Abstract
We have shown that expression of Human Papillomavirus type 16 E7 (HPV16.E7) protein within epithelial cells, as occurs in HPV associated-premalignancy and cancers, results in local immune suppression, and a weak and ineffective immune response to E7 protein. However, a robust acute inflammatory stimulus can overcome this to enable immune elimination of HPV16.E7 transformed epithelial cells. 2,4-Dinitrochlorobenzene (DNCB) can elicit acute inflammation and has been shown to initiate the regression of HPV-associated genital warts. Although clinical use of DNCB is discouraged due to its mutagenic potential, understanding how DNCB induced acute inflammation alters local HPV16.E7 mediated-immune suppression might lead to better treatments. Here, we show that topical DNCB application to skin expressing HPV16.E7 as a transgene induces a hyperinflammatory response, not seen in non-transgenic control animals. The E7 associated-inflammatory response is characterized by enhanced expression of Th2 cytokines and increased infiltration of CD11b+Gr1intF4/80+Ly6ChiLy6Glow myeloid cells, producing arginase-1. Inhibition of arginase with an arginase specific inhibitor, N(omega)-hydroxy-nor-L-arginine, ameliorates the DNCB-induced inflammatory response. Our results demonstrate that HPV16.E7 protein enhances DNCB associated-production of arginase-1 by myeloid cells and consequent inflammatory cellular infiltration of skin.
Keywords: arginase, human papillomavirus, DNCB, inflammation, skin
Introduction
Persistent infection with oncogenic human papillomaviruses (HPV), particularly HPV16, is associated with selective expression of two virally encoded proteins (E6 and E7). One action of HPV16.E7 protein is to subvert the innate immune system(Frazer et al., 1998) through down-regulation of IFNγ pathways, modulation of antigen presentation, and suppression of Toll Like Receptor 9 protein (Bhat et al., 2011).
K14.E7 transgenic mice which express HPV16.E7 oncoprotein within basal keratinocytes under the control of the keratin 14 transcriptional promoter have extensively used as a model of HPV oncoprotein-induced immune suppression associated with human squamous cancers, in which only the E7 genes of the papillomavirus are expressed (Trimble and Frazer, 2009). We have previously shown that skin grafts expressing HPV16.E7 oncoprotein are not spontaneously rejected when transplanted onto syngeneic animals, but are rejected when certain components of the innate immune system are unavailable, confirming that expression of HPV16.E7 in the epithelium results in the establishment of a local suppressive environment and the subversion of antigen specific T cells (Mattarolloet al., 2010; Mittal et al., 2013). Therefore, successful strategies targeting HPV-associated cancer need to circumvent or disrupt the local suppressive environment.
Topical immunotherapy with immunostimulatory agents has been used clinically to treat cancerous lesions including squamous cell and basal cell carcinoma in immunocompetent and immunosuppressed patients (Hengge et al., 2001). Topical application of 2,4-dinitrochlorobenzene (DNCB) is an effective therapy for condylomata acuminata caused by HPV infection. DNCB induced the complete clearance of HPV-associated warts in 13/15 patients (Georgala et al., 1989). The efficacy of this treatment is attributed to the immunostimulatory role of DNCB that might activate cell-mediated immunity (Belij et al., 2012). Although the use of DNCB for treatment has been discouraged because of its mutagenic potential (Black et al., 1985). We speculated that understanding how induction of a vigorous acute inflammatory response can break the locally immune-suppressive environment and restore the effector function of adaptive immunity might lead to more acceptable treatments for persisting HPV infection.
Arginase, which metabolizes L-arginine to n-ornithine and urea, has been identified as a crucial regulator of inflammation. Mammalian cells express two different isoforms; arginase-1 and arginase-2, which are encoded by two distinct genes and are different in their tissue distribution and subcellular localization (Bronte and Zanovello, 2005). Arginase can function as an immunosuppressive factor in the tumor environment (Ochoa et al., 2007), virus (Tacke et al., 2012) or parasite infection (Mou et al., 2013). However, this enzyme has also been identified as an important proinflammatory factor in numerous disease models (Kenyon et al., 2008; Stoermer et al., 2012; Zhang et al., 2009). As inflammation decides the fate of HPV infection and arginase is an important regulator of inflammation and immunity, we investigated the interaction between DNCB induced-inflammation and HPV16.E7 protein in induction of arginase in K14.E7 transgenic mice. We show here that K14.E7 transgenic mice exhibit an enhanced local inflammatory response to DNCB treatment, compared to non-transgenic mice, and that myeloid cells express increased arginase-1, which specifically promotes DNCB-induced inflammation.
