Abstract
Insulin performs unique functions within the CNS. Produced nearly exclusively by the pancreas, insulin crosses the blood-brain barrier (BBB) using a saturable transporter, affecting feeding and cognition through CNS mechanisms largely independent of glucose utilization. Whereas peripheral insulin acts primarily as a metabolic regulatory hormone, CNS insulin has an array of effects on brain that may more closely resemble the actions of the ancestral insulin molecule. Brain endothelial cells (BEC), the cells that form the vascular BBB and contain the transporter that translocates insulin from blood to brain, is itself regulated by insulin. The insulin transporter is altered by physiological and pathological factors including hyperglycemia and the diabetic state. The latter can lead to BBB disruption. Pericytes, pluripotent cells in intimate contact with the BEC, protect the integrity of the BBB and its ability to transport insulin. Most of insulin’s known actions within the CNS are mediated through two canonical pathways, the phosphoinositide-3 kinase (PI3)/Akt and Ras/mitogen activated kinase (MAPK) cascades. Resistance to insulin action within the CNS, sometimes referred to as diabetes mellitus type III, is associated with peripheral insulin resistance, but it is possible that variable hormonal resistance syndromes exist so that resistance at one tissue bed may be independent of that at others. CNS insulin resistance is associated with Alzheimer’s disease, depression, and impaired baroreceptor gain in pregnancy. These aspects of CNS insulin action and the control of its entry by the BBB are likely only a small part of the story of insulin within the brain.
Keywords: Blood-brain barrier, insulin, glucose, brain, central nervous system, resistance, transport
1. Introduction
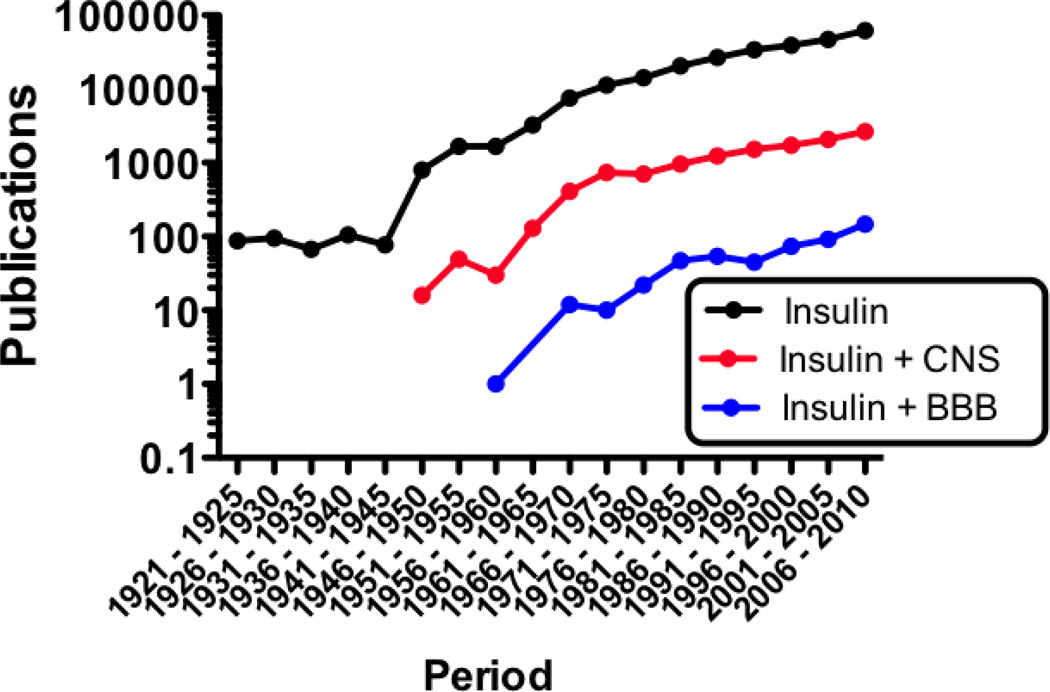
Insulin has emerged as a major regulatory substance within the central nervous system. This is evidenced by the rate of increase in publications relating insulin to the CNS and to the blood-brain barrier (BBB) that was slow to start but is now growing exponentially (Figure 1). First isolated by Banting and Best nearly a century ago (Banting & Best, 1922), insulin is well known as the major, immediate regulator of blood glucose levels, stimulating the uptake of glucose by liver, muscle, and adipose tissue. In contrast, glucose transport into the brain and the use of glucose by the majority of cells within the brain are independent of insulin. Furthermore, insulin is made almost exclusively by the pancreatic beta cell and, being a protein, it is logical to assume that insulin could not cross the BBB. Glucose use independent of insulin stimulation, lack of insulin production, and the presence of the BBB all supported the view that the CNS should be an insulin-insensitive tissue.
Figure 1.
Relative growth of the fields reviewed here as evidenced by publications per 5 year period. Publications on insulin greatly increased in the later half of the 1940’s and publications on insulin and the brain surged in the early 1960’s. Insulin and the BBB remains a little explored topic, but publications in all three fields are growing exponentially.
Yet insulin receptors are distributed throughout the brain, insulin is transported across the BBB by a saturable transport system, and an increasing number of non-metabolic functions for insulin within the CNS and at the BBB are being discovered. This suggests that whereas insulin acts largely as a metabolic regulator in the periphery, its actions within the CNS are largely unrelated to facilitating glucose utilization. Insulin and insulin-like growth factor-1 (IGF1) are descended from a common ancestral protein that acted as a mitogenic growth factor and metabolic regulatory hormone. It seems, then, that the evolution of insulin function has taken a divergent course in the periphery and in the CNS. This review will explore important aspects of insulin and the CNS, including the relation between CNS insulin and CNS glucose, the evolutionary history of insulin and its receptors, the effects of insulin on the BBB, the characteristics of insulin transport across the BBB, the actions of insulin in the brain, and what happens when insulin BBB transport or insulin function within the CNS goes awry. This review reveals insulin to be a major signal between the peripheral tissues and the CNS, having a regulated transport across the BBB, and controlling important events in the CNS that are related to metabolism, neural plasticity, and cognition.
2. The Relation Between Glucose and Insulin: The Periphery
Despite its various effects on many tissues and organs, a single cell type located in a single tissue determines the level of insulin in blood: the beta cell in the pancreas. It is this cell that secretes insulin, packaged and co-secreted with amylin, and does so in response to the blood glucose level that it perceives through GLUT-2. It is important to note that GLUT-2 is one of the glucose transporters that is not stimulated or regulated by insulin. Loss of pancreatic beta cells as in diabetes mellitus type I (DM1) or in animals treated with streptozotocin results in no or deficient insulin secretion and loss of blood glucose control (hypoinsulinemic hyperglycemia). Insulin levels are also indirectly affected when resistance to the actions of insulin occur as in diabetes mellitus type II (DM2) or obesity. In these scenarios, insulin is inefficient in activating its receptors that control the uptake of glucose by muscle and other insulin sensitive tissues. Blood glucose levels rise, stimulating the pancreatic beta cell to release more insulin until either glucose levels return to normal or pancreatic secretion reaches its maximum. The former condition of hyperinsulinemic euglycemia is termed the insulin resistance syndrome (IRS) (Anonymous, 2003) and the latter condition of hyperinsulinemic hyperglycemia has had many terms suggested for it over the decades and will be referred to here as DM2.
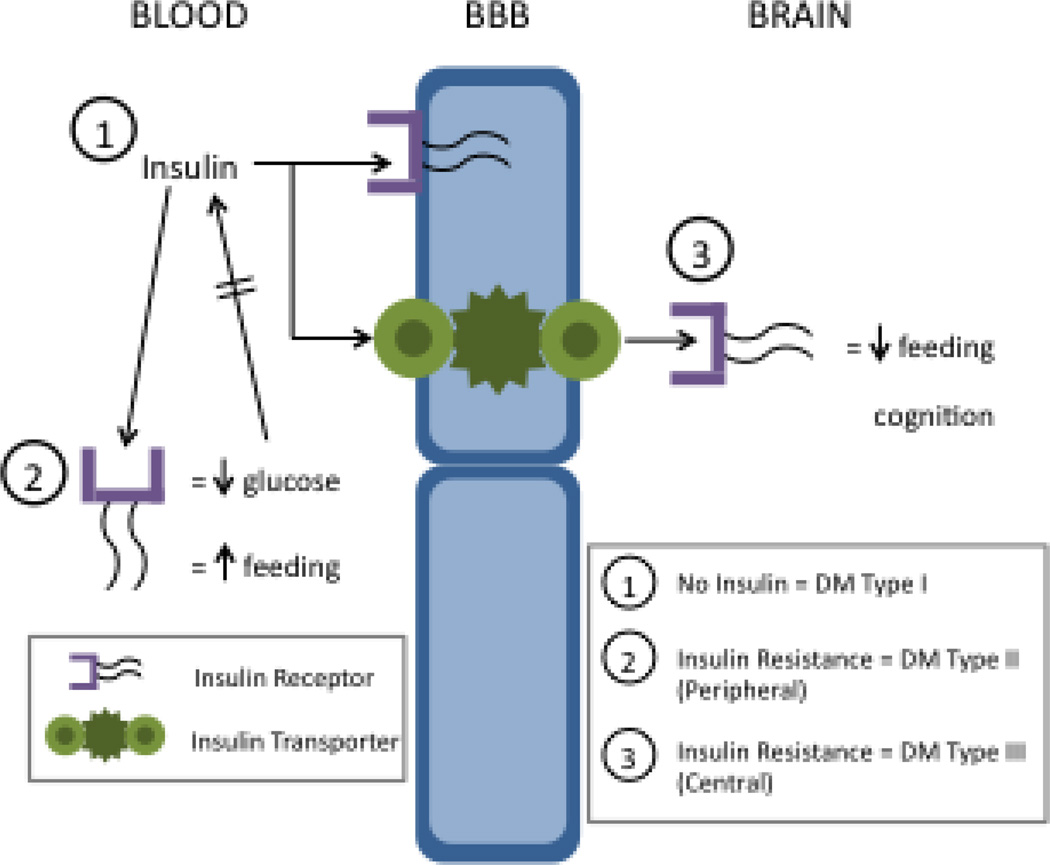
Thus, a classic negative feedback loop is formed from the intimate links that occur among insulin receptor sensitivity, pancreatic beta cell activity, blood glucose levels, and glucose use by insulin-sensitive tissues (Figure 2). In brief, insulin secreted by pancreatic beta cells drives glucose uptake by those tissues possessing the insulin-sensitive glucose transporter GLUT-4, this glucose uptake lowers blood glucose levels, and the detection of lower levels of glucose by the beta cells through insulin-insensitive GLUT-2 results in a decreased secretion of insulin.
Figure 2.
Schematic of relation among concepts of peripheral and central insulin receptors, forms of diabetes, and major actions of insulin. In the blood, insulin, the insulin receptor, and the GLUT-2 receptor are engaged in a negative feedback loop with glucose. At the BBB, the insulin receptor and the insulin transporter are both present with the receptor affecting several aspects of brain endothelial cell function and the transporter involved in the blood-to-brain translocation of insulin. Within the brain, the insulin receptor rarely induces glucose uptake by brain cells. Instead, it has effects on feeding that are largely opposite to those produced by insulin in the periphery. CNS insulin also has effects on cognition. Lack of insulin production by the pancreas induces diabetes mellitus (DM) type I. Resistance to peripheral insulin is well recognized in DM type II and resistance to CNS insulin is often referred to as DM type III. The relation between DM type II and type III requires further investigation.
