking on Days 4 and 5 for each dose independently were used to systematically assess the treatment effect of the microinjection on drinking. These analyses revealed no main effects of time or day, nor was there an interaction for the aCSF and 0.16 S(-)- groups (p's > .05) (Fig. 4B & 4F). However, the 0.02 R(+)- group showed a main effect of day and time, with animals drinking less on the microinjection day and drinking being lower in the middle of the drinking session (p's < .05). There would have been an effect of day*time bin, except Mauchly's Test for Sphericity was significant (p = .049) and Greenhouse-Geisser statistics indicated no interaction was present; F(1.99,15.918) = 2.99, p = .079 (Fig. 4C). There was also a main effect of day for the 0.04 R(+)-group, with animals drinking less overall on the microinjection day (p < .05). There was no main effect of time (p >.05). Again, a significant interaction of Day*Time bin was reduced by a significant Mauchly's Test for Sphericity (p < .05), with the Greenhouse-Geisser correction only trending towards significant; F(1.858,13.077) = 3.591, p = .06 (Fig. 4D). Analysis of the 0.08 S(-)- data only revealed a significant main effect of time, with higher drinking in the first (1-20) and last (101-120) 20 minute bins (p < .05). There was also an interaction of day*time; F(5,40) = 3.635, p < .01. Post-hoc paired samples t-tests revealed that drinking on the microinjection day was significantly lower only at the 1-20 minute point; t(8) = 4.218, p < .01 (Fig. 4E).
3.2 Saccharin Consumption
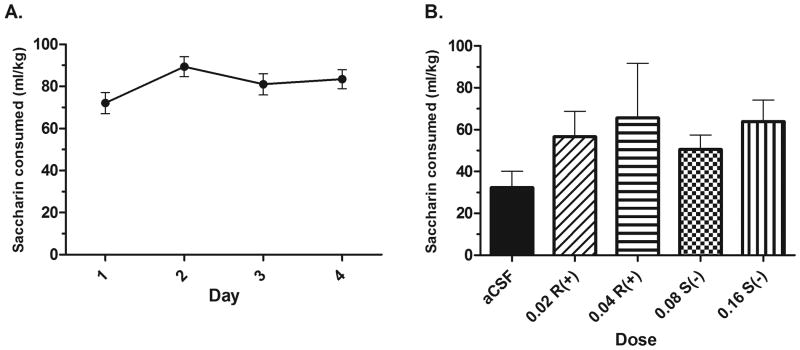
The ANOVA on Days 1-4 acquisition data revealed no significant effect of day on acquisition of drinking; F(3,45) = 2.206, p > .05 (Fig. 5A). Total two-hour saccharin drinking on Day 4 revealed no baseline differences in drinking between treatment groups; F(4,41) = 2.043, p > .05, data not shown. There were no effects of drug treatment on total Day 5 drinking; F(4,41) = 0.981, p > .05 (Fig 5B).
Figure 5.
Effects of microinjection of R(+)- and S(-)-baclofen in the NACsh on saccharin intake. A) Acquisition of saccharin intake. B) Microinjections of R(+)- and S(-)- baclofen do not alter ethanol intake compared to a microinjection of aCSF (n's = 7-10 per group).
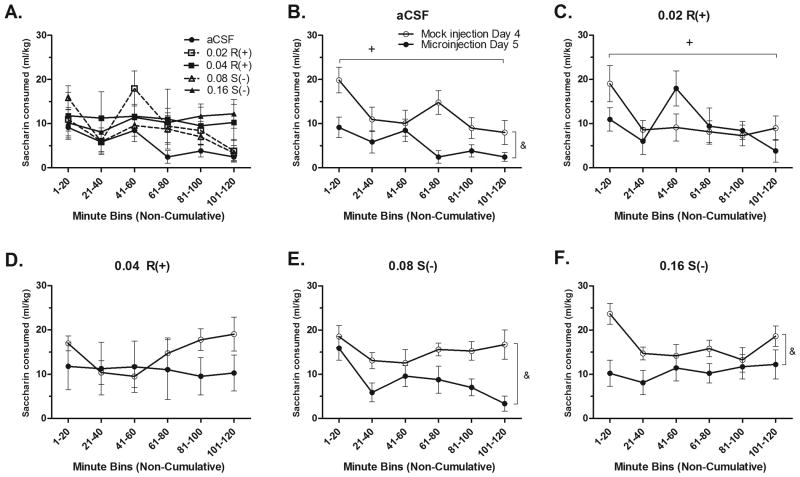
The time course of saccharin intake was then statistically compared using a two-way repeated measures ANOVA on Days 4 and 5. There were no baseline differences in the time course of saccharin intake on Day 4; F(13.85,128.14) = 1.194, p > .05, data not shown. Further, there were no differences in time course of intake on Day 5; F(14.89,137.74) = 1.096, p > .05 (Fig. 6A). Greenhouse-Geisser statistics are reported for both of these repeated measures ANOVAs, as Mauchly's Test of Sphericity was significant for each test (p's < .01).