Results
K14.E7 mice develop a robust inflammation response to DNCB
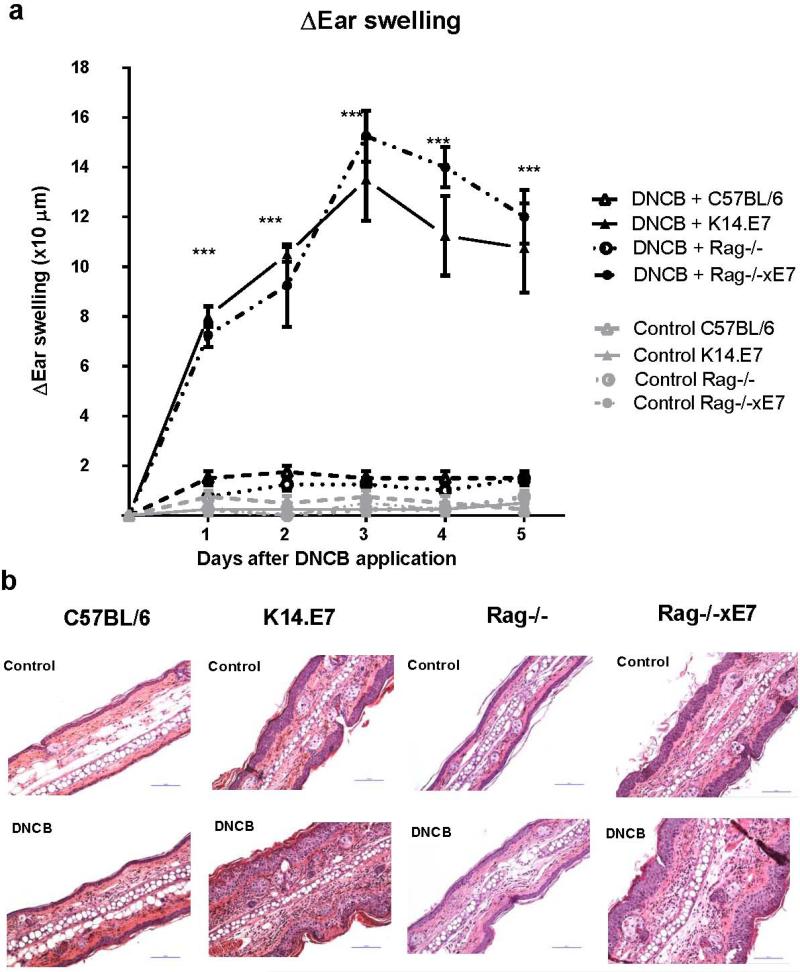
An inflammatory response was induced in wild type non-transgenic C57BL/6 and in E7 transgenic K14.E7 mice by applying DNCB topically to ear skin. The mean increase in ear thickness was monitored as an indicator of inflammatory reaction (Pinto et al., 2010). K14.E7 mice displayed a significantly higher degree of ear swelling than C57BL/6 mice which peaked at day 3 in response to a single application of DNCB (Figure 1a).
Figure 1. K14.E7 mice exhibit an enhanced inflammatory response to DNCB.
Ear swelling of C57BL/6, K14.E7, Rag−/−, and Rag−/−xE7 mice in response to DNCB or vehicle over 5 consecutive days (a) measured by caliper. Data are means ± SEM, and are representative of two independent experiments with 4 mice per group. ***p<0.001. Histology of representative sections from C57BL/6, K14.E7, Rag−/−xE7 and Rag−/−mice (b) at one day post DNCB or vehicle application. Hematoxylin and Eosin stain, original magnification x200, scale bar = 100 μm (representative of 4 mice per group).
T lymphocytes have been found in several skin inflammation diseases and play an important role in the production of inflammatory cytokines and in the recruitment of innate immune cells (Bromley et al., 2013). They also drive the enhanced inflammatory response seen on repeated exposure to DNCB (Wang et al., 2000). Additionally, our previous study showed that lymphocytes are increased in number in K14.E7 skin (Choyce et al., 2013). Therefore, we investigated whether the enhanced inflammatory response to first exposure to DNCB in K14.E7 skin was dependent on local lymphocyte function. K14.E7 mice deficient in lymphocytes (Rag−/−xE7), when exposed to DNCB, exhibited a similar level of ear swelling to K14.E7 mice, and stronger than control Rag−/− mice over five days after DNCB treatment (Figure 1a). Thus, DNCB treated K14.E7 mice exhibit a hyperinflammatory response on first exposure to DNCB which is independent of an adaptive immune response.
DNCB treated K14.E7 skin displays an enhanced infiltration of myeloid cells and Th2 cytokine expression
To characterize further the inflammation in DNCB treated K14.E7 skin, we examined the infiltration of immune cells. Histological examination suggested that the greater ear swelling in K14.E7 mice corresponded to a significant increase in the number of immune cells infiltrating the dermis (Figure 1b). As expected, the thickness of K14.E7 epidermal layer remained unchanged following DNCB treatment (Control: 42.4 ± 3.7 μm, DNCB: 40 ± 3.5 μm). Rag−/−xE7 mice develop similar ear swelling and inflammatory cell infiltration in the dermis to K14.E7 mice whereas control Rag−/− mice do not (Figure 1a, 1b). Thus, the increased ear swelling in K14.E7 in response to DNCB mice was mainly contributed by infiltration of cells of innate immune system in the dermis, and was independent of lymphocytes.
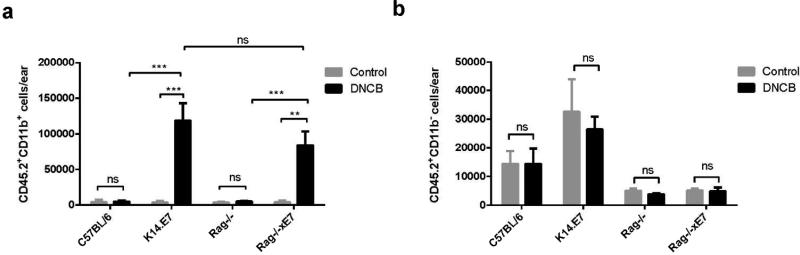
Flow cytometry analysis revealed that DNCB treated K14.E7 ears recruited significantly higher numbers of myeloid cells (CD45.2+CD11b+) than similarly treated C57BL/6 ears. We observed the same trend when compared Rag−/−xE7 with Rag−/− mice (Figure 2a). In contrast, the number of non-myeloid bone marrow derived cells (CD45.2+CD11b−) remained unchanged and was comparable in all four groups following DNCB treatment (Figure 2b). Thus, the expression of HPV16.E7 in the skin mediates the enhanced recruitment of myeloid cells and this effect is independent of adaptive immunity.