3. The Relation Between Glucose and Insulin: The CNS
In contrast to the peripheral tissues, glucose acquisition by the most metabolically active organ, the CNS, is independent of insulin. Furthermore, the CNS does not depend on an ultrafiltrate produced at the capillary bed as its source of glucose; rather its capillary bed transports glucose from blood into the brain. The capillary bed of the brain is specially modified to prevent the unregulated leakage of substances from blood to brain, thus forming the BBB. Hence, no ultrafiltrate is produced at the capillary bed. Instead, GLUT-1, an insulin-insensitive glucose transporter, conveys about 50 times more glucose into the CNS than would otherwise enter (Oldendorf, 1971). Glucose transport is saturable, but not active; that is, it does not use energy in its transfer of glucose from blood into brain. Instead, GLUT-1 is a facilitated diffusion system, conveying glucose from the compartment of higher concentration to that of lower concentration.
In most cases, glucose levels are about twice as high in blood as in brain interstitial and cerebrospinal fluids, thus driving by mass action glucose in the blood-to-brain direction. In studies in which glucose levels are raised in the CNS or isotopes of glucose are introduced into the CNS, the brain-to-blood movement (efflux) of glucose can be demonstrated. Glucose that is transported into but not used by the CNS is also returned to the blood. Thus, uptake, retention, and metabolism of glucose are nearly synonymous measures; this allows the metabolic rate of the CNS to be inferred from the rate at which glucose probes such as 2-fluoro-2-deoxy-d-glucose are taken up by brain.
Although the CNS can use ketones during starvation, glucose is the main fuel of the CNS and is required for its normal function (White & Venkatesh, 2011). Reduction in blood glucose levels below that for which GLUT-1 can compensate results immediately in the signs and symptoms of hypoglycemia. The brain responds by increasing sympathetic outflow and releasing hypothalamic regulatory factors, all of which directly or indirectly result in the release of counter-regulatory hormones that oppose the action of insulin (Frizzell, et al., 1993).
Just as acquisition (i.e., transport) of glucose across the BBB is not affected by insulin, so the usage of glucose by the cells within the CNS is largely unaffected by insulin. The non-insulin sensitive glucose transporters GLUT-1 (astrocytes), GLUT-3 (neurons), and GLUT-5 (microglia) account for the majority of glucose uptake by the CNS (McEwen & Reagan, 2004). Interestingly, insulin-insensitive GLUT-2, the glucose transporter used by the pancreas to sense blood glucose levels, is located on some cells in the hypothalamus, raising the possibility that this transporter is critical to the CNS reaction to hypoglycemia. It has long been known that CNS sensing of hypoglycemia induces a profound, CNS-based, vagally-mediated counter regulatory response to restore blood glucose levels (Clark, 1925).
Some cells in the cerebellum, hypothalamus, and hippocampus do possess the insulin-sensitive GLUT-4 (C.A. Grillo, Piroli, Hendry, & Reagan, 2009). Another insulin-sensitive insulin transporter, GLUT-8, has an intracellular location and seems to be involved in protein glycosylation at the rough endoplasmic reticulum rather than in glucose internalization (McEwen & Reagan, 2004). Although GLUT-4 likely plays an important role in the CNS cells possessing it, its presence in the CNS does not alter the conclusion that glucose acquisition and use is largely independent of insulin. Thus, insulin must have other actions in the brain.
4. Evolution of Insulin and Its Major Receptors
Clues to what functions insulin has within the CNS and how those functions arose can be informed by considering the evolutionary history of insulin and its actions. Insulin belongs to a superfamily that immediately includes insulin-like growth factor (IGF)-I and IGF-II; more distant relatives include relaxin and the invertebrate hormones bombyxin, locust insulin-related peptide, and molluscan insulin-like peptide (Chan, Nagamatsu, Cao, & Steiner, 1992). All members share a common insulin-like tertiary structure. Insulin has had a separate gene lineage from the IGF’s throughout chordate evolution, whereas the invertebrate amphioxus has a single insulin-like peptide (ILP) with hybrid characteristics of insulin and the IGF’s. The amphioxi, or lancelets, are fish-like marine proto-chordates considered the archetypal vertebrate form, splitting from vertebrates about 520 million years ago. Although the predicted amino acid sequence of ILP shows a hybrid insulin/IGF molecule, the structure of the ILP gene is more homologous to the structure of the vertebrate insulin gene than that of the IGF gene. Similar work on molluscan insulin-like peptide further suggests that the ancestral gene giving rise to the various members of the insulin superfamily was organized much like the vetebrate insulin gene.
Minor mutations in the amphioxus ILP gene could have easily given rise to the insulin and the IGF lineages. Human insulin is 51 amino acids in length and forms two polypeptide chains linked together by disulfide bonds, whereas human IGF-I and IGF-II are single polypeptide chains of 70 and 67 amino acids each.
In humans, the two IGF’s share a 66% homology with each other and share a 50% sequence homology in their A and B domains with insulin. In humans, insulin is primarily secreted from the pancreas when blood glucose levels are perceived to be high, whereas IGF-I is secreted primarily from the liver under the influence of growth hormone. In the tunicates, mRNA for insulin and IGF are expressed in the digestive tract as well as the heart and nervous system (McRory & Sherwood, 1997); insulin is expressed by the pancreas, brain, pituitary, gastrointestinal tract, and adipose tissue of fish (Caruso & Sheridan, 2011). Hence, the evolution of the insulin superfamily involves interesting, largely unexplored divergences in the hormonal controls of secretion, tissues of secretion, targeted tissues, and biological actions.
The ancestral insulin-like peptide is postulated to have functioned as a mitogenic growth factor. In mammals, the IGF’s have assumed the role of mitogenic growth factors while insulin evolved to be a metabolic regulatory hormone (Chan & Steiner, 2000). However, this distinction may not be so clear in the CNS where the major function of insulin is not that of stimulating uptake of glucose.
The human insulin and IGF-I receptors are also similar, sharing a 55% identity according to cDNA analysis (Navarro, et al., 1999). Amphioxus contains a single insulin-like receptor with approximately equal homology to human insulin and IGF-I receptors. Separation of insulin and IGF receptors may have occurred in sponges prior to the Cambrian explosion (Skorokhod, et al., 1999). IGF-II binds to the cation-independent mannose-6-phosphate receptor (Castonguay, Olson, & Dahms, 2011). The mannose-6-phosphate receptor has recently been found to be expressed by the BBB early in development where it transports lysosomal enzymes (Urayama, Grubb, Sly, & Banks, 2004) and is used by the AIDS virus to cross the BBB (Dohgu, Ryerse, Robinson, & Banks, 2012). As a net result of homologies among insulin and IGF receptors and proteins, insulin can bind to the IGF-I receptor but much less well to the IGF-II receptor. In comparison, the IGF’s bind less well to the insulin receptor.
Evolutionary pressures have induced a differential distribution of IGF and insulin receptors to arise, but that pattern has not been pushed to its extreme conclusion. IGF and insulin receptors are found throughout the CNS and their relative distributions vary, but overlap. Werther et al. (Werther, et al., 1987) reported several regions of brain including the amygdala, cerebellum, and entorhinal cortex where the insulin receptor level was much lower than the receptor level of IGF-1; in comparison, insulin receptor levels were higher than those for IGF-1 in the CA1 hippocampal field and arcuate nucleus.
Thus, it may be that insulin in the CNS is more closely related to ancestral insulin in terms of function than is insulin in the periphery. Likewise, the anatomical separation of insulin and IGF-I receptors in the CNS is less distinct than in the peripheral tissues.
5. Effects of Insulin on BECs and BBB Function
The cells that form the BBB are unique in that they have cell membranes that face into the blood stream and into the CNS. Indeed, the BEC is a sort of cellular portmanteau, residing simultaneously in both the peripheral and CNS compartments. For this reason, BECs are exposed to both peripheral and CNS signals. The BECs and ependymal cells that comprise the BBB and blood-CSF barrier possess insulin-binding sites (Baskin, et al., 1986; Frank, Jankovic-Vokes, Pardridge, & Morris, 1985; Frank & Pardridge, 1981; Miller & Borchardt, 1991). Those binding sites at the BBB and probably those at the blood-CSF barrier perform two distinct functions: some of them act as transporters of insulin across the BBB and others act as classic receptor sites, affecting the function of the barrier cell by activating intracellular machinery (Figure 2). As previously reviewed (Banks, 2004), the question of whether insulin transporters and insulin receptors are derived from the same gene product or represent unique proteins has not been addressed experimentally (see below for further discussion).
Insulin has several effects on barrier cell function. Insulin enhances transport of the amino acids tyrosine and tryptophan (Cangiano, et al., 1983; Daniel, Love, Moorhouse, & Pratt, 1981; Tagliamonte, DeMontis, Olianas, Onali, & Gessa, 1976), increases the blood-to-brain transport of leptin (Kastin & Akerstrom, 2001), increases expression and function of the efflux transporter P-glycoprotein, but decreases expression and function of another efflux transporter, breast cancer resistance protein (H. Liu, Yang, Wang, Liu, Li, et al., 2009; X. Liu, et al., 2011). Insulin’s effects on tryptophan transport are indirect, acting by reducing in the circulating levels of other amino acids competing for the same transporter, whereas the effects on P-glycoprotein are direct, acting at the BEC insulin receptor, initiating a PKC/NFkB dependent pathway (H. Liu, Yang, Wang, Liu, Li, et al., 2009). Insulin increases transport of azidothymidine (Ayre, Skaletski, & Mosnaim, 1989), a drug used to treat AIDS, and a ligand for a probenecid-sensitive transporter (Takasawa, Terasaki, Suzuki, Ooie, & Sugiyama, 1997). Insulin does not seem to be important in maintaining tight junction proteins or BBB integrity (Kondo, Hafezi-Moghadam, Thomas, Wagner, & Kahn, 2004). Insulin is a noncompetitive inhibitor of alkaline phosphatase (Catalan, Martinez, Aragones, Miguel, & Robles, 1988) and can increase expression of the glutamate cysteine ligase catalytic subunit, the latter important in maintaining cellular levels of glutathione (Langston, Li, Harrison, & Aw, 2011). At the choroid plexus, insulin may affect CSF production through its inhibition of serotonin receptor 5-HT2c activity through a MAP kinasedependent pathway (Hurley, et al., 2003) and its stimulation of sodium uptake from blood (Johanson & Murphy, 1990). From this, it is clear that insulin affects many barrier cell functions and exerts those effects through various cellular pathways.
Insulin degrading enzyme is present on BEC (Keller & Borchardt, 1987; Miller & Borchardt, 1991). Its location is primarily on the abluminal, or brain-side, membrane (Lynch & Banks, 2006), consistent with a role in the degradation of amyloid beta peptide and other substances (Kurochkin & Goto, 1994). However, it is still possible that insulin degrading enzyme limits insulin’s action at the BEC, affects its transport across the BBB, or degrades insulin that is within the CNS.