Figure 6.
Time-course of saccharin intakes. A) Following the microinjection, the time course of saccharin intake was not significantly different between dose groups. Time course of saccharin intake following mock and microinjections for the aCSF (n=9) (B), R(+)- 0.02 (n=7) (C), R(+)-0.04 (n=8) (D), S(-)- 0.08 (n=10) (E), S(-)- 0.16 (n=8) (F) groups. Plus sign (+) signifies a main effect of time, p < .05. Ampersand (&) signifies a main effect of day, p < .05.
Next, the time course of Day 4 saccharin drinking was compared to that on Day 5. Analysis of the aCSF data revealed significant main effects of day and time on drinking (p's < .05), with consumption being lower on Day 5, and drinking being lower as time went on (Fig. 6B). There was no interaction between day*time bin (p > .05). Analysis of 0.02 R(+) data showed no effect of day or an interaction of day*time, but did find a main effect of time (p < .05) (Fig. 6C). No main effects or interactions were found for the 0.04 R(+)- group (p's > .05) (Fig. 6D). Analysis of 0.08 S(-)- and 0.16 S(-)- data revealed only a main effect of day (p < .05), with animals consuming less saccharin following the microinjection versus the mock injection (Fig. 6E and 6F, respectively).
4. Discussion
Overall, these data show that intra-NACsh microinjection of R(+)- and S(-)-baclofen bidirectionally alter ethanol, but not saccharin intake. Total ethanol intake is decreased by the high dose 0.04 μg R(+)-baclofen/side, but increased following microinjection of the high dose 0.16μg S(-)-baclofen /side. These drug effects also show a distinct time course of action, with R(+)-baclofen reducing ethanol intake early compared to aCSF, but S(-)-baclofen increasing ethanol intake later compared to aCSF. It is nevertheless important to note that although the high R(+)-baclofen treatment trended towards an interaction, the high R(+)- and S(-)-baclofen treatments did not show time-specific effects in the within-groups analyses (Fig. 4D and 4F, respectively). Moreover, although ethanol and saccharin animals did not show statistically significant difference in time-course of intake on Day 4, they were not matched to Day 5 treatment group by time-course of intake. Thus, for a clearer interpretation of time-course of drug effects, future studies should match treatment groups by both total intake and time-course of intake. Saccharin intake was not affected by either baclofen enantiomer. Therefore, the current results are not likely an artifact of alterations in locomotor activity following drug administration. This point is especially well documented in that the high R(+)-baclofen dose did not reduce saccharin intake compared to the mock injection day, although aCSF injection did (Figs. 6D and 6B, respectively).
4.1 Effect of the Microinjection
As demonstrated in the mock- versus microinjection time course data, the microinjection of aCSF did not reduce ethanol consumption compared to the mock injection day, suggesting that the reduction of ethanol consumption in the high R(+)-baclofen dose group was a drug effect, and not simply an aversive response to the microinjection. Conversely, the aCSF microinjection did reduce saccharin intake compared to consumption following the mock injection. These data may suggest that the aCSF injection reduced the motivation of the mice to consume saccharin, and that B6 mice may be more motivated to consume ethanol than saccharin following an aversive experience, which has been previously demonstrated.