Figure 2. K14.E7 mouse skin has an enhanced myeloid cell infiltration in response to DNCB.
Absolute counts of leukocyte cells in the skin of C57BL/6 (n=4), K14.E7 (n=6), Rag−/− (n=5) and Rag−/−xE7 (n=5) mice following DNCB treatment. Absolute numbers of CD45.2+CD11b+ myeloid cells (a) and CD45.2+CD11b− cells (b) were determined by flow cytometry. Data are presented as means ± SEM and are representative of two independent experiments. ** p< 0.01, ***p<0.001, ns= not significant.
Interleukin-1β (IL-1β) and interleukin-6 (IL-6) are major cytokines secreted from the local inflammation site and promote the recruitment of innate immune cells (Fielding et al., 2008; Zohlnhofer et al., 2000). DNCB treatment resulted in a significant increase (7-fold) in IL-1β mRNA expression in both C57BL/6 and K14.E7 skin (Table 1). In addition, IL-6 mRNA expression was significantly increased in DNCB treated K14.E7 skin but not in non-transgenic skin, suggesting that IL-6 might be responsible for the enhanced recruitment of myeloid cells in K14.E7 skin.
Table 1.
Expression of Cytokine mRNA in response to DNCB or vehicle in K14E7 and C57 mice
| Expression relative to house keeping gene RPL32 | |||||
|---|---|---|---|---|---|
| C57BL/6 | K14.E7 | ||||
| Cytokine | Vehicle | DNCB | Vehicle | DNCB | P |
| IL-1β (× 104) | 6.8 ± 0.8 | 51 ± 4 | 221 ± 132 | 1490 ± 900 | # +* |
| IL-6 (× 104) | 50 ± 8 | 55 ± 29 | 142 ± 103 | 5310 ± 2870 | + @ |
| IL-4 (× 1011) | 1.3 ± 2.2 | 3.0 ± 3.1 | 15.1 ± 14.2 | 74.5 ± 53.3 | + @ * |
| Ptge2s (× 104) | 75 ± 74 | 91 ± 127 | 158 ± 34 | 480 ± 124 | + @ |
| IL-10 (× 104) | 0.4 ± 0.3 | 0.8 ± 0.8 | 4.0 ± 1.1 | 7.0 ± 1.6 | + * |
| IFNγ (× 104) | 0.2 ± 0.2 | 0.11 ± 0.12 | 1.9 ± 1.1 | 1.1 ± 0.9 | * |
| TNFα (× 104) | 3.2 ± 2.9 | 2.7 ± 1.6 | 41.3± 15.2 | 3.4 ± 2.2 | * @ |
P< 0.05 C57 DNCB v vehicle
p< 0.05 K14E7 DNCB v vehicle
p < 0.05 K14E7 DNCB v C57 vehicle
p < 0.05 change in expression for DNCB treated K14E7 v DNCB treated C57 Ptge2s: prostaglandinE2 synthase
Further, DNCB treatment of K14.E7 mice significantly induced mRNA expression of Prostaglandin E2 synthase (Ptge2s) and Th2 cytokines IL-4 and IL-10. In contrast, there was no significant change in the expression of these factors in DNCB treated C57BL/6 mice. Th1 cytokines displayed decreased (TNFα) or unaltered (IFNγ) expression after DNCB treatment (Table 1).
Together, these results suggest that after a single DNCB treatment to previously unexposed animals, K14.E7 mice mounted an enhanced inflammatory response, which is accompanied by CD45.2+CD11b+ myeloid cell infiltration and amplified Th2 cytokine expression.
Arginase -1 is specifically induced in DNCB treated K14.E7 mice
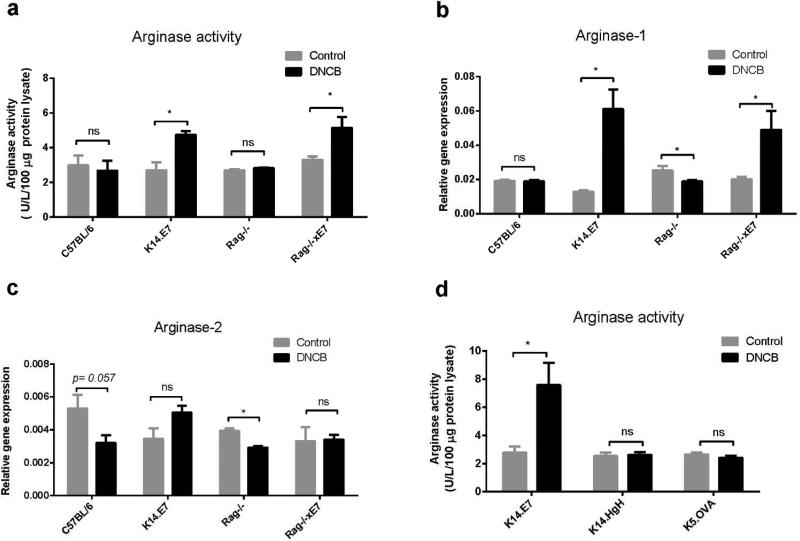
Inflammation and myeloid cell activation can be associated with the induction of arginase (Bronte et al., 2003). We speculate that arginase activity might contribute to enhanced inflammation of K14.E7 skin in response to DNCB. To test this hypothesis, we first investigated arginase activity in the skin of C57BL/6 and K14.E7 mice following DNCB treatment. Arginase activity was comparable in control K14E7 and C57BL/6 ear skin (Figure 3a), as was arginase mRNA expression (Figure 3b and RNAseq data not shown). DNCB treated K14.E7 skin however demonstrated a substantially higher amount of arginase activity than DNCB treated C57BL/6 skin (Figure 3a). Arginase activity is contributed by two arginase isoforms. Increased arginase activity in K14.E7 mice corresponded with markedly increased (4-fold) arginase-1 mRNA expression. Conversely, the expression of arginase-1 mRNA remained unchanged in DNCB treated C57BL/6 mice (Figure 3b). In contrast to arginase-1, there was no significant induction of arginase-2 mRNA in either C57BL/6 or K14.E7 skin (Figure 3c).