6. A History of the Hunt for Insulin in the CNS: Is It There and What’s It’s Origin?
The question of whether insulin could cross the BBB was considered as early as 1954. In that year, two studies examined the ability of peripherally injected radioactively labeled insulin to enter the CNS (Elgee, Williams, & Lee, 1954; Haugaard, Vaughan, Haugaard, & Stadie, 1954), one study injecting the radioactive insulin into the blood stream of agonal patients, obtaining brain samples at autopsy. Both studies concluded that insulin did not cross the BBB. Several other authors revisiting this issue well into the mid 1980’s similarly concluded that insulin did not cross the BBB (Havrankova, Brownstein, & Roth, 1981; Havrankova, Roth, & Brownstein, 1979).
In contrast, studies by Margolis and Altszuler, Greco et al, and Woods and Porte (Greco, Ghirlanda, Fedeli, & Gambassi, 1970; Margolis & Altszuler, 1967; Woods & Porte, 1977) found insulin in the CSF, noting a correlation between the levels of insulin in CSF and serum. These authors noted that the CSF versus serum relation began to flatten as insulin levels increased out of the physiologic range and concluded that insulin was crossing the BBB by a saturable mechanism. The counterargument made by several authors to this interpretation was that CSF insulin was derived from CNS sources, not from the blood (Havrankova, et al., 1981; Havrankova, et al., 1979; Reiser, Lenz, Bernstein, & Dorn, 1985). The question of whether insulin is made in the brain of mammals is supported by the expression in the brains of so called lower species. For example, insulin mRNA is expressed in the nervous system of tunicates and insulin protein is found in the brains of fish (Caruso & Sheridan, 2011; McRory & Sherwood, 1997). However, in mammals, the only extra-pancreatic source of insulin to be clearly demonstrated is by a small group of cells in the olfactory mucosa (Lacroix, et al., 2008). With no source of insulin production in the CNS, its presence in the CSF is clear evidence that insulin crosses the BBB. Later, studies using exogenous or radioactively labeled insulin confirmed that insulin crossed the BBB by a saturable mechanism (Banks, Jaspan, Huang, & Kastin, 1997; Banks, Jaspan, & Kastin, 1997b; Baura, et al., 1993; Poduslo, Curran, & Berg, 1994; Schwartz, et al., 1991).
In contrast to the saturable blood-to-brain influx, no evidence for a saturable component to the brain-to-blood efflux of insulin has been found (Cashion, Banks, & Kastin, 1996). Instead, the best evidence suggests that CNS insulin enters the bloodstream with the reabsorption of CSF.
7. Characteristics of Insulin Transport Across the BBB
Insulin crosses the BBB by a saturable mechanism, the rate averaging about 0.5–0.6 microl/g-min but ranging from 0.2 to 1.7 microl/g-min among the various studies, the rate being influenced by a host of factors that include aluminum treatment (Banks & Kastin, 1985), dexamethasone treatment (Baura, et al., 1996), strain of mouse (Banks, Farr, & Morley, 2000), brain region (Banks & Kastin, 1998; Banks, Kastin, & Pan, 1999), overexpression of APP (Poduslo, Curran, Wengenack, Malester, & Duff, 2001), hibernation (Florant, Richardson, Mahan, Singer, & Woods, 1991), starvation (Urayama & Banks, 2008), obesity (Israel, et al., 1993; Kaiyala, Prigeon, Kahn, Woods, & Schwartz, 2000), triglycerides (Urayama & Banks, 2008), iron staus (Ben-Shachar, Yehuda, Finberg, Spanier, & Youdim, 1988), hyperglycemia (Banks, Jaspan, & Kastin, 1997a; Nakaoke, Verma, Niwa, Dohgu, & Banks, 2007), the diabetic state independently of insulin or glucose levels (Banks, Jaspan, et al., 1997a), nitric oxide levels (Banks, Dohgu, et al., 2008; Xaio, Banks, Niehoff, & Morley, 2001), and activation of the innate immune system (Banks, Dohgu, et al., 2008; Xaio, et al., 2001). About 0.05% of an intravenously injected dose of insulin is taken up per gram of whole brain by the mouse (Banks, Jaspan, Huang, et al., 1997; Banks & Kastin, 1998).
The insulin transporter is not known to transport other hormones with the exception of small amounts of IGF-I (Yu, Kastin, & Pan, 2006); it has been formally demonstrated that it does not transport leptin or amylin (Banks & Kastin, 1998; Banks, Kastin, Huang, Jaspan, & Maness, 1996). The insulin transport protein is assumed to be the same protein as that which functions as the receptor (Duffy & Pardridge, 1987; Frank, et al., 1985). However, no direct evidence supports or refutes this untested assumption. As for other peptide and regulatory proteins, in some cases BBB transport is lost when the receptor(s) are knocked-out as exemplified by tumor necrosis alpha (Pan & Kastin, 2002), whereas in other cases the BBB transporter is not the same protein as the receptor as exemplified by epidermal growth factor and leptin (Banks, Niehoff, Martin, & Farrell, 2002; Pan & Kastin, 1999).
A saturable mechanism for insulin transport across the BBB means that at some point, increasing serum insulin will not result in meaningful increases in brain insulin. A critical question is whether significant levels of insulin enter the brain at serum levels that are above or below those needed to produce hypoglycemia. If on the one hand brain levels of insulin are significant only when serum levels of insulin are so high that they produce hypoglycemia, then this could be taken as evidence that CNS insulin acts to counter hypoglycemia. Consistent with this, CNS insulin in several studies does have opposite effects to those of serum insulin on serum glucose and feeding, increasing glucose rather than decreasing it and decreasing serum insulin and feeding rather than increasing them (Ajaya & Haranath, 1982; Brief & Davis, 1984; Bruning, et al., 2000a; Debons, Krimsky, & From, 1970; D. P. Figlewicz, et al., 1995; Florant, Singer, et al., 1991; Hatfield, Millard, & Smith, 1974; Schwartz, Figlewicz, Baskin, Woods, & Porte, 1992; Strubbe & Mein, 1977; Woods & Porte, 1983). Such paradoxical effects have been noted for several peptides and proteins, suggesting a form of brain-body communication in which the substance within the CNS acts in counter-regulatory fashion to its functions at peripheral tissues (Banks & Kastin, 1993). Furthermore, rats given antibody to insulin into the brain have increased feeding and gain weight and mice with the brain insulin receptor knocked out have increased serum insulin and can develop diet-sensitive obesity (Bruning, et al., 2000a; McGowan, Andrews, & Grossman, 1992; Strubbe & Mein, 1977). As hypoglycemia is not a physiologic event, it could be concluded that CNS insulin acts as a counterregulatory hormone to serum insulin, helping to curb hypoglycemia. If on the other hand brain levels of insulin are significant when serum levels are in the physiologic, euglycemic range, then this could be taken as evidence that CNS insulin has physiologic effects. The evidence seems to support this latter physiologic role for CNS insulin. A linear relation exists between serum and CSF levels of insulin, demonstrating that insulin is crossing the BBB under physiologic conditions (Greco, et al., 1970; Margolis & Altszuler, 1967; Woods & Porte, 1977). Studies with exogenous and radioactively labeled insulin suggest that at least 50% of maximal transport capacity is reached at euglycemic levels of serum insulin (Banks, Jaspan, Huang, et al., 1997; Banks, Jaspan, et al., 1997b). Insulin has multiple effects in the CNS, so it could be that CNS insulin functions at both physiologic, euglycemic levels and as a counter-regulatory hormone during hypoglycemia.
Insulin transport varies among brain regions. The olfactory bulb tends to have the fastest rate of transport, being about 2–6 times faster than the rate of uptake in the remaining brain. The olfactory bulb also has the highest concentration of insulin protein (Baskin, Porte, Guest, & Dorsa, 1983), the highest concentration of insulin receptors (Gupta, Azam, & Baquer, 1992; Hill, Lesniak, Pert, & Roth, 1998), and the highest rate of insulin degradation. Uptake into spinal cord is usually faster than into whole brain (Banks, et al., 1999). Not all regions of the brain transport insulin. In ICR mice from Charles River laboratories, the BBB does not transport insulin into at the midbrain, thalamus, or occipital cortex (Banks & Kastin, 1998). In contrast, amylin is transported into all regions of the brain. In the SAMP8 mouse, insulin is not transported into the occipital cortex, pons-medulla, hippocampus, or hypothalamus (Banks, et al., 2000).
Treating mice with lipopolysaccharide (LPS), a derivative of the bacterial coat from gram negative bacteria and an activator of the innate immune system, enhances the saturable component of insulin uptake by brain by about 3–5 fold (Xaio, et al., 2001). This uptake is dependent on nitric oxide but is not affected by indomethacin, an inhibitor of prostaglandin synthesis. LPS has complex effects on nitric oxide synthases (NOSs), increasing mRNA or protein levels for inducible (iNOS) and endothelial (eNOS) NOS, but decreasing those for neuronal NOS (nNOS). The source of nitric oxide is important, that generated from nNOS inhibiting insulin transport and that generated from eNOS and iNOS stimulating transport (Banks, Dohgu, et al., 2008). Since nitric oxide is the same compound regardless of the isoenzyme which generates it, this suggests that some of the effects of nitric oxide are indirect, exerted through other cells of the neurovascular unit (NVU) that then affect BBB transport. This is confirmed in that LPS incubated with brain endothelial cells in vitro does not increase insulin transport unless another cell type is also present (Banks, Dohgu, et al., 2008). This is not because brain endothelial cells in culture are unable to respond to LPS; indeed, they robustly release cytokines in the presence of LPS (Reyes, Fabry, & Coe, 1999; Verma, Nakaoke, Dohgu, & Banks, 2006). Instead, LPS likely releases nitric oxide from other cells of the NVU, these cells in turn releasing substances that act on the transport of insulin across the BBB. Evidence suggests that the insulin resistance induced by LPS is mediated through NOS (Sugita, et al., 2002). As discussed elsewhere, CNS insulin may act in a counter-regulatory fashion to insulin in the periphery. Hence, one mechanism by which LPS and NOS could induce insulin resistance is by increasing the influx of insulin into the brain.
The above considerations have a therapeutic importance, suggesting that an enhanced BBB-penetrating synthetic insulin might produce less hypoglycemia and less weight gain. This mechanism has been suggested to explain why insulin detemir, the synthetic long-acting insulin analogue, produces less hypoglycemia and weight gain than many other insulins (Davies, Derezinski, Pedersen, & Clauson, 2008; Fajardo Montanana, Hernandex Herrero, & Rivas Fernandez, 2008; Rossetti, et al., 2008). However, insulin detemir does not cross the BBB (Banks, Morley, Lynch, Lynch, & Mooradian, 2010), suggesting than some other mechanism is protecting against hypoglycemia.
Although there is no saturable efflux on insulin administered directly into the CNS, retention by brain of insulin given as an intracerebroventricular (ICV) injection is altered by certain events. Mice that are starved, given intraperitoneal injections of tumor necrosis factor-alpha (TNF), or treated with aluminum all have increased retention of ICV administered insulin, whereas mice given ICV TNF, fasted, or starved and refed do not have an altered retention of insulin (Cashion, et al., 1996). Starvation increased retention of ICV insulin in TNF double receptor knockout mice and infusions of TNF for 48 hours decreased body weight but did not alter insulin retention; these results show that the effect of starvation on insulin retention are not mediated through TNF.