Aversive shock stimuli have been shown to increase ethanol consumption [37, 38], whereas chronic mild stressors inhibit development of conditioned place preference for sucrose [39], as well as decrease sucrose and saccharin intake [40, 41]. Intake of the sweet reinforcers was recovered using a tricyclic antidepressant, but this effect was blocked when dopamine antagonists were concurrently administered [40]. As discussed in section 1 and further in section 4.4, the NACsh and dopamine of the NACsh are integral parts of motivation to obtain a reinforcer [6]. The presence of dopamine in the NACsh following ethanol presentation [7] may have reduced the aversive consequences of the microinjection procedure, leading animals to continue consuming ethanol at their previous drinking rates, whereas the saccharin animals reduced consumption.
4.2 Locomotor Sedative Effects of Baclofen
In our experience, ethanol consumption and locomotion do not exhibit the same behavioral pattern. Within the DID paradigm, home cage locomotion markedly decreases by the 14th day of ethanol access, whereas ethanol consumption remains stable [42]. Moreover, baclofen is typically thought of as possessing sedative properties. Cryan and colleagues [43] demonstrated that baclofen potentiates locomotor sedation in naïve animals when systemically administered. However, Spano et al. [44] established that systemic administration of baclofen reduced reinstatement of heroin-seeking behavior in rats re-exposed to heroin, without affecting inactive lever presses or locomotor activity. Further, Agmo and Paredes [45, 46] demonstrated that while baclofen reduced sociosexual behavior and locomotion, other drugs also decreased locomotion without affecting sociosexual behavior, suggesting that reductions in locomotor activity are not causative of reductions in motivated behavior. Using home-cage locomotor activity boxes, our lab has recently demonstrated that systemic administration of R(+)- and S(-)-baclofen do not alter locomotor activity during ethanol or saccharin consumption in the same 5 day DID paradigm used in the current study [47]. Thus, our results, taken with those of Spano et al. and Agmo and Paredes, suggest that baclofen is working to reduce ethanol consumption via mechanisms alternative to those producing sedation.
4.3 Stereospecific Drug Action
The major highlight of these results is the stereospecific, bidirectional effects of the baclofen enantiomers on ethanol intake when microinjected into the NACsh. One explanation of this result may be differential binding. Other molecules, such as type 1 antiarrhythmic drugs and rolipram exhibit stereospecific binding that is related to molecular rotation of the molecule and differing binding affinity [48, 49]. In the case of rolipram, the high affinity binding sites mitigate the antidepressant effects of the drug [50]. Mecamylamine, a nicotinic parasympathetic ganglionic blocker that has been investigated for treating AUDs, also shows stereospecific binding which appears to be related to differing outcomes in behavioral assays [51].
As such, the actions of baclofen may depend on whether high and low affinity GABAB receptors exist, whether R(+)- and S(-)-baclofen binding is preferential to one type of receptor over the other, and whether this affinity, sensitivity, or binding preference is related to behavioral effects of the drug. Binding of baclofen relies not only on the absolute configuration of the drug (d/l or S/R), but also on the rotation of the molecule (+ or -). Both R- and S-baclofen are equally efficacious in displacing GABA from high and low affinity binding sites, but (-)baclofen is much more efficacious than (+)baclofen at displacing racemic baclofen at low affinity sites, which appear to be selective for (-)baclofen [52-54]. Further, these enantiomers interact differently with other neurotransmitters. Karbon and colleagues [55] suggest that the (-)baclofen isomer interacts with norepinephrine, whereas Waddington and Cross [52] suggest that non-GABAergic effects of baclofen lie with the R- configuration.