Figure 3. Arginase-1 but not arginase-2 is induced in K14.E7 mice following DNCB treatment, and this regulation is lymphocyte independent.
Arginase activity was measured by determining the release of urea product from 100 μg of protein lysate per sample. Protein lysates were prepared from ear tissues of DNCB or vehicle treated C57BL/6, K14.E7, Rag−/−, Rag−/−xE7, K14.HgH and K5.OVA ear tissues. Relative gene expression of (b) arginase-1 and (c) arginase-2 mRNAs was determined by real-time PCR in the skin of C57BL/6, K14.E7, Rag−/− and Rag−/−xE7 mice 1 day following DNCB treatment, normalized against the housekeeping gene RPL32. Data are presented as means ± SEM and are representative of two independent experiments (n=4 mice per group). *p<0.05, ns=not significant.
K14.E7 mice lacking lymphocytes (Rag−/−xE7) not only exhibited a similar level of ear swelling as K14.E7 mice but also of arginase-1 mRNA and arginase activity following DNCB treatment. Notably, Arginase-1 and arginase-2 mRNA expression are not increased in control Rag−/− mice following DNCB treatment (Figure 3b, 3c). Thus, the induction of arginase in K14.E7 skin is independent of adaptive immunity.
To examine whether the induction of arginase was unique to skin expressing HPV16.E7 oncoprotein as a transgene, K14.hGh and K5.OVA mice expressing human growth hormone or ovalbumin, respectively, under the control of keratin promoters were treated with DNCB. In contrast to K14.E7 mice, there was no change in the level of arginase activity of K14.hGh or K5.OVA mice following DNCB treatment (Figure 3d). Furthermore, the ear swelling level of these transgenic mice (K5.OVA: 2.25 ± 0.5 μm; K14.hGh: 1.75 ± 0.5 μm, day 5) was similar to C57BL/6 (1.75 ± 0.5 μm, day 5) and markedly lower than in K14.E7 mice (19.8 ± 9.9 μm, day 5). Thus, these results suggest that increased arginase activity, likely derived from activated myeloid cells, might be a consequence of exposure of K14.E7 expressing epithelial cells to DNCB.
CD11b+Gr1intF4/80+Ly6C+Ly6G− cells are the major source of arginase-1 in DNCB treated K14.E7 mice
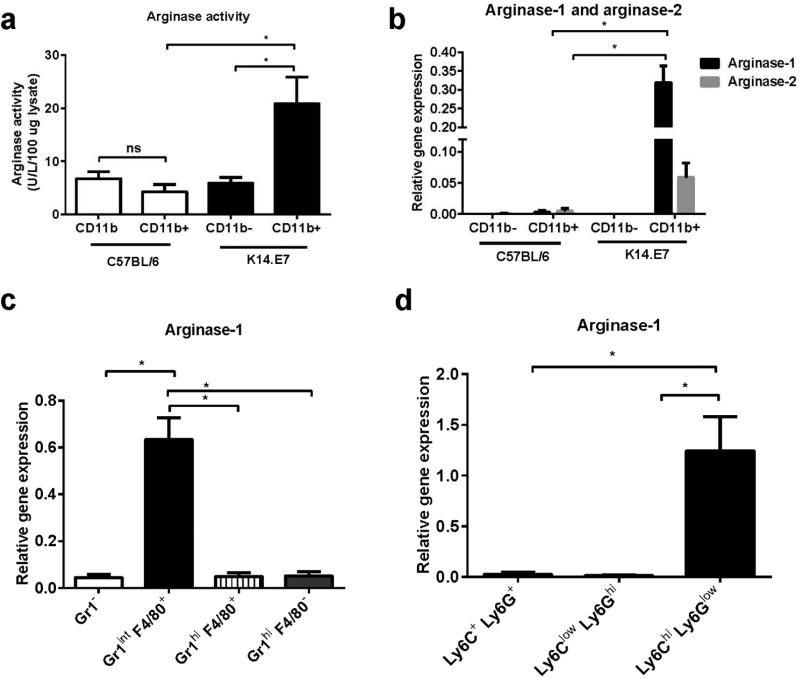
Arginase-1 can be induced in myeloid cells including macrophages, dendritic cells and myeloid derived suppressive cells in response to a wide range of stimuli (Chang et al., 2013; Sindrilaru et al., 2011). These myeloid cells express surface CD11b (Hammad and Lambrecht, 2008). To confirm whether myeloid cells were the source of DNCB induced-arginase-1 in K14.E7 skin, CD45.2+CD11b− and CD45.2+CD11b+ cells (Supplementary figure 1) were isolated from DNCB treated C57BL/6 and K14.E7 mice and analyzed for arginase activity and arginase-1 mRNA expression. CD11b+ myeloid cells from DNCB treated K14.E7 mice produced significantly higher level of arginase activity per cell than CD11b− cells, and than CD11b+ myeloid cells from DNCB treated C57BL/6 mice (Figure 4a). In addition, arginase-1 mRNA expression was substantially detected in CD45.2+CD11b+ cells in DNCB treated K14.E7 mice, but not in CD11b− or CD11b+ cells from C57BL/6 mice (Figure 4b). Although arginase-2 mRNA was also detected in CD45.2+CD11b+ cells in K14.E7 skin, arginase-2 mRNA expression was 10 times lower than arginase-1 (Figure 4b). Thus, DNCB activated myeloid cells (CD45.2+CD11b+) produce the increased arginase-1 observed in DNCB treated K14.E7 skin.