8. Insulin Action in the Brain: Behavioral Effects
CNS Insulin is emerging as a substance with widespread effects on brain. Broadly speaking, two major areas of behavior affected by insulin are those related to feeding and to cognition. These are areas of intensive research and only a brief overview of some of the significant findings can be presented here. In some, but not all, cases, work was performed by delivery to brain of small doses of insulin, making it unlikely that insulin was acting through peripheral mechanisms such as lowering blood glucose or stimulating vagal afferents. Insulin thus belongs to a category of peptides that have effects both on feeding and cognition, that category including leptin, ghrelin, glucagonlike peptide/exendin, melanocyte stimulating hormone, vasoactive intestinal peptide, pituitary adenylate cyclase activating polypeptide, secretion, cholecystokinin, substance P, and orexin A. The evolutionary underpinnings of this relation have been explored elsewhere (Banks, in press).
One of the earliest actions found for CNS insulin is its effects on feeding and serum glucose. In general, CNS insulin has the opposite effects of peripheral insulin, increasing blood glucose levels, decreasing feeding and body weight, and even decreasing blood levels of insulin (Ajaya & Haranath, 1982; Brief & Davis, 1984; Bruning, et al., 2000a; Debons, et al., 1970; D. P. Figlewicz, et al., 1995; Florant, Singer, et al., 1991; Hatfield, et al., 1974; Schwartz, et al., 1992; Strubbe & Mein, 1977; Woods & Porte, 1983). Insulin is intimately connected with other brain peptides and circuits, including those related to reward such as that of the opiates (D.P. Figlewicz & Sipols, 2010), another system long known to be involved in feeding and reward (Gosnell, Levine, & Morley, 1986). Consistent with this model of interactive, overlapping neuronal circuitries, insulin given by ICV administration enhances the meal-suppressive efficacy of ICV cholecystokinin octapeptide in the baboon (D. P. Figlewicz, et al., 1995) and short-term or long-term ICV infusion of insulin exhibits a discrete anabolic effect on cerebral energy metabolism in the rat (Henneberg & Hoyer, 1994).
The effects of CNS insulin on aspects related to cognition are well documented in vivo and in vitro, in animals and man, in disease states and in health. Although peripheral increases in insulin, insulin resistance, and other aspects of the metabolic syndrome are risk factors for AD (Luschsinger, Tang, Shea, & Mayeux, 2004), it seems that in brain it is a deficiency in insulin and insulin action that occurs in AD. Insulin, insulin receptor, and C peptide concentrations in brain correlate with and decrease with aging and in patients with AD (Frolich, et al., 1998a). Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid beta peptide in memory-impaired adults (Reger, et al., 2008), normal volunteers (Benedict, et al., 2004), and in patients with AD (Craft, et al., 1999). In rats, ICV administered insulin reverses place preference conditioning with high-fat diet (D. P. Figlewicz, 2004).
Many mechanisms have been suggested that could explain the link between AD and insulin. As noted in the section on evolution, insulin in the CNS seems to have retained its role as a growth factor, including effects on synaptogenesis and nerve growth (T.J. Nelson, M.K. Sun, J. Hongpaisan, & D.L. Alkon, 2008). Thus, a resistance to insulin or its actions could lead to cognitive decline (Hoyer, 1998, 2004). Consistent with this modeling, diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of AD (Ho, et al., 2004), suggesting that insulin and amyloid beta peptide production are closely related. Both peptides are substrates for insulin degrading enzyme (Qui, et al., 1998) and amyloid beta peptide may be able to bind to the insulin receptor (Xie, et al., 2002). Furthermore, insulin transport across the BBB is increased in the majority of brain regions examined in transgenic mice overexpressing APP (Poduslo, et al., 2001). This is consistent with a report showing that insulin increases CSF amyloid-beta 42 levels in normal older adults (Watson, et al., 2003).
Low-density lipoprotein receptor related protein-1 (LRP-1), a multifunctional receptor expressed in a variety of tissues (Lillis, Van Duyn, Murphy-Ullrich, & Strickland, 2008), also connects insulin and amyloid beta protein on a number of levels. In the periphery, insulin facilitates the hepatic clearance of plasma amyloid beta peptide (Banks, Maness, Banks, & Kastin) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP1) to the plasma membrane in hepatocytes (Tamaki, Ohtsuki, & Terasaki, 2007). LRP is also responsible for the brain-to-blood clearance of amyloid beta peptide (Deane, Sagare, & Zlokovic, 2008; Deane, et al., 2004b), raising the possibility that CNS insulin may affect efflux in the brain. Insulin deficiency induced by streptozotocin also downregulates LRP-1 at the BBB (Hong, et al., 2009) and the insulin-sensitizing drug rosiglitazone upregulates LRP-1 expression in cultured human brain endothelial cells (Moon, et al., 2011).
Neuronal LRP-1 is also involved in insulin signaling and AD. LRP-1, either alone or in conjunction with adaptor proteins such as heparin sulfate proteoglycan facilitates the intraneuronal internalization of amyloid beta peptide (Deane, et al., 2004a; Fuentealba, et al., 2010; Kanekiyo, et al., 2011). LRP-1 also enhances neuronal production of amyloid beta peptide by routing APP to endosomal compartments where β-secretase is most active (Kinoshita, et al., 2001; Kounnas, et al., 1995; Pietrzik, et al., 2004; Waldron, et al., 2008; Yoon, Pietrzik, Kang, & Koo, 2005). Increased neuronal expression of LRP-1 has been observed both in the SAMP8 model of AD as well as in human AD hippocampus (Donahue, et al., 2006; Erickson, et al., 2012), suggesting a potential cause for increased neuronal uptake of amyloid beta peptide. LRP-1 also regulates insulin signaling in the CNS, as neuron-specific knockout or knockdown of LRP-1 results in decreased neuronal expression of the insulin receptor and decreased insulin signaling (Fuentealba, Liu, Kanekiyo, Zhang, & Bu, 2009). Decreases in LRP-1 are also associated with increased neuronal susceptibility to apoptosis (Fuentealba, et al., 2009). This indicates that the neuroprotective effects of insulin signaling are dependent on LRP-1 expression. Although increased expression of neuronal LRP-1 is observed in AD hippocampus compared to age-matched controls (Donahue, et al., 2006), decreases in LRP-1 expression in the brain occur with aging (Kang, et al., 2000). Therefore, increases or decreases in neuronal LRP-1 can cause neuronal dysfunction. It has yet to be determined whether increased LRP-1 expression in AD is related to aberrant insulin signaling. Also unclear is whether insulin levels in the CNS or periphery affect neuronal LRP-1 expression.
Insulin has been proposed to have other effects on brain function. Down regulation of hypothalamic insulin receptor expression elicits depressive-like behavior in rats (C. A. Grillo, et al., 2011). Brain insulin modulates baroreceptors and insulin-baroreceptor regulatory dysfunction may underlie the increased risk for hypotension in pregnancy as well as preeclampsia, the hypertensive emergency of pregnancy (Azar & Brooks, 2011). Future work is likely to find additional roles for insulin in the CNS.
9. Insulin Action in the Brain: Receptors and Signal Transduction Pathways
The discovery of the presence of receptors for insulin and the insulin-like growth factors in the brain (Baskin, et al., 1986; Havrankova, Roth, & Brownstein, 1978; J. L. Marks, Porte, Stahl, & Baskin, 1990; Unger, et al., 1989) led to studies that recognized the importance of insulin and IGF signaling in non-metabolic functions including neuronal survival (Valenciano, Corrochano, de Pablo, de la Villa, & de la Rosa, 2006), synaptic and dendritic plasticity (Beattie, et al., 2000; Chiu, Chen, & Cline, 2008; Dou, Chen, Dufour, Alkon, & Zhao, 2005; Man, et al., 2000; Passafaro, Piech, & Sheng, 2001; Skeberdis, Lan, Zheng, Zukin, & Bennett, 2001; Valenciano, et al., 2006; Wan, et al., 1997; Zhao, et al., 1999), learning and memory (Dou, et al., 2005; Zhao, et al., 1999), and neuronal circuitry formation (Chiu, et al., 2008).
Insulin receptor (IR) signaling is well defined in the periphery and the mechanism of insulin signal transduction through the IR is mediated by the action of insulin receptor substrate (IRS) molecules on two canonical pathways, the phosphoinositide-3 kinase (PI3)/Akt and Ras/mitogen activated kinase (MAPK) cascades. These pathways are essential for glucose, lipid, and protein metabolism in the liver, muscle and adipose tissue and are the subject of many excellent reviews (Bell, Pilkis, Weber, & Polonsky, 1996; Saltiel, 2001; Saltiel & Kahn, 2001; Withers & White, 2000). These canonical pathways are also involved in mediating insulin signaling in the CNS (Niswender, et al., 2003), but the task of understanding the role of IR signaling in the brain has only recently been undertaken.
The IR is distributed throughout the brain, but has the highest concentration in the olfactory bulb, cerebral cortex, hypothalamus, hippocampus, and cerebellum (Havrankova, et al., 1978; Unger, et al., 1989; Wozniak, Rydzewski, Baker, & Raizada, 1993). The IR levels are also higher in neurons compared to glial cells (Unger, et al., 1989) and levels decrease with age, suggesting that insulin signaling and IR levels are involved in the aging process (Bosco, Fava, Plastino, Montalcini, & Pujia, 2011; Chung, Shin, Joo, Kim, & Cha, 2002; Frolich, et al., 1998b). Recent studies have shown that IR signaling is important for synaptic function and dendritic morphology.
Neuronal Survival
Studies conducted in rat hippocampal cell culture have shown that IR signaling promotes cell survival under oxygen and glucose deprivation (OGD) (Mielke, Taghibiglou, & Wang, 2006). A potential mechanism of insulin action to protect neurons from OGD-induced cell death was proposed by the authors involving increased γ-aminobutyric acid (GABA) signaling, but an exact mechanism remains to be elucidated. In addition to OGD-induced cell death, insulin protects embryonic retinal cells during development from caspase and cathepsin-mediated apoptosis by reducing the levels of these pro-apoptotic proteins (Valenciano, et al., 2006).
Synaptic Maintenance
Insulin modifies post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor levels. Insulin accelerates the clatharin-dependent endocytosis of AMPA receptors containing the glutamate GluR2 subunit, which is critical for the process of long-term depression in rat hippocampal cell culture (Beattie, et al., 2000; Lin, et al., 2000; Man, et al., 2000; Zhou, Xiao, & Nicoll, 2001). However, cell culture studies have also shown that insulin leads to the insertion of GluR1 subunit containing AMPA receptors (Passafaro, et al., 2001). IR signaling also promotes the addition of GABA receptors to the post-synaptic membrane (Wan, et al., 1997). These findings support the role of insulin signaling in recruiting the machinery necessary for both excitatory and inhibitory neurotransmission.
Dendritic Arbor Development
The canonical insulin signaling PI3/Akt and Ras/MAPK cascades include components that have been implicated in dynamics of dendritic arbor formation. IRSp53, a novel insulin receptor substrate found in the brain, has been shown to localize to the post-synaptic density (Abbott, Wells, & Fallon, 1999) and has been hypothesized to interact and stabilize the scaffolding proteins of the cytoskeleton in the post-synaptic density region (Chiu & Cline, 2010). In addition, cell culture studies have shown that increased expression of IRSp53 promotes dendritic arbor development (Govind, Kozma, Monfries, Lim, & Ahmed, 2001), whereas cells treated with RNA interference against IRSp53 exhibits the converse effect (Choi, et al., 2005). These studies show that a key component in IR signaling pathways plays a key role in the dynamics of dendrite formation.