These literature sources suggest that the R- absolute configuration and the (-) molecular rotation are important for binding affinity and interaction with other receptor systems in the brain. Yet, the mixtures used in this study combine the R- configuration with the (+) rotation, and the S- configuration with the (-) rotation, leading to an extremely muddled interpretation of binding and affinity results. In B6 mice, it can be surmised that there is a representation of GABAB receptors that are susceptible to both R(+)- and S(-)-baclofen in the NACsh. The increased ethanol consumption following S(-)-baclofen microinjection may be related to low affinity binding sites that are selective for (-)-baclofen, whereas decreased ethanol consumption following R(+)-baclofen may be related to sites that are selective for that baclofen molecule, or the interactive effects of R-baclofen on non-GABAergic mechanisms. As B6 mice are an inbred strain, they represent an isogenic population that may not offer the ability to see individual differences in response to each enantiomer of baclofen, although their ethanol intake may be quite variable. As such, it is possible that individual differences in receptor subtypes leads to the differences of baclofen efficacy reported in human literature [56], or that increased severity of AUDs leads to neuroadaptive changes that cause an individual to be more susceptible to the R-baclofen enantiomer. It may therefore be of interested to assess the enantioselective effects of baclofen in an animal model that represents a homozygous population, such as the selectively bred High Alcohol Preferring (HAP) lines [57].
4.4 Neurotransmitter Involvement
Involvement of different neurotransmitter systems may explain why intra-NACsh baclofen altered ethanol, but not saccharin intake. Ethanol, but not saccharin, is known to interact with GABAA receptors. Extrasynaptic GABAA receptor subunit knock down has been demonstrated to reduce ethanol intake, but not saccharin intake [12, 13]. As previously discussed, the NACsh has been deemed an area of maladaptive dopamine release in response to drugs of abuse [6]. Considering the work done by Bassareo et al. [7, 10], it is possible to conclude that by the fifth day of ethanol access compared to the first day of access, the NACsh is releasing greater amounts of dopamine. Whereas it can be concluded that there is no release of dopamine in response to saccharin presentation on the fifth day of access in the NACsh, dopamine is still released in the NACcore and PFC. Furthermore, the GABA and dopamine systems of the NAC are known to interact; dopaminergic terminals are located on GABAergic neurons and form junctions with GABAergic dendrites, and dopamine agonists inhibit GABA release in the NAC [58-60]. Therefore, the effects of baclofen on ethanol, but not saccharin, intake may be contingent upon the presence of GABA and/or dopamine in the NACsh that would be released in response to the presentation of the ethanol reinforcer. Evidence of this has been shown in the VTA, where baclofen microinjection reduced microdialysate dopamine [61] and reduced binge-like ethanol intake in the DID paradigm when injected into the anterior VTA [23]. The role of dopamine release on reinforcer intake would be further implicated if microinjection of baclofen into the NACcore or PFC on the fifth of saccharin intake altered saccharin consumption.
4.5 Neurocircuitry
There are reciprocal efferent and afferent GABAergic and dopaminergic projections to and from the NACsh. GABAergic efferents from the NACsh project to the ventral pallidum and the VTA [2, 3]. Both of these areas are believed to be involved in reinforcer-directed behavior. Lesions of the ventral pallidum reduce development of conditioned place preference and decrease self-administration of reinforcers and drugs of abuse [62, 63]. Approximately 35-55% of the projections from the NACsh to the VTA are GABAergic projections, and these projections synapse on both dopaminergic and non-dopaminergic neurons of the VTA, including GABAergic interneurons which function to disinhibit VTA dopamine neurons [3]. The dopaminergic feedback loop between the NACsh and VTA is also considered to be especially important, with the NAC receiving dopaminergic afferent projections to its GABAergic cells [1]. This projection has been implicated as playing a role in the reduction of ethanol intake observed following microinjections of baclofen in to the anterior VTA [23]. The current results suggest that the reciprocal NACsh-to-VTA projection is also playing an important role in modulating ethanol intake. Further, projections to the VTA and ventral pallidum may also be involved in the motivation to consume the reinforcer or the perceived value of the reinforcer, and baclofen may be altering how the NACsh is communicating with these areas as well.