Figure 4. CD11b+ Gr1int F4/80+ Ly6GlowLy6Chi cells produce arginase in DNCB treated K14.E7 skin.
Arginase activity produced by CD45.2+CD11b+ and CD45.2+CD11b− populations from DNCB treated C57BL/6 and K14.E7 mice (a) was determined as described in Materials and Methods. Arginase-1 and arginase-2 mRNA expression (b) was determined by real-time PCR. Arginase-1 mRNA expression in Gr1−F4/80+; Gr1intF4/80+; Gr1hiF4/80+ and Gr1−F4/80− subsets from DNCB treated K14.E7 mice was determined (c) by real time PCR. Real time PCR analysis of arginase-1 mRNA expression in different cell subsets (d) was based on the expression of Ly6C and Ly6G antigens. Data were pooled from four independent experiments. In each experiment, six C57BL/6 and two K14.E7 mice were treated with DNCB or vehicle. Means ± SEM. *p<0.05, **p<0.01, *** p<0.001, ns= not significant.
CD11b marker is expressed on different myeloid subsets including macrophages/monocytes and granulocytes. To further define which cell subset expresses arginase-1 in DNCB treated K14.E7 mice, we examined the expression level of arginase-1 in different populations of CD11b+ cells based on the expression of macrophage marker F4/80 and granulocyte marker Gr1. We found that F4/80+Gr1int cells expressed significantly higher level of arginase-1 than other cell populations (Figure 4c). Furthermore, arginase-1 was abundantly expressed in inflammatory monocytes which express Ly6ChiLy6Glow but not in Ly6ClowLy6Ghi granulocytes (Figure 4d).
Suppression of arginase ameliorates the ear swelling of inflamed K14.E7 skins
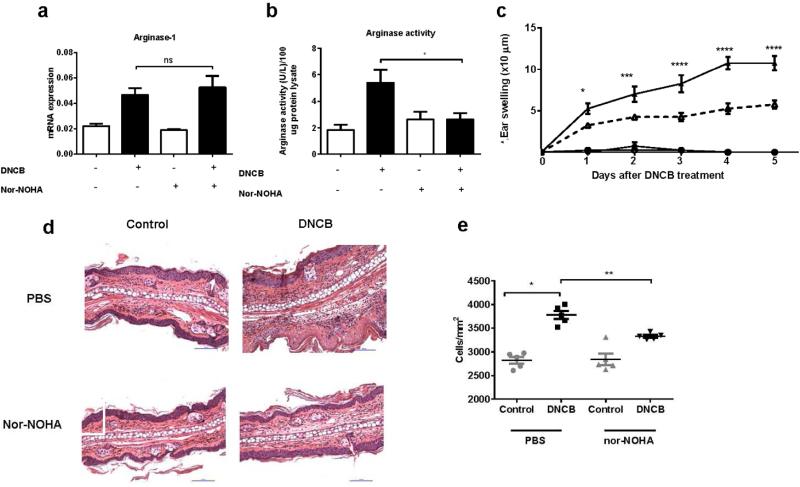
Arginase can be paradoxically involved in up- or down-regulation of inflammatory responses. N(omega)-hydroxy-nor-L-arginine (nor-NOHA), which is an intermediate in the L-arginine/NO pathway is widely used as a specific, reversible inhibitor of arginase both in-vitro and in-vivo (Bratt et al., 2009; Tenu et al., 1999). To understand whether arginase promoted or suppressed the inflammatory response of K14.E7 mice to DNCB, we administered nor-NOHA or saline buffer to K14.E7 mice one day prior to DNCB treatment and daily for 4 days.
Ear tissue was harvested after 24 hours of DNCB treatment with or without arginase inhibitor and the expression and activity of arginase were determined. Nor-NOHA, as expected, did not suppress the transcription level of arginase-1 gene (Figure 5a), but reduced arginase activity in DNCB treated K14.E7 skin (Figure 5b).
Figure 5. Inhibition of arginase ameliorates the ear swelling of DNCB treated K14.E7 mice.