Learning and Memory
Behavioral studies have shown that IR levels are affected by both short and long-term memory consolidation in rat hippocampus (Dou, et al., 2005; Zhao, et al., 1999). These behavioral studies show that rats that have been trained in water maze tests have altered IR patterns in both the CA1 and CA3 regions of the hippocampus. Interestingly, the authors showed that IR levels change while other neurotransmitter receptors levels, including the NMDA and AMPA receptors, do not (Dou, et al., 2005). These findings suggest that IR signaling modulates the activity of known synaptic maintenance receptors.
A study examining the effects of intranasal insulin delivery over the course of 1 week in mice showed an increased expression of potassium ion channel Kv1.3 in the olfactory bulb (D. R. Marks, Tucker, Cavallin, Mast, & Fadool, 2009). The olfactory bulb has the highest level of IR in the brain and the effects of insulin in glucose responsive (i.e., neuronal action potentials stimulated by glucose binding to IR) and glucose sensitive neurons (i.e., neuron action potentials are inhibited by glucose binding to IR) have been hypothesized to be mediated by the action of Kv1.3 activity (Plum, Schubert, & Bruning, 2005). Mice that received intranasal insulin have improved cognition, as shown by short and long-term object recognition. These findings suggest that insulin delivered to the CNS via an intranasal route increases neuronal activity and improves memory by mechanisms involving changes in Kv1.3 levels.
Neuronal circuitry formation
Experiments conducted in Xenopus tadpoles have shown that IR signaling is critical for the optic neuronal circuit formation (Chiu, et al., 2008). The authors showed that IR knockout in the tectal neurons led to decreased electrophysiological responses to light stimulation. In addition, studies have shown that IR signaling is important for controlling body weight and reproduction (Bruning, et al., 2000b). The authors used neuron specific disruption of the IR gene mice to show that the absence of insulin receptors leads to an increase in body fat and plasma leptin levels. Also, male and female mice with the neuron specific diruption of the IR gene showed decreased spermatogenesis and ovarian follicle formation, respectively; these effects were associated with impaired hypothalamic luteinizing hormone signaling. These findings show that brain IR signaling is necessary for the CNS response to both environmental and peripheral tissue stimuli.
Non-canonical IR signaling
The effects of insulin have traditionally been thought to occur through the action of the PI3/Akt and Ras/MAPK signaling cascades. However, studies elucidating insulin effects transduced through the protein kinase C (PKC)/NF-κB pathway have started to surface. One such study showed that insulin regulates pglycoprotein in rat brain microvessel endothethial cells via the PKC/NF-κB cascade and not through the PI3/Akt pathway (H. Liu, Yang, Wang, Liu, Liu, et al., 2009). The authors showed that using pharmacological inhibitors of PKC/NK-κB lead to decreased p-glycoprotein levels and activity. Conversely, P-glycoprotein levels and activity were not affected by PI3/Akt inhibition. The PKC/NF-κB pathway has been overlooked as a potential effector of insulin activity. Potential reasons for this oversight include the overlap in the mechanisms of PKC and PI3 activity, including PKC activity leading to increased levels of PI3 directly (T. J. Nelson, M. K. Sun, J. Hongpaisan, & D. L. Alkon, 2008). Many PKC signaling pathways, such as the activation of PKC by inositol triphosphate and diacyl glycerol directly, are integrally linked to PI3 signaling pathways making the resolution of PKC pathways independent of PKC/Akt signaling difficult (T. J. Nelson, et al., 2008). However, the availability of specific pharmacological inhibitors of the components of PI3/Akt and PKC/NF-κB machinery will make elucidating the specific pathways of insulin action possible in future studies.
10. When Things Go Wrong Part I: Altered BBB Transport of Insulin
Obese animals have a decreased transport of insulin into brain (Israel, et al., 1993; Kaiyala, et al., 2000; Stein, et al., 1987; Urayama & Banks, 2008) as well as resistance to the ability of CNS insulin to reduce food intake (Bruning, et al., 2000a; Clegg, et al., 2005). The obese Zucker rat has reduced levels of insulin in the brain, especially in the olfactory bulb and hypothalamus (Baskin, et al., 1985). Starvation for 48 hours reversed the inhibition in insulin transport in obese mice, even though the obese mice only lost about 10% of body weight and still weighed almost twice as much as the thin mice (Urayama & Banks, 2008). When mice were studied by the brain perfusion method, a technique that removes the immediate influence of circulating factors on BBB activity, the thin, obese, and obese-starved mice all transported insulin at the same rate (Urayama & Banks, 2008). This shows that circulating factors influence insulin transport across the BBB. One such factor known to influence ghrelin and leptin transport is triglycerides (Banks, Burney, & Robinson, 2008; Banks, et al., 2004). Triglycerides are elevated in both starvation and obesity and hypertriglyceridemia is the dyslipidemia most associated with the metabolic syndrome (Morley, 2004). When included in the brain perfusion, triglycerides stimulated insulin transport across the BBB (Urayama & Banks, 2008).
Diabetic animals have increased transport of insulin into the brain even when studied by the brain perfusion method, a technique that removes the immediate influence of serum glucose, serum insulin, and other circulating factors on BBB transporters (Banks, Jaspan, et al., 1997a). In fact, both in vivo and in vitro evidence suggests that glucose can have an inhibitory influence on insulin transport across the BBB. In vivo, mice treated with glucose have a decreased transport rate of insulin across the BBB. Dexamethasone also inhibits insulin transport (Baura, et al., 1996); whether this is mediated through dexamethasone’s ability to increase serum glucose is unclear.
Diabetes mellitus, hyperglycemia in vivo, or altered glycemic concentrations in vitro have been associated with other alterations in insulin-BEC interactions. For example, insulin’s ability to enhance leptin transport across the BBB is lost in diabetic mice (Kastin & Akerstrom, 2001), and insulin-enhanced expression of the glutamate-cysteine ligase catalytic subunit is dependent on glucose concentration (Langston, et al., 2011). Huber et al (Huber, VanGilder, & Houser, 2006) and Bendayan (Bouchard, Ghitescu, & Bendayan, 2002) have shown that the BBB is disrupted in diabetic mice. This disruption varies among brain regions, is molecular weight dependent, and progresses over time. Shah et al. (Price, Eranki, Banks, Ercal, & Shah, 2012) have presented evidence that vascular pericytes protect the BBB from the disruption of diabetes, but that pericytes eventually succumb to the oxidative stress of hyperglycemia, leaving the brain endothelial cells susceptible to the diabetic state.
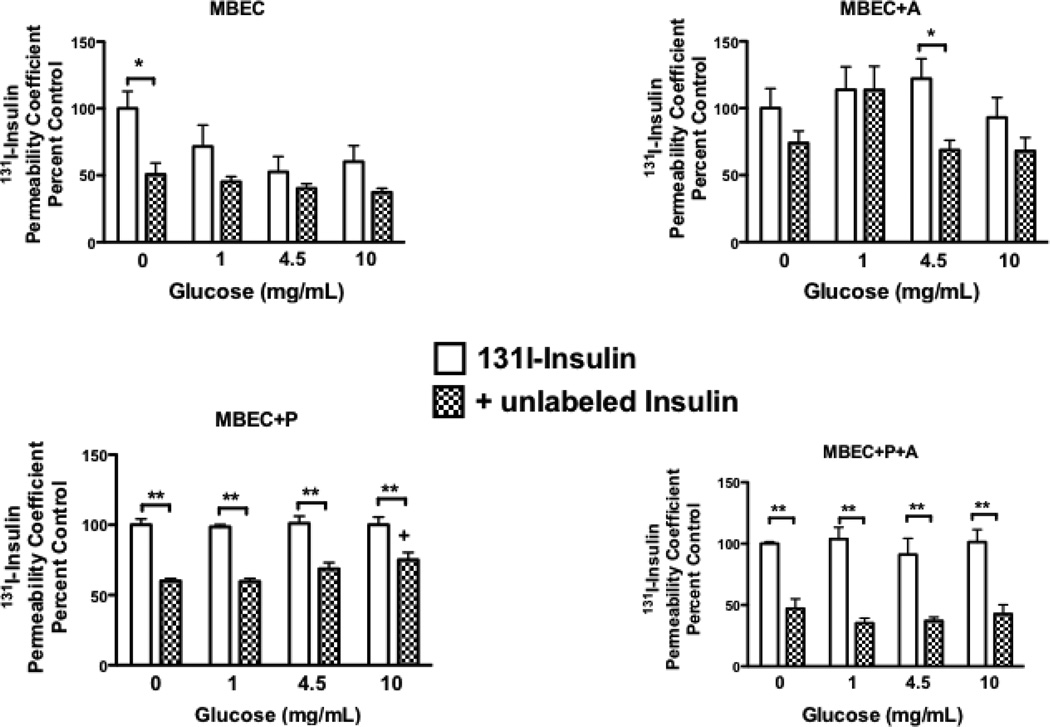
As noted above, pericytes may play a crucial role in maintaining the ability of BECs to transport insulin (Nakaoke, et al., 2007). We examined in vitro the ability of monolayers of BECs to transport insulin by a saturable mechanism at various concentrations of glucose (figure 3). We found that endothelial cells grown alone or co-cultured with astrocytes demonstrated minimal saturable transport of insulin at most concentrations of glucose (Nakaoke, et al., 2007). When grown with pericytes, however, BECs transported insulin at all concentrations of glucose. Tricultures of pericytes and astrocytes with the BECs did not seem to improve transport of insulin in comparison to co-cultures of pericytes with BECs (Nakaoke, et al., 2007).
Figure 3.
Effect of Glucose on Insulin Transport Across Brain Endothelial Cells: Protective Effect of Pericytes. Primary monolayer cultures of mouse (CD-1 strain) brain endothelial cells were cultured alone (MBEC), in co-culture with astrocytes (MBEC + A) or with pericytes (MBEC + P), or in triculture with pericytes and astrocytes (MBEC + P + A). Cultures were incubated for 2 h at various concentrations of glucose ranging from 0 to 10 mg/ml. After the two hours, the glucose concentration was returned to 4.5 mg/ml, adioactive insulin with our without unlabeled insulin added to the luminal chamber, and abluminal contents sampled at 5, 10, 20, and 30 min. The permeability coefficient was calculated and expressed relative to the (0 mg/ml glucose radioactive insulin only group). A difference between the radioactive and the + unlabeled insulin group demonstrates the presence of saturable insulin transport. The results indicate that pericytes are important to the saturable transport of insulin across brain endothelial cells. *p<0.05 and **p<0.01 between indicated comparisons; + p<0.05 incomparison to the panel’s “0 mg/ml unlabeled insulin” group.
Altered transport across the BBB has been suggested in other CNS insulin-mediated behaviors. For example, lower CSF levels in both pregnancy (Azar & Brooks, 2011; Daubert, Chung, & Brooks, 2007) and AD suggest decreased transport of insulin across the BBB in these conditions. Increased levels of fasting plasma insulin, lower levels of insulin in CSF, and decreased CSF/serum ratios for insulin in AD are consistent with impaired transport of insulin at the BBB (Craft, et al., 1998).