When discussing the neurocircuitry considerations of microinjection studies, it is important to note possible drug diffusion outside of the target brain area. The 200 nl/side of fluid injected in the current study is less than what has been injected into the NACsh in previous studies [64, 65]. A built-in control for determining diffusion is histological misses. In the present study, the high R(+)- and high S(-)-baclofen treatments each had a miss-rate of n=2 in the ethanol animals. In these animals, the microinjector tips bilaterally hit the NACcore. These animals showed no drug effect on ethanol consumption, which replicates previous lack of effects on ethanol consumption following GABAergic modulation in the core [12,13]. Further, our lab has previously shown anatomical specificity of baclofen when microinjected into the VTA at the same fluid volume given in the current study. Moore & Boehm [23] demonstrated that baclofen injected into the anterior, but not posterior, VTA reduced ethanol consumption. As such it is possible to infer that the fluid volume given does not diffuse so widely, as the VTA is a much smaller anatomical structure than the nucleus accumbens.
4.6 Drawbacks to DID
When interpreting the differences between drug effects on ethanol and saccharin intake, it is important to keep in mind that DID only accounts for consumption of the reinforcer, but not the reinforcer value. This poses a problem for assessing whether the chosen reinforcers, ethanol and saccharin, are similarly reinforcing and whether drug effects are truly reinforcer specific, or if they are due to increased motivation to obtain saccharin. While mice freely ingest saccharin and a 0.2% solution is often used as a standard reinforcer control group [66-68], minimal data exists on the reinforcing quality of different saccharin concentrations as seen in operant paradigms. One study [67] found that animals responded 2.5 times more in an FR4 operant paradigm for a 0.2% w/v saccharin solution than a 20% v/v ethanol concentration.
At a 0.2% w/v concentration, B6 mice have between a 50% to a 94.5% saccharin preference [66, 68]. As seen in Risinger et al. [67], similar preference does not mean animals will work to similar amounts to obtain the reinforcer. Although 0.2% saccharin and 20% ethanol have similar preference ratios, yet B6 mice work 2.5 times harder to receive saccharin. Working to overcome negative drug effects to consume saccharin would explain why animals drank even after receiving R(+)-baclofen, but it does not explain why S(-)-baclofen did not produce an increase in saccharin consumption or why aCSF reduced saccharin intake. Further, our lab has observed reductions in saccharin intake following systemic R(+)-baclofen [47], so concluding that R(+)-baclofen is unable to reduce saccharin consumption would seem unsatisfactory. Perhaps, the best explanation is that the NACsh is not responsible for the control of saccharin intake, likely due to a combination of a transfer of control to another brain region and the possible lack of dopamine response on Day 5. This hypothesis could be tested by microinjecting baclofen into different brain regions or at different time-points.
4.7 Conclusion
The hypothesis that R(+)- and S(-)-baclofen would bidirectionally alter ethanol intake, but not saccharin intake, was upheld. It is not likely that the effects of baclofen on consummatory behavior were related to locomotion, as baclofen did not alter saccharin intake. Overall, GABAergic interactions with dopamine in the NACsh may have contributed to the drug effects seen in this study. Maladaptive dopamine release in the NACsh occurs with consumption of drugs of abuse, like ethanol, but not to simple tastants, like saccharin. This maladaptive dopamine release may be necessary for altering consummatory behavior, explaining the lack of drug effect on saccharin intake. When administered systemically, R(+)-baclofen reduces saccharin intake. Systemic administration presumably allows these drugs to act elsewhere, like the NACcore or the PFC where there is still release of dopamine in response of the tastant.
The stereospecific effects of baclofen on ethanol intake may be related to a myriad of enantioselective properties like those seen in other compounds. These include differential binding to high/low affinity receptors which may influence differential receptor effects or which behaviors receptors may modify. Overall, these results continue to support the important role of the GABAB receptor in the modulation of ethanol intake. They also reinforce the notion that drugs exert enantioselective actions on behaviors, and that these actions are extremely important in considering the efficacy or potency of a drug for clinical treatment. Enantioselective effects may indeed help explain the inconsistent results reported in the human literature on the use of baclofen for the treatment of AUDs.
Highlights.
R(+)- and S(-)-baclofen were microinjected into the nucleus accumbens shell.
Binge-like ethanol and saccharin intake were monitored.
Time-course of ethanol and saccharin intake was monitored.
We report a bidirectional, enantioselective effect on binge-like ethanol intake.
Acknowledgments
Funding Support: NIAAA grant #AA016789 and the Indiana Alcohol Research Center (AA007611).