Mice were injected with 500 μg arginase inhibitor (nor-NOHA) or saline buffer one day prior to DNCB treatment and daily for 5 days. Arginase-1 mRNA expression (a) and arginase activity (b) in saline or nor-NOHA treated K14.E7 mice after 24 hours of exposure to DNCB, determined by real-time PCR and arginase assay, respectively. Ear swelling of K14.E7 mice treated with saline/nor-NOHA monitored during 5 days after DNCB treatment (c). Histological sections (d) and quantification of cell numbers infiltrating into the dermis from Nor-NOHA or saline treated K14.E7 skins one day following DNCB application (e). Means ± SEM, data show result from two independent experiments (n=5). *p<0.05, **p<0.01, *** p<0.001, ****p<0.0001, ns= not significant.
Furthermore, swelling of DNCB treated K14.E7 skin was reduced in animals treated with arginase inhibitor (Figure 5c). Consistent with the ear swelling data, arginase inhibition decreased the number of infiltrating cells in the dermis of DNCB treated K14.E7 mice (Figure 5d, 5e). These results demonstrate that arginase-1 induced by DNCB in K14.E7 transgenic ear skin, but not in C57BL/6 ear skin, itself contributes to exacerbated inflammatory response to DNCB in K14.E7 skin by recruitment of further inflammatory cells.
Discussion
DNCB triggers a T cell independent inflammatory response through activation of the NALP-3 inflammasome in keratinocytes (Schuepbach-Mallepell et al., 2013), and has been used to treat HPV associated-genital warts. Despite its efficacy, this chemical is potentially mutagenic and carcinogenic (Black et al., 1985). Therefore, we sought to understand the influence of expression of the major oncogenic protein of HPV16, E7, on inflammatory mechanism induced by DNCB using mice transgenic for the E7 protein expressed in keratinocytes. Here, we show that the acute response to DNCB in previously unexposed mice is significantly higher in K14.E7 transgenic than in non-transgenic mice. In response to DNCB-induced inflammasome activation, keratinocytes have been shown to produce a wide range of proinflammatory cytokines including IL-6 and IL-1β (Enk and Katz, 1992; Rambukkana et al., 1996), known to be responsible for the recruitment of polymorphonuclear cells (Fielding et al., 2008; Zohlnhofer et al., 2000). We indeed found an increased number of inflammatory myeloid cells in the dermis and enhanced mRNA expression levels of IL-6 and IL-1β, in DNCB treated K14E7 transgenic mice, and show further that DNCB induced IL-1β and IL-6 production was greatly enhanced in K14E7 skin when compared with non-transgenic skin. We could not detect significant induction of IL-6 in non-transgenic skin. Therefore, we hypothesize that this cytokine might be a regulator of inflammatory cell infiltration in K14.E7 skin and its ear swelling. Premalignant skin of HPV16.E7 transgenic mice is manifested by hyperplastic epidermis and infiltration of innate immune cells of myeloid origin. The presence of these cells in K14.E7 skin might be responsible for the dramatic inflammatory response to short term DNCB treatment and inflammasome activation. Interestingly, we were not able to detect these responses to DNCB in other transgenic skin including K14.hGh and K5.OVA, suggesting that they are unique to K14.E7 mice, a consequence of the presence of HPV.E7 oncoprotein in the skin.
DNCB treatment of K14.E7 mice significantly induced mRNA expression of Th2 cytokines IL-4, IL-10, when compared with wild type skin. This finding is consistent with the finding in a mouse model of allergic skin inflammation, in which Th2 cytokines are responsible for the amplification and chronicity of allergic skin inflammation (Masuoka et al., 2012), and with a study in which IL-4 deficient mice displayed an impaired ear swelling response to DNCB application (Traidl et al., 1999). Furthermore, we show that DNCB treated K14.E7 skin exhibited an accumulation of CD11b+ myeloid cells, but not of non-myeloid bone marrow-derived cells which are mainly lymphocytes. Th2 cytokines induce arginase activity in myeloid cells including macrophages and dendritic cells (Barron et al., 2013; Munder et al., 1999). Arginase-2 can be expressed by macrophages and has structural and enzyme characteristics similar to arginase-1 (Khallou-Laschet et al., 2010), but remained unchanged in K14.E7 skin. Thus, the higher level of arginase activity in DNCB treated K14.E7 mice is mainly contributed by arginase-1, and promoted possibly by higher level of Th2 cytokines and by accumulation of myeloid cells.
One predicted source of enhanced Th2 cytokine production in DNCB treated K14E7 skin would be Th2 CD4+ lymphocytes. Indeed, K14E7 skin has a large number of CD4+ lymphocytes (Choyce et al., 2013). However, we demonstrate here that the induction of arginase-1 and enhanced ear swelling response of K14.E7 skin are independent of lymphocytes. Th2 cytokines which induce arginase-1 expression and hyperinflammatory response in DNCB treated K14.E7 might therefore be derived from innate immune cells (Bradding et al., 1992; Kuroda et al., 2009) or epithelial cells (Balato et al., 2012). We also detected the induction of Ptge2s in DNCB treated K14.E7 skin. Our data are consistent with a previous study that HPV16.E7 oncoprotein induced cyclooxygenase-2 (COX-2) transcription and Ptge2s production (Subbaramaiah and Dannenberg, 2007). Furthermore, the COX-2–Pteg2s synthase axis has been shown to induce arginase-1 in myeloid cells in a tumor environment (Rodriguez et al., 2005). However, further studies are needed to address the induction mechanism of arginase-1 in DNCB treated K14.E7 skin.