11. When Things Go Wrong Part II: CNS Insulin Receptor Resistance/Diabetes Mellitus Type III
The introduction discussed DM1 and DM2 as conditions of insulin deficiency and insulin resistance, respectively (Figure 2). More specifically, DM2 is a resistance at insulin receptors in the peripheral tissues. By extension, then, diabetes mellitus type III (DM3) is a resistance at the insulin receptors in the CNS. Several studies have reported resistance to insulin at CNS receptors; basically, these studies have shown less activation of cellular machinery when the cells or tissues are exposed to insulin. It is becoming increasingly clear that DM2 involves dysfunction of the CNS circuitries that help to control appetite and hepatic glucose production (Sandoval, Obici, & Seeley, 2009). An important question is whether animals that have DM2 automatically have DM3.
The reason that DM2 (insulin resistance at peripheral tissues) and DM3 (insulin resistance at the CNS) should not be assumed to occur together can be explained if one considers other resistance states such as that to thyroid hormones. Like insulin, thyroid hormones have a largely metabolic role in peripheral tissues, being secreted largely by a single tissue, having that secretion controlled by way of a negative feedback loop, and crossing the BBB by a saturable transporter to affect feeding and cognition through CNS receptors. Like insulin, resistance syndromes to the thyroid hormones exist. In the generalized resistance syndrome, the level of thyroid hormones is elevated to maintain the euthyroid state, just as in the insulin resistance syndrome (IRS), the level of insulin is elevated to maintain the euglycemic state. Variable tissue resistance occurs when the degree of tissue sensitivity to thyroid hormone varies among tissues (Refetoff, 1982). The degree of elevation in thyroid hormone is dictated by the degree to which thyroid hormone resistance is sensed at the pituitary, just as the degree of elevation in insulin is dictated by the degree to which insulin resistance is sensed by the pancreas. Thus, a tissue with more resistance than the pituitary is in a relative state of hypothyroidism, while a tissue with less resistance than the pituitary is in a relative state of hyperthyroidism. An animal with variable tissue resistance to thyroid hormone can present with a mixed clinical state with, for example, impaired cognition but normal bone growth.
Can variable tissue resistance exist for insulin? If so, DM2 (i.e., resistance at peripheral tissue receptors) and DM3 (i.e., resistance at CNS receptors) may occur together to varying degrees, as well as independently. Such an interdependent relation is a prerequisite to the extent that DM3 is synonymous with AD as DM2 is both a risk factor for AD and yet is absent in many AD patients. Variable tissue resistance provides a mechanism by which the CNS can have a functional excess or deficiency in insulin relative to peripheral tissues, explaining why administration of insulin to the brain by the intranasal route can improve cognition in AD, and how impaired insulin action in the brain can contribute to orthostastis in pregnancy (Azar & Brooks, 2011; Daubert, et al., 2007).
The counterregulatory mechanisms for CNS insulin resistance are dramatically different from those for peripheral resistance. CNS resistance does not reduce blood glucose; therefore, it would not result in a compensatory elevation in insulin through any known mechanism. In comparison, elevations in serum insulin resulting from IRS and DM2 have an attenuated impact on CNS levels of insulin because transport of insulin across the BBB is saturable.
These considerations raise the question of whether the functional consequences of DM2 and DM3 on cognition are similar. Although cognitive impairment is common to DM2 and Alzheimer’s disease, the domains affected are dissimilar with, for example, executive function being more affected in DM2 (Yau, et al., 2010). Magnetic resonance imaging in either disease shows white matter changes and cerebral atrophy (Manschot, et al., 2006), but infarcts are more common in diabetics with cognitive decline than nondiabetics with cognitive decline (Biessels, Koffeman, & Scheltens, 2006). Cognitive changes in DM2 occur at earlier ages than does that of Alzheimer’s disease, being detectable in middle age or even in adolescence (Hassenstab, Sweat, Bruehl, & Convit, 2010; Yau, et al., 2010). Other than the common feature of “insulin resistance”, DM2 and DM3 differ greatly in accompanying conditions and so likely have very different mechanisms leading to cognitive impairment; for example, DM2 by definition has hyperglycemia and is often accompanied by hypertension, obesity, and even iatrogenic hypoglycemia.
In summary, compartmentalization of CNS versus peripheral insulin resistance has important implications for how we view and investigate the relations among AD, DM2, and the metabolic syndrome. First, it suggests that measures of peripheral insulin resistance are imperfect reflections of the CNS state. Second, it advocates that direct measures of CNS status are needed in evaluation of insulin’s role in neurodegenerative diseases. Third, it provides a rationale for considering that causes or mechanisms of CNS resistance could be different from those of peripheral resistance. Fourth, it underscores the importance and rationale for directly treating the CNS with insulin or insulin-like drugs.
12. Conclusions
Insulin leads parallel lives, performing one set of actions among the peripheral tissues and another within the CNS. The evolution of insulin and its receptors from their ancestral forms gives clues to how this dichotomy may have arisen. It remains unclear, however, why a hormone once produced and acting within the CNS and peripheral tissues has retained functions in both compartments but production only by a single peripheral tissue. This makes insulin action with the CNS dependent on insulin production by the pancreas and on transport across the BBB. Whether resistance to insulin within the CNS is also immediately a result of insulin resistance at peripheral tissues is unclear. What is clear is that insulin is of major importance to the health of the CNS and that this importance may explain its therapeutic effects in neurodegenerative disease.
Acknowledgments
Supported by VA Merit Review (WAB), RO1 AG029839 (WAB), and RO1 DK083485 (WAB). We thank Emily E. Wing for help with figures. JBO was supported by NIH/NIA 5T32AG000258.
Abbreviations
- AD
Alzheimer’s disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APP
Amyloid precursor protein
- BBB
Blood-brain barrier
- BEC
Brain endothelial cell
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DM1
Diabetes mellitus type I
- DM2
Diabetes mellitus type II
- DM3
Diabetes mellitus type III
- GABA
gamma-aminobutyric acid
- GLUT
Glucose transporter
- ICV
Intracerebroventricular
- IGF
Insulin-like growth factor
- ILP
Insulin-like peptide
- IR
Insulin receptor
- IRS
Insulin resistance syndrome
- LPS
Lipopolysaccharide
- LRP-1
Low-density lipoprotein receptor related protein-1
- eNOS
Endothelial nitric oxide synthase
- iNOS
Inducible nitric oxide synthase
- nNOS
Neuronal nitric oxide synthase
- MAPK
Mitogen activated kinase
- NVU
Neurovascular unit
- OGD
Oxygen and glucose deprivation
- PI3
Phosphoinositide-3 kinase
- PKC
Protein kinase C
- TNF
Tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300–7308. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajaya B, Haranath PS. Effects of insulin administered into cerebrospinal fluid spaces on blood glucose in unanaesthetized and anaesthestized dogs. Indian Journal of Medical Research. 1982;75:607–615. [PubMed] [Google Scholar]
- Anonymous. American College of Endocrinology position statement on the insulin resistance syndrome: position statement. Endocrine Practice. 2003;9(Suppl 2):9–21. [PubMed] [Google Scholar]
- Ayre SG, Skaletski B, Mosnaim AD. Blood-brain barrier passage of azidothymidine in rats: effect of insulin. Research Communications in Chemical Pathology and Pharmacology. 1989;63:45–52. [PubMed] [Google Scholar]
- Azar AS, Brooks VL. Impaired baroreflex gain during pregnancy in concscious rats: role of brain insulin. Hypertension. 2011;57:283–288. doi: 10.1161/HYPERTENSIONAHA.110.162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. European Journal of Pharmacology. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Banks WA. Role of the blood-brain barrier in the evolution of feeding and cognition. New York Academy of Sciences. doi: 10.1111/j.1749-6632.2012.06568.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29:2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Banks WA, Dohgu S, Nakaoke R, Lynch JL, Fleegal-DeMotta MA, Erickson MA, Vo TQ. Nitric oxide isoenzymes regulate LPS-enhanced insulin transport across the blood-brain barrier. Endocrinology. 2008;149:1514–1523. doi: 10.1210/en.2007-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. Journal of Gerontology: Biological Science. 2000;55A:B601–B606. doi: 10.1093/gerona/55.12.b601. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: Saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997a;18:1577–1584. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific enzyme immunoassays. Peptides. 1997b;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Research Bulletin. 1985;15:287–292. doi: 10.1016/0361-9230(85)90153-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Physiological consequences of the passage of peptides across the blood-brain barrier. Reviews in the Neurosciences. 1993;4:365–372. doi: 10.1515/revneuro.1993.4.4.365. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Pan W. Uptake and degradation of blood-borne insulin by the olfactory bulb. Peptides. 1999;20:373–378. doi: 10.1016/s0196-9781(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Banks WA, Maness LM, Banks MF, Kastin AJ. Aluminum-sensitive degradation of amyloid ·-protein 1–40 by murine and human intracellular enzymes. Neurotoxicology and Teratology. 1996;18:671–677. doi: 10.1016/s0892-0362(96)00084-0. [DOI] [PubMed] [Google Scholar]
- Banks WA, Morley JE, Lynch JA, Lynch KM, Mooradian AD. Insulin detemir is not transported across the blood-brain barrier. Peptides. 2010;31:2284–2288. doi: 10.1016/j.peptides.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Niehoff ML, Martin D, Farrell CL. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Research. 2002;950:130–136. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]
- Banting FG, Best CH. The internal secretion of the pancreas. Journal of Laboratory and Clinical Medicine. 1922;7:251–266. [Google Scholar]
- Baskin DG, Brewitt B, Davidson DA, Corp E, Paquette T, Figlewicz DP, Lewellen TK, Graham MK, Woods SC, Dorsa DM. Quantitative autoradiographic evidence for insulin receptors in the choroid plexus of the rat brain. Diabetes. 1986;35:246–249. doi: 10.2337/diab.35.2.246. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Porte D, Jr, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain. Endocrinology. 1983;112:898–903. doi: 10.1210/endo-112-3-898. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr, Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sciences. 1985;36:627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Kaiyala K, Porte D, Jr, Kahn SE, Schwartz MW. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45:86–90. doi: 10.2337/diab.45.1.86. [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. Journal of Clinical Investigation. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bell GI, Pilkis SJ, Weber IT, Polonsky KS. Glucokinase mutations, insulin secretion, and diabetes mellitus. Annu Rev Physiol. 1996;58:171–186. doi: 10.1146/annurev.ph.58.030196.001131. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Yehuda S, Finberg JP, Spanier I, Youdim MB. Selective alteration in blood-brain barrier and insulin transport in iron-deficient rats. Journal of Neurochemisrty. 1988;50:1434–1437. doi: 10.1111/j.1471-4159.1988.tb03027.x. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Koffeman A, Scheltens P. Diabetes and cognitve impairment. Clinical diagnosis and brain imaging in patients attending a memory clinic. J Neurol. 2006;253:477–482. doi: 10.1007/s00415-005-0036-4. [DOI] [PubMed] [Google Scholar]
- Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer's disease pathogenesis. J Cell Mol Med. 2011;15:1807–1821. doi: 10.1111/j.1582-4934.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard P, Ghitescu LD, Bendayan M. Morpho-function studies of the blood-brain barrier in streptozotocin-induced diabetic rats. Diabetologia. 2002;45:1017–1025. doi: 10.1007/s00125-002-0853-2. [DOI] [PubMed] [Google Scholar]
- Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Research Bulletin. 1984;12:571–575. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science (Washington DC) 2000a;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000b;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Cangiano C, Cardelli-Cangiano P, Cascino A, Patrizi MA, Barberini F, Rossi F, Capocaccia L, Strom R. On the stimulation by insulin of tryptophan transport across the blood-brain barrier. Biochemistry International. 1983;7:617–627. [PubMed] [Google Scholar]
- Caruso MA, Sheridan MA. New insights into the signaling system and function of insulin in fish. Gen Comp Endocrinol. 2011;173:227–247. doi: 10.1016/j.ygcen.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Cashion MF, Banks WA, Kastin AJ. Sequestration of centrally administered insulin by the brain: effects of starvation, aluminum, and TNF-alpha. Hormones and Behavior. 1996;30:280–286. doi: 10.1006/hbeh.1996.0034. [DOI] [PubMed] [Google Scholar]
- Castonguay AC, Olson LJ, Dahms NM. Mannose 6-phosphate receptor homology (MRH) domain-containing lectins in the secretory pathway. Biochimica et Biophysica Acta. 2011;1810:815–826. doi: 10.1016/j.bbagen.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan RE, Martinez AM, Aragones MD, Miguel BG, Robles A. Insulin action on brain microvessels; effect on alkaline phosphatase. Biochemical and Biophysical Research Communications. 1988;150:583–590. doi: 10.1016/0006-291x(88)90433-0. [DOI] [PubMed] [Google Scholar]
- Chan SJ, Nagamatsu S, Cao Q-P, Steiner DF. Structure and evolution of insulin and insulin-like growth factors in chordates. Progress in Brain Research. 1992;92:15–24. doi: 10.1016/s0079-6123(08)61161-9. [DOI] [PubMed] [Google Scholar]
- Chan SJ, Steiner DF. Insulin through the ages: Phylogeny of a growth promoting and metabolic regulatory hormone. American Zoologist. 2000;40:213–222. [Google Scholar]
- Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ko J, Racz B, Burette A, Lee JR, Kim S, Na M, Lee HW, Kim K, Weinberg RJ, Kim E. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J Neurosci. 2005;25:869–879. doi: 10.1523/JNEUROSCI.3212-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Shin CM, Joo KM, Kim MJ, Cha CI. Region-specific alterations in insulin-like growth factor receptor type I in the cerebral cortex and hippocampus of aged rats. Brain Res. 2002;946:307–313. doi: 10.1016/s0006-8993(02)03041-x. [DOI] [PubMed] [Google Scholar]
- Clark GA. The influence ofthe vagus on the islets of Langerhans. Part I. Vagus hypoglycemia. J. Physiology. 1925;59:466–471. doi: 10.1113/jphysiol.1925.sp002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol. 2005;288:R981–R986. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Phychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrosinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- Daniel PM, Love ER, Moorhouse SR, Pratt OE. The effect of insulin upon the influx of tryptophan into the brain of the rabbit. J Physiol. 1981;312:551–562. doi: 10.1113/jphysiol.1981.sp013643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert DL, Chung M-Y, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol. 2007;292:R2188–R2195. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Derezinski T, Pedersen CB, Clauson P. Reduced weight gain with insulin detemir compared to NPH insulin is not explained by a reduction in hypoglycemica. Diabetes Technol Ther. 2008;10:273–277. doi: 10.1089/dia.2008.0282. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Zlokovic B. The role of the cell surface LRP and soluble LRP in blood-brain barrier A· clearance in Alzheimer's disease. Current Pharmaceutical Design. 2008;14:1601–1605. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004a;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004b;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Debons AF, Krimsky I, From A. A direct action of insulin on the hypothalamic satiety center. Am J Physiol. 1970;219:938–943. doi: 10.1152/ajplegacy.1970.219.4.938. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Ryerse JS, Robinson SM, Banks WA. Human immunodeficiency virus-1 uses the mannos-6-phosphate receptor to cross the blood-brain barrier. PLOS one. 2012 doi: 10.1371/journal.pone.0039565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem. 2005;12:646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Research. 1987;420:32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- Elgee NJ, Williams RH, Lee ND. Distribution and degradation studies with insulin-I131. Journal of Clinical Investigation. 1954;33:1252–1260. doi: 10.1172/JCI103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Niehoff ML, Farr SA, Morley JE, Dillman LA, Lynch KM, Banks WA. Peripheral administration of antisense oligonucleotides targeting the amyloid-beta protein precursor reverses AbetaPP and LRP-1 overexpression in the aged SAMP8 mouse brain. J Alzheimers Dis. 2012;28:951–960. doi: 10.3233/JAD-2011-111517. [DOI] [PubMed] [Google Scholar]
- Fajardo Montanana F, Hernandex Herrero C, Rivas Fernandez M. Less weight gain and hypoglycemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight Type 2 diabetes patients: the PREDICTIVE BMI clinical trial. Diabet med. 2008;25:916–923. doi: 10.1111/j.1464-5491.2008.02483.x. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ. Energy regulatory signals and food reward. Pharmacol Biochem Behav. 2010;97:15–24. doi: 10.1016/j.pbb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ, Seeley RJ, Chaves M, Woods SC, Porte D., Jr Intraventricular insulin enhances the meal-suppressive efficacy of intraventricular cholecystokinin octapeptide in the baboon. Behav.Neurosci. 1995;109:567–569. doi: 10.1037//0735-7044.109.3.567. [DOI] [PubMed] [Google Scholar]
- Florant GL, Richardson RD, Mahan S, Singer L, Woods SC. Seasonal changes in CSF insulin levels in marmots: insulin may not be a satiety signal for fasting in winter. American Journal of Physiology. 1991;260:R712–R716. doi: 10.1152/ajpregu.1991.260.4.R712. [DOI] [PubMed] [Google Scholar]
- Florant GL, Singer L, Scheurink AJW, Park CR, Richardson RD, Woods SC. Intraventricular insulin reduces food intake and body weight of marmots during the summer feeding period. Physiology and Behavior. 1991;49:335–338. doi: 10.1016/0031-9384(91)90053-q. [DOI] [PubMed] [Google Scholar]
- Frank HJL, Jankovic-Vokes T, Pardridge WM, Morris WL. Enhanced insulin binding to blood-brain barrier in vivo and to brain microvessels in vitro in newborn rabbits. Diabetes. 1985;34:728–733. doi: 10.2337/diab.34.8.728. [DOI] [PubMed] [Google Scholar]
- Frank HJL, Pardridge WM. A direct in vitro demonstration of insulin binding to isolated brain microvessel. Diabetes. 1981;30:757–761. doi: 10.2337/diab.30.9.757. [DOI] [PubMed] [Google Scholar]
- Frizzell RT, Jones EM, Davis SN, Biggers DW, Myers SR, Connolly CC, Neal DW, Jaspan JB, Cherrington AD. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes. 1993;42:1253–1261. doi: 10.2337/diab.42.9.1253. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. Journal of Neural Transmission. 1998a;105:423–428. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm. 1998b;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Fuentealba RA, Liu Q, Kanekiyo T, Zhang J, Bu G. Low density lipoprotein receptor-related protein 1 promotes anti-apoptotic signaling in neurons by activating Akt survival pathway. J Biol Chem. 2009;284:34045–34053. doi: 10.1074/jbc.M109.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Liu Q, Zhang J, Kanekiyo T, Hu X, Lee JM, LaDu MJ, Bu G. Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One. 2010;5:e11884. doi: 10.1371/journal.pone.0011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS, Morley JE. The stimulation of food intake by selective agaonists of mu, kappa and delta opiod receptors. Life Sci. 1986;38:1081–1088. doi: 10.1016/0024-3205(86)90243-2. [DOI] [PubMed] [Google Scholar]
- Govind S, Kozma R, Monfries C, Lim L, Ahmed S. Cdc42Hs facilitates cytoskeletal reorganization and neurite outgrowth by localizing the 58-kD insulin receptor substrate to filamentous actin. J Cell Biol. 2001;152:579–594. doi: 10.1083/jcb.152.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco AV, Ghirlanda G, Fedeli G, Gambassi G. Insulin in the cerebro spinal fluid of man. Eur Neurol. 1970;3:303–307. doi: 10.1159/000113983. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Research. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP. Downregulation of hypothalamic insulin receptor expression elicts depressive-like behavior in rats. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Azam M, Baquer NZ. Modulation of rat brain insulin receptor kinase activity in diabetes. Neurochemistry International. 1992;20:487–492. doi: 10.1016/0197-0186(92)90027-o. [DOI] [PubMed] [Google Scholar]
- Hassenstab JJ, Sweat V, Bruehl H, Convit A. Metabolic syndrome is associated with learning and recall impairment in middle age. Dement Geriatr Cogn Disord. 2010;29:356–362. doi: 10.1159/000296071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JS, Millard WJ, Smith CJV. Short-term influence of intraventromedial hypothalamic administration of insulin on feeding in normal and diabetic rats. Pharmacology Biochemistry and Behavior. 1974;2:223–226. doi: 10.1016/0091-3057(74)90056-2. [DOI] [PubMed] [Google Scholar]
- Haugaard N, Vaughan M, Haugaard ES, Stadie WC. Studies of radioactive injected labeled insulin. Journal of Biological Chemistry. 1954;208:549–563. [PubMed] [Google Scholar]
- Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20(suppl):268–273. [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein MJ. Concentrations of insulin and of insulin receptors in the brain are independent of peripheral insulin levels. Journal of Clinical Investigation. 1979;64:636–642. doi: 10.1172/JCI109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberg N, Hoyer S. Short-term or long-term intracerebroventricular (i.c.v.) infusion of insulin exhibits a discrete anabolic effect on cerebral energy metabolism in the rat. Neuroscience Letters. 1994;175:153–156. doi: 10.1016/0304-3940(94)91102-9. [DOI] [PubMed] [Google Scholar]
- Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1998;17:1127–1138. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hong H, Liu LP, Liao JM, Wang TS, Ye FY, Wu J, Wang YY, Wang Y, Li YQ, Long Y, Xia YZ. Downregulation of LRP1 [correction of LPR1] at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology. 2009;56:1054–1059. doi: 10.1016/j.neuropharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Is sporadic Alzheimer's disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. Journal of Neural Transmission. 1998;105:415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer's disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. American Journal of Physiology. 2006;291:H2660–H2668. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- Hurley JH, Shengwen Z, Bye LS, Marshall MS, DePaoli-Roach AA, Guan K, Fox AP, Yu L. Insulin signaling inhibits the 5-HT2c receptor in choroid plexus via MAP kinase. BMC Neuroscience. 2003;4:10. doi: 10.1186/1471-2202-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, Porte D, Jr, Figlewicz DP. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Research Bulletin. 1993;30:571–575. doi: 10.1016/0361-9230(93)90084-o. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Murphy VA. Acetazolamide and insulin alter choroid plexus epithelial cell [Na+], pH, and volume. Am J Physiol. 1990;258:F1538–F1546. doi: 10.1152/ajprenal.1990.258.6.F1538. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci. 2011;31:1644–1651. doi: 10.1523/JNEUROSCI.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer's disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 2001;73:237–242. doi: 10.1159/000054640. [DOI] [PubMed] [Google Scholar]
- Keller BT, Borchardt RT. Cultured bovine brain capillary endothelial cells (BBCEC) - a blood-brain barrier model for studying the binding and internalization of insulin and insulin-like growth factor 1. Federation Proceedings. 1987;46:416. [Google Scholar]