Footnotes
Artificial cerebrospinal fluid (aCSF), Alcohol use disorders (AUDs), Blood ethanol concentration (BEC), C57Bl/6J (B6), Drinking-in-the-Dark (DID), High Alcohol Preferring (HAP), nucleus accumbens core (NACcore), nucleus accumbens shell (NACsh), prefrontal cortex (PFC), ventral tegmental area (VTA)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF, Sanna PP, Bloom FE. Neuroscience of Addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 2.Dray A, Oakley NR. Projections from nucleus accumbens to globus pallidus and substantia nigra in the rat. Experientia. 1978;34:68–70. doi: 10.1007/BF01921907. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–60. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- 4.Bardo MT. Neuropharmacological Mechanisms of Drug Reward: Beyond Dopamine in the Nucleus Accumbens. 1998;12:37–68. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 5.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 6.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Supplement 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Bassareo V, De Luca MA, Aresu M, Aste A, Ariu T, Di Chiara G. Differential adaptive properties of accumbens shell dopamine responses to ethanol as a drug and as a motivational stimulus. European Journal of Neuroscience. 2003;17:1465–72. doi: 10.1046/j.1460-9568.2003.02556.x. [DOI] [PubMed] [Google Scholar]
- 8.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proceedings of the National Academy of Sciences. 1995;92:12304–8. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanda G, Pontieri FE, Chiara GD. Cannabinoid and Heroin Activation of Mesolimbic Dopamine Transmission by a Common μ1 Opioid Receptor Mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 10.Bassareo V, De Luca MA, Di Chiara G. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. The Journal of Neuroscience. 2002;22:4709–19. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leriche M, Méndez M, Zimmer L, Bérod A. Acute ethanol induces Fos in GABAergic and non-GABAergic forebrain neurons: A double-labeling study in the medial prefrontal cortex and extended amygdala. Neuroscience. 2008;153:259–67. doi: 10.1016/j.neuroscience.2008.01.069. [DOI] [PubMed] [Google Scholar]
- 12.Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. α4-Containing GABAA Receptors in the Nucleus Accumbens Mediate Moderate Intake of Alcohol. The Journal of Neuroscience. 2009;29:543–9. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proceedings of the National Academy of Sciences. 2011;108:4459–64. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–83. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 15.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen Efficacy in Reducing Alcohol Craving and Intake: A Prelimnary Double-Blind Randomized Controlled Study. Alcohol and Alcoholism. 2002;37:504–8. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- 16.Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and Safety of Baclofen for Alcohol Dependence: A Randomized, Double-Blind, Placebo-Controlled Trial. Alcoholism: Clinical and Experimental Research. 2010;34:1849–57. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon JE, Khan RA, Gessert CE, Larson PM, Renier CM. Treating alcohol withdrawal with oral baclofen: A randomized, double-blind, placebo-controlled trial. Journal of Hospital Medicine. 2011;6:469–74. doi: 10.1002/jhm.928. [DOI] [PubMed] [Google Scholar]
- 18.Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of Baclofen on Alcohol and Sucrose Self-Administration in Rats. Alcoholism: Clinical and Experimental Research. 2003;27:900–8. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- 19.Janak PH, Michael Gill T. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 20.Maccioni P, Fantini N, Froestl W, Carai MAM, Gessa GL, Colombo G. Specific Reduction of Alcohol's Motivational Properties by the Positive Allosteric Modulator of the GABAB Receptor, GS39783—Comparison With the Effect of the GABAB Receptor Direct Agonist, Baclofen. Alcoholism: Clinical and Experimental Research. 2008;32:1558–64. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 21.Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G, et al. The GABAB receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol and Alcoholism. 2002;37:499–503. doi: 10.1093/alcalc/37.5.499. [DOI] [PubMed] [Google Scholar]