Cells producing arginase-1 in K14.E7 skin were positive for F4/80 and Ly6C antigen and express Gr1 at intermediate level. This population has been defined as monocytic myeloid suppressive cells or inflammatory monocytes, which appear to adopt an immune stimulatory or suppressive function depending on the local environment (Kallberg et al., 2012). Indeed, CD11b+Gr1+ cells during the early phase of polymicrobial sepsis exhibit proinflammatory phenotypes, whereas this cell population becomes immature and immune suppressive in the late phase (Brudecki et al., 2012).
To understand the role of arginase in the hyperinflammation in K14.E7 skin, we used the compound nor-NOHA, which efficiently suppresses arginase-1 and arginase-2 activity in-vitro and in-vivo studies (Bratt et al., 2009; Tenu et al., 1999). Nor-NOHA abrogates the function of arginase by modifying the structure of the enzyme and does not affect the transcription of arginase gene, consistent with an unaltered arginase mRNA level (Krotova et al., 2010). Arginase inhibitor treatment decreased arginase activity and the level of leucocyte infiltrate and ear swelling in DNCB treated K14.E7 mice. This suggests that enhanced arginase activity is critically required for the strong and sustained inflammatory response and that arginase inhibitor alleviates DNCB-induced inflammation by decreasing the recruitment of leukocytes in the skin. Arginase efficiently competes with iNOS for the common substrate L-arginine, leading to inhibition of iNOS expression and NO production. Since endothelium derived NO is reported to suppress expression of adhesion molecules such as VCAM-1, ICAM-1 (Peng et al., 1998), limiting of NO bioavailability through arginase results in enhanced vascular inflammation (White et al., 2006). In a mouse model of atherogenesis, arginase-2 but not arginase-1 enhances monocyte adhesion to endothelial cells and trigger the production of proinflammatory cytokines through mitochondrial reactive oxygen species (Ming et al., 2012). Liu et al. show that infiltrating myeloid cells produce arginase-1 which promotes angiogenesis and further recruitment of monocytes in laser-induced injury murine model (Liu et al., 2013).
Taken together, our findings demonstrate that HPV16.E7 oncoprotein expressing skin develops a hyperinflammatory response to DNCB via an arginase-1 dependent mechanism. These findings provide insights into the proinflammatory role of arginase-1 in HPV16.E7 expressing skin in response to immunostimulation by DNCB.
Materials and Methods
Mice
K14.HPV16E7 (K14.E7) mice were generated from inbred strain, C57BL/6 (Narayan et al., 2009). K14.E7, K14.HgH, and Rag−/− , all on a C57BL/6 background, were purchased from Animal Resources Center (Perth, Australia). K5.mOVA mice on a C57BL/6 background were kindly provided by H. Azukizawa (Azukizawa et al., 2003). Rag−/−xE7 mice were generated by crossing male K14.E7 with female Rag1−/− knockout C57BL/6 mice, heterozygous K14E7 mice were crossed and then backcrossed with homozygous Rag1−/− mice to an F2 generation (Narayan et al., 2009). All mice were maintained under specific pathogen-free conditions at Princess Alexandra Hospital Biological Research Facility. Experimental mice were sex matched and used at 6-9 week of age. All animal procedures complied with guidelines approved by the University of Queensland Animal Ethics Committee.
DNCB treatment
2,4-dinitrochlorobenzene (Sigma, New South Wales, Australia) was dissolved in vehicle [acetone: olive oil (4:1)] immediately prior to use. Six- to nine-week-old mice were treated with 20 μl of 1% DNCB or vehicle on the left ear and right ear, respectively. After 24 hours, the ear tissues were harvested for mRNA and protein analysis. Ear thickness was measured by using the digital caliper and change in ear swelling was determined by calculating the mean increase in ear thickness compared to untreated ears.
Histological analysis
Ear tissues were fixed by 4% paraformaldehyde. Tissues were embedded in paraffin and 7 μm sections were prepared and stained with Hematoxylin and Eosin. Immune cell infiltration was evaluated by light microscopy and quantified by using Nis-elements Br 3.2 software (Nikon Instruments Inc., New York, United States).
Realtime- PCR
RNA was isolated from homogenized tissues by using RNaeasy Mini kit (Qiagen, Victoria, Australia). RNA extracts were quantified using absorption of light at 260 and 280 nm (A260/280). Details of the procedures and primers used for the quantitative real time PCR are described in supplementary methods.
Arginase activity
Arginase activity was measured by colorimetric determination of urea formed from L-arginine as previously described (Chang et al., 2000). Details of the procedures are desbribed in supplementary methods.
Flow cytometry and cell sorting
Flow cytometry staining was performed as previously described (Mattarollo et al., 2010). Details of flow cytometry and cell sorting are described in supplementary methods.
Statistical Analysis
Each data point represents the mean ± SEM and is representative of two independent experiments with at least 4 mice per group. Prism (Graph pad Software, La Jolla, CA) was used for graphs and statistical analysis: * p<0.05; ** p<0.01; ***; p<0.001; **** p<0.0001. Multiple comparisons of ear swelling data were derived by two-way ANOVA. For other data, statistically significantly differences between groups were analysed by nonparametric t-test (Mann-Whitney test).