- Kinoshita A, Whelan CM, Smith CJ, Mikhailenko I, Rebeck GW, Strickland DK, Hyman BT. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J Neurosci. 2001;21:8354–8361. doi: 10.1523/JNEUROSCI.21-21-08354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Hafezi-Moghadam A, Thomas K, Wagner DD, Kahn CR. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood-brain barrier. Biochem Biophys Res Commun. 2004;317:315–320. doi: 10.1016/j.bbrc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Kurochkin IV, Goto S. Alzheimer's ·-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Letters. 1994;345:33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- Lacroix MC, Badonnel K, Meunier N, Tan F, Schlegel-Le Poupon C, Durieux D, Monnerie R, Baly C, Congar P, Salesse R, Cailol M. Expression of insulin system in the olfactory epithelium: first approaches to its role and regulation. J Neuroendocrinol. 2008;20:1176–1190. doi: 10.1111/j.1365-2826.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- Langston JW, Li W, Harrison L, Aw TY. Activation of promoter activity of the catalytic subunit of gamma-glutamylcysteine ligase (GCL) in brain endothelial cells by insulin requires antioxidant response element 4 and altered glycemic status: implication for GCL expression and GSH synthesis. Fee Radic Biol Med. 2011;51:1749–1757. doi: 10.1016/j.freeradbiomed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang H, Wang D, Liu Y, Li Y, Xie L, G W. Insulin regulates P-glycoprotein in rat brain microvessel endothelial cells via an insulin receptor-mediated PKC/NF-kappaB pathway but not a PI3KAkt pathway. Eur J Pharmacol. 2009;2009:277–282. doi: 10.1016/j.ejphar.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang H, Wang D, Liu Y, Liu X, Li Y, Xie L, Wang G. Insulin regulates P-glycoprotein in rat brain microvessel endothelial cells via an insulin receptor-mediated PKC/NF-kappaB pathway but not a PI3K/Akt pathway. European journal of pharmacology. 2009;602:277–282. doi: 10.1016/j.ejphar.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Liu X, Jing XY, Jin S, Li Y, Liu L, Yu YL, Liu XD, Xie L. Insulin suppresses the expression and function of breast cancer resistance protein in primary cultures of rat brain microvessel endothelial cells. Pharmacol Rep. 2011;63:487–493. doi: 10.1016/s1734-1140(11)70515-1. [DOI] [PubMed] [Google Scholar]
- Luschsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and the risk of Alzheimer's disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- Lynch JA, Banks WA. Opiate modulation of IL-1 alpha, IL-2, and TNF-alpha transport across the blood-brain barrier. Brain, Behavior, & Immunity. 2006;22:1096–1102. doi: 10.1016/j.bbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967;215:1375–1376. doi: 10.1038/2151375a0. [DOI] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6734–6751. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- McGowan MK, Andrews KM, Grossman SP. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiology and Behavior. 1992;51:753–766. doi: 10.1016/0031-9384(92)90112-f. [DOI] [PubMed] [Google Scholar]
- McRory JE, Sherwood NM. Ancient divergence of insulin and insulin-like growth factor. DNA and Cell Biology. 1997;16:939–949. doi: 10.1089/dna.1997.16.939. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Taghibiglou C, Wang YT. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience. 2006;143:165–173. doi: 10.1016/j.neuroscience.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Miller DW, Borchardt RT. Distribution of insulin binding sites on cultured bovine brain microvessel endothelial cells and their possible role in the transport of insulin across the blood-brain barrier. Journal of Cell Biology. 1991;115:261a. [Google Scholar]
- Moon JH, Kim HJ, Yang AH, Kim HM, Lee BW, Kang ES, Lee HC, Cha BS. The effect of rosiglitazone on LRP1 expression and amyloid beta uptake in human brain microvascular endothelial cells: a possible role of a low-dose thiazolidinedione for dementia treatment. Int J Neuropsychopharmacol. 2011:1–8. doi: 10.1017/S1461145711001611. [DOI] [PubMed] [Google Scholar]
- Morley JE. The metabolic syndrome and aging. J Gerontol Med Sci. 2004;59A:139–142. doi: 10.1093/gerona/59.2.m139. [DOI] [PubMed] [Google Scholar]
- Nakaoke R, Verma S, Niwa M, Dohgu S, Banks WA. Glucose-regulated blood-brain barrier transport of insulin: Pericyte-astrocyte-endothelial cell cross talk. International Journal of Neuroprotection and Neuroregeneration. 2007;3:195–200. [Google Scholar]
- Navarro I, Leibush B, Moon TW, Plisetskaya EM, Banos N, Mendez E, Planas JV, Gutierrez J. Insulin, insulin-like growth factor-I (IGF-I) and glucagon: the evolution of their receptors. Comp Biochem Physiol B Biochem Mol Biol. 1999;122:137–153. doi: 10.1016/s0305-0491(98)10163-3. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Sun MK, Hongpaisan J, Alkon DL. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. European journal of pharmacology. 2008;585:76–87. doi: 10.1016/j.ejphar.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Sun MK, Hongpaisan J, Alkon DL. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol. 2008;585:76–87. doi: 10.1016/j.ejphar.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radio-labelled amino acids, amines and hexoses after arterial injection. American Journal of Physiology. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Entry of EGF into brain is rapid and saturable. Peptides. 1999;20:1091–1098. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. TNF alpha transport across the blood-brain barrier is abolished in receptor knockout mice. Experimental Neurology. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends in endocrinology and metabolism: TEM. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood-brain barrier. Proceedings of the National Academy of Sciences USA. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Wengenack TM, Malester B, Duff K. Permeability of proteins at the blood-brain barrier in the normal adult mouse and double transgenic mouse model of Alzheimer's disease. Neurobiology of Aging. 2001;8:555–567. doi: 10.1006/nbdi.2001.0402. [DOI] [PubMed] [Google Scholar]
- Price TO, Eranki V, Banks WA, Ercal N, Shah GN. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology. 2012;153:362–372. doi: 10.1210/en.2011-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid-beta protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Refetoff S. Syndromes of thyroid hormone resistance. Am J Physiol. 1982;243:E88–E98. doi: 10.1152/ajpendo.1982.243.2.E88. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, II, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired adults. Journal of Alzheimer's Disease. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser M, Lenz E, Bernstein HG, Dorn A. Insulin-like immunoactivity in human cerebrospinal-fluid is independent of insulin blood levels. Human Neurobiology. 1985;4:53–55. [PubMed] [Google Scholar]
- Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Research. 1999;851:215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- Rossetti P, Porcellati F, Ricci NB, Candeloro P, Cioli P, Bolli GB, Fanelli CG. Different brain responses to hypoglycemia induced by equipotent doses of the long-acting insulin analog detemir and human regular insulin in humans. Diabetes. 2008;57:746–756. doi: 10.2337/db07-1433. [DOI] [PubMed] [Google Scholar]
- Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Obici S, Seeley RJ. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov. 2009;8:386–398. doi: 10.1038/nrd2874. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Bergman RN, Kahn SE, Taborsky GL, Jr, Fisher LD, Sipols AJ, Woods SC, Steil GM, Porte D., Jr Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Journal of Clinical Investigation. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocrine Reviews. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorokhod A, Gamulin V, Gundacker D, Kavsan V, Muller IM, Muller WE. Origin of insulin receptor-like tyrosine kinases in marine sponges. Biol Bull. 1999;197:198–206. doi: 10.2307/1542615. [DOI] [PubMed] [Google Scholar]
- Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte D, Jr, Woods SC. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of Obese Zucker rats. Endocrinology. 1987;121:1611–1615. doi: 10.1210/endo-121-5-1611. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiology and Behavior. 1977;19:309–313. doi: 10.1016/0031-9384(77)90343-2. [DOI] [PubMed] [Google Scholar]
- Sugita H, Kaneki M, Tokunaga E, Sugita M, Koike C, Yasuhara S, Tompkins RG, Martyn JA. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am J Physiology. 2002;282:E386–E394. doi: 10.1152/ajpendo.00087.2001. [DOI] [PubMed] [Google Scholar]
- Tagliamonte A, DeMontis MG, Olianas M, Onali PL, Gessa GL. Role of insulin in the transport of tyrosine and tryptophan from blood to brain. Advances in Experimental Medicine and Biology. 1976;69:89–94. doi: 10.1007/978-1-4684-3264-0_7. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Terasaki T, Suzuki H, Ooie T, Sugiyama Y. Distributed model analysis of 3'-azido-3'deoxythymidine and 2',3'-dideoxyinosine across the blood-brain barrier via a probenecid-sensitive transport system. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1509–1517. [PubMed] [Google Scholar]
- Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1–40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Molecular Pharmacology. 2007;72:850–855. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- Unger J, McNeill TH, Moxley RT, 3rd, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31:143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proceedings of the National Academy of Sciences USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenciano AI, Corrochano S, de Pablo F, de la Villa P, de la Rosa EJ. Proinsulin/insulin is synthesized locally and prevents caspase- and cathepsin-mediated cell death in the embryonic mouse retina. J Neurochem. 2006;99:524–536. doi: 10.1111/j.1471-4159.2006.04043.x. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain,Behavior, and Immunity. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol Dis. 2008;31:188–197. doi: 10.1016/j.nbd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Watson GS, Peskind ER, Asthana S, Purganon K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S. Insulin increases CSF amyloid-beta 42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- White H, Venkatesh B. Clinical review: ketones and brain injury. Critical Care. 2011;15:219. doi: 10.1186/cc10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DJ, White M. Perspective: The insulin signaling system--a common link in the pathogenesis of type 2 diabetes. Endocrinology. 2000;141:1917–1921. doi: 10.1210/endo.141.6.7584. [DOI] [PubMed] [Google Scholar]
- Woods SC, Porte D., Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. American Journal of Physiology. 1977;233:E331–E334. doi: 10.1152/ajpendo.1977.233.4.E331. [DOI] [PubMed] [Google Scholar]
- Woods SC, Porte D., Jr . The role of insulin as a satiety factor in the central nervous system. In: Szabo AJ, editor. Advances in Metabolic Disorders, CNS Regulation of Carbohydrate Metabolism. Vol. 10. New York: Academic Press; 1983. pp. 457–482. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem Int. 1993;22:1–10. doi: 10.1016/0197-0186(93)90062-a. [DOI] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Research. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- Xie L, Helmerhorst E, Taddel K, Plewright B, van Bronswijk W, Martins R. Alzheimer's beta-amyloid peptides compete for insulin binding to the insulin receptor. Neurosci. 2002;22(RC221):1–5. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PL, Javier DC, Ryan CM, Tsui WH, Ardekani BA, Ten S, Convit A. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IS, Pietrzik CU, Kang DE, Koo EH. Sequences from the low density lipoprotein receptor-related protein (LRP) cytoplasmic domain enhance amyloid beta protein production via the beta-secretase pathway without altering amyloid precursor protein/LRP nuclear signaling. J Biol Chem. 2005;280:20140–20147. doi: 10.1074/jbc.M413729200. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kastin AJ, Pan W. Reciprocal interactions of insulin and insulin-like growth factor I in receptor-mediated transport across the blood-brain barrier. Endocrinology. 2006;147:2611–2615. doi: 10.1210/en.2006-0020. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]