- 22.Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacology Biochemistry and Behavior. 2004;78:743–50. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Moore EM, Boehm SL., Ii Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behavioral Neuroscience. 2009;123:555–63. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Experimental and Clinical Psychopharmacology. 1997;5:183–94. [PubMed] [Google Scholar]
- 25.Smith BR, Boyle AEL, Amit Z. The Effects of the GABAB Agonist Baclofen on the Temporal and Structural Characteristics of Ethanol Intake. Alcohol. 1999;17:231–40. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- 26.Czachowski CL, Legg BH, Stansfield KH. Ethanol and Sucrose Seeking and Consumption Following Repeated Administration of the GABAB Agonist Baclofen in Rats. Alcoholism: Clinical and Experimental Research. 2006;30:812–8. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 27.Olpe HR, Demiéville H, Baltzer V, Bencze WL, Koella WP, Wolf P, et al. The biological activity of d-baclofen (Lipresal®) European Journal of Pharmacology. 1978;52:133–6. doi: 10.1016/0014-2999(78)90032-8. [DOI] [PubMed] [Google Scholar]
- 28.Paredes R, Agmo A. Stereospecific actions of baclofen on sociosexual behavior, locomotor activity and motor execution. Psychopharmacology. 1989;97:358–64. doi: 10.1007/BF00439451. [DOI] [PubMed] [Google Scholar]
- 29.Fromm GH, Shibuya T, Nakata M, Terrence CF. Effects of d-baclofen and l-baclofen on the trigeminal nucleus. Neuropharmacology. 1990;29:249–54. doi: 10.1016/0028-3908(90)90009-g. [DOI] [PubMed] [Google Scholar]
- 30.Jamali F, Mehvar R, Pasutto FM. Enantioselective aspects of drug action and disposition: Therapeutic pitfalls. Journal of Pharmaceutical Sciences. 1989;78:695–715. doi: 10.1002/jps.2600780902. [DOI] [PubMed] [Google Scholar]
- 31.Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., Ii GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacology Biochemistry and Behavior. 2007;88:105–13. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linsenbardt DN, Boehm SL., Ii Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–34. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melón LC, Boehm SL. Role of Genotype in the Development of Locomotor Sensitization to Alcohol in Adult and Adolescent Mice: Comparison of the DBA/2J and C57BL/6J Inbred Mouse Strains. Alcoholism: Clinical and Experimental Research. 2011;35:1351–60. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192:207–17. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- 37.Cicero TJ, Myers RD, Black WC. Increase in volitional ethanol consumption following interference with a learned avoidance response. Physiology & Behavior. 1968;3:657–60. [Google Scholar]
- 38.Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. The Journal of Physiology. 1978;285:455–70. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology. 1991;104:255–9. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 40.Sampson D, Willner P, Muscat R. Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology. 1991;104:491–5. doi: 10.1007/BF02245655. [DOI] [PubMed] [Google Scholar]
- 41.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 42.Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to Ethanol's Ataxic Effects and Alterations in Ethanol-Induced Locomotion Following Repeated Binge-Like Ethanol Intake Using the DID Model. Alcoholism: Clinical and Experimental Research. 2011;35:1246–55. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N, N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4, 6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. Journal of Pharmacology and Experimental Therapeutics. 2004;310:952–63. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- 44.Spano MS, Fattore L, Fratta W, Fadda P. The GABAB receptor agonist baclofen prevents heroin-induced reinstatement of heroin-seeking behavior in rats. Neuropharmacology. 2007;52:1555–62. doi: 10.1016/j.neuropharm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Agmo A, Paredes R. GABAergic drugs and sexual behaviour in the male rat. European Journal of Pharmacology. 1985;112:371–8. doi: 10.1016/0014-2999(85)90783-6. [DOI] [PubMed] [Google Scholar]
- 46.Agmo A, Paredes R, Fernández H. Differential effects of GABA transaminase inhibitors on sexual behavior, locomotor activity, and motor execution in the male rat. Pharmacology Biochemistry and Behavior. 1987;28:47–52. doi: 10.1016/0091-3057(87)90010-4. [DOI] [PubMed] [Google Scholar]