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH (5U01CA141583), National Health and Medical Research Council of Australia (569938), Australian Research Council, Cancer Council Queensland, and Australian Cancer Research Foundation. Tran is a recipient of University of Queensland fellowship for international students. We thank staff of the Biological Research Facility at Princess Alexandra Hospital for excellent technical assistance with animal care.
Abbreviations
- CIN
cervical intraepithelial neoplasia
- DNCB
2,4-Dinitrochlorobenzene
- HPV
human papillomavirus
- Nor-NOHA
Nω-Hydroxy-nor-L-arginine
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–88. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- Balato A, Lembo S, Mattii M, Schiattarella M, Marino R, De Paulis A, et al. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol. 2012;21:892–4. doi: 10.1111/exd.12027. [DOI] [PubMed] [Google Scholar]
- Barron L, Smith AM, El Kasmi KC, Qualls JE, Huang X, Cheever A, et al. Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS One. 2013;8:e61961. doi: 10.1371/journal.pone.0061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belij S, Popov A, Zolotarevski L, Mirkov I, Djokic J, Kataranovski D, et al. Systemic immunomodulatory effects of topical dinitrochlorobenzene (DNCB) in rats. Activity of peripheral blood polymorphonuclear cells. Environ Toxicol Pharmacol. 2012;33:168–80. doi: 10.1016/j.etap.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Bhat P, Mattarollo SR, Gosmann C, Frazer IH, Leggatt GR. Regulation of immune responses to HPV infection and during HPV-directed immunotherapy. Immunol Rev. 2011;239:85–98. doi: 10.1111/j.1600-065X.2010.00966.x. [DOI] [PubMed] [Google Scholar]
- Black HS, Castrow FF, 2nd, Gerguis J. The mutagenicity of dinitrochlorobenzene. Arch Dermatol. 1985;121:348–9. [PubMed] [Google Scholar]
- Bradding P, Feather IH, Howarth PH, Mueller R, Roberts JA, Britten K, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–6. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234:273–80. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Larson RP, Ziegler SF, Luster AD. IL-23 induces atopic dermatitis-like inflammation instead of psoriasis-like inflammation in CCR2-deficient mice. PLoS One. 2013;8:e58196. doi: 10.1371/journal.pone.0058196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Chang CI, Zoghi B, Liao JC, Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J Immunol. 2000;165:2134–41. doi: 10.4049/jimmunol.165.4.2134. [DOI] [PubMed] [Google Scholar]
- Chang J, Thangamani S, Kim MH, Ulrich B, Morris SM, Jr., Kim CH. Retinoic acid promotes the development of Arg1-expressing dendritic cells for the regulation of T-cell differentiation. Eur J Immunol. 2013;43:967–78. doi: 10.1002/eji.201242772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choyce A, Yong M, Narayan S, Mattarollo SR, Liem A, Lambert PF, et al. Expression of a single, viral oncoprotein in skin epithelium is sufficient to recruit lymphocytes. PLoS One. 2013;8:e57798. doi: 10.1371/journal.pone.0057798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custot J, Moali C, Brollo J, Boucher L, Delaforge M, Mansuy D, et al. The New α-Amino Acid Nω-Hydroxy-nor-l-arginine: a High-Affinity Inhibitor of Arginase Well Adapted To Bind to Its Manganese Cluster. J Am Chem So. 1997;119:4086–7. [Google Scholar]
- Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992;89:1398–402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–95. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- Frazer IH, Fernando GJ, Fowler N, Leggatt GR, Lambert PF, Liem A, et al. Split tolerance to a viral antigen expressed in thymic epithelium and keratinocytes. Eur J Immunol. 1998;28:2791–800. doi: 10.1002/(SICI)1521-4141(199809)28:09<2791::AID-IMMU2791>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Georgala S, Danopoulou I, Katsarou A. Dinitrochlorobenzene treatment of condylomata acuminata. Australas J Dermatol. 1989;30:103–5. doi: 10.1111/j.1440-0960.1989.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Benninghoff B, Ruzicka T, Goos M. Topical immunomodulators--progress towards treating inflammation, infection, and cancer. Lancet Infect Dis. 2001;1:189–98. doi: 10.1016/s1473-3099(01)00095-0. [DOI] [PubMed] [Google Scholar]
- Hsiao YP, Yang JH, Wu WJ, Lin MH, Sheu GT. E6 and E7 of human papillomavirus type 18 and UVB irradiation corporately regulate interleukin-6 and interleukin-8 expressions in basal cell carcinoma. Exp Dermatol. 2013;22:672–4. doi: 10.1111/exd.12223. [DOI] [PubMed] [Google Scholar]
- Kallberg E, Stenstrom M, Liberg D, Ivars F, Leanderson T. CD11b+Ly6C++Ly6G- cells show distinct function in mice with chronic inflammation or tumor burden. BMC Immunol. 2012;13:69. doi: 10.1186/1471-2172-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon NJ, Bratt JM, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol. 2008;230:269–75. doi: 10.1016/j.taap.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotova K, Patel JM, Block ER, Zharikov S. Endothelial arginase II responds to pharmacological inhibition by elevation in protein level. Mol Cell Biochem. 2010;343:211–6. doi: 10.1007/s11010-010-0515-5. [DOI] [PubMed] [Google Scholar]
- Kulidjian AA, Issekutz AC, Issekutz TB. Differential role of E-selectin and P-selectin in T lymphocyte migration to cutaneous inflammatory reactions induced by cytokines. Int Immunol. 2002;14:751–60. doi: 10.1093/intimm/