- 47.Kasten CR, Blasingame SN, Boehm SL., 2nd Bidirectional enantioselective effects of the GABABagonist baclofen in two mouse models of excessive ethanol consumption. doi: 10.1016/j.alcohol.2014.11.005. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill RJ, Duff HJ, Sheldon RS. Determinants of stereospecific binding of type I antiarrhythmic drugs to cardiac sodium channels. Molecular Pharmacology. 1988;34:659–63. [PubMed] [Google Scholar]
- 49.Schneider HH, Schmiechen R, Brezinski M, Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. European Journal of Pharmacology. 1986;127:105–15. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HT, Zhao Y, Huang Y, Deng C, Hopper A, Vivo M, et al. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology. 2006;186:209–17. doi: 10.1007/s00213-006-0369-4. [DOI] [PubMed] [Google Scholar]
- 51.Nickell JR, Grinevich VP, Siripurapu KB, Smith AM, Dwoskin LP. Potential therapeutic uses of mecamylamine and its stereoisomers. Pharmacology Biochemistry and Behavior. 2013;108:28–43. doi: 10.1016/j.pbb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waddington JL, Cross AJ. GABAergic properties of baclofen in vivo and in vitro. Brain Research Bulletin. 1980;(Supplement 2):5. 503–5. [Google Scholar]
- 53.Bowery NG, Hill DR, Hudson AL. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. British Journal of Pharmacology. 1983;78:191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drew CA, Johnston GAR, Weatherby RP. Bicuculline-insensitive GABA receptors: Studies on the binding of (−)-baclofen to rat cerebellar membranes. Neuroscience Letters. 1984;52:317–21. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- 55.Karbon EW, Duman RS, Enna SJ. GABAB receptors and norepinephrine-stimulated cAMP production in rat brain cortex. Brain Research. 1984;306:327–32. doi: 10.1016/0006-8993(84)90382-2. [DOI] [PubMed] [Google Scholar]
- 56.Agabio R, Maccioni P, Carai MAM, Gessa G Luigi, Froestl W, Colombo G. The Development of Medications for Alcohol-Use Disorders Targeting the GABAB Receptor System. Recent Patents on CNS Drug Discovery. 2012;7:113–28. doi: 10.2174/157488912800673137. [DOI] [PubMed] [Google Scholar]
- 57.Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and Characterization of Replicate High- and Low-Alcohol Preferring Lines of Mice and a High-Drinking Crossed HAP Line. Behav Genet. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pickel VM, Towle AC, Joh TH, Chan J. Gamma-aminobutyric acid in the medial rat nucleus accumbens: Ultrastructural localization in neurons receiving monosynaptic input from catecholaminergic afferents. The Journal of Comparative Neurology. 1988;272:1–14. doi: 10.1002/cne.902720102. [DOI] [PubMed] [Google Scholar]
- 59.Beart PM, McDonald D, Gundlach AL. Mesolimbic dopaminergic neurones and somatodendritic mechanisms. Neuroscience Letters. 1979;15:165–70. doi: 10.1016/0304-3940(79)96107-x. [DOI] [PubMed] [Google Scholar]
- 60.Belleroche J, Gardiner IM. Action of apomorphine, bromocriptine and lergotrile onγ-aminobutyric acid and acetylcholine release in nucleus accumbens and corpus striatum. J Neural Transmission. 1983;58:153–68. doi: 10.1007/BF01252802. [DOI] [PubMed] [Google Scholar]
- 61.Klitenick M, DeWitte P, Kalivas P. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. The Journal of Neuroscience. 1992;12:2623–32. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: Further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience. 1993;52:605–20. doi: 10.1016/0306-4522(93)90410-h. [DOI] [PubMed] [Google Scholar]
- 63.Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Research. 1990;508:20–9. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- 64.Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. Binge Drinking Upregulates Accumbens mGluR5–Homer2–PI3K Signaling: Functional Implications for Alcoholism. The Journal of Neuroscience. 2009;29:8655–68. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lum EN, Campbell RR, Rostock C, Szumlinski KK. mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology. 2014;79:679–87. doi: 10.1016/j.neuropharm.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lush IE. The genetics of tasting in mice: VI. Saccharin, acesulfame, dulcin and sucrose. Genetics Research. 1989;53:95–9. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 67.Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse Strain Differences in Oral Operant Ethanol Reinforcement under Continuous Access Conditions. Alcoholism: Clinical and Experimental Research. 1998;22:677–84. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- 68.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–60. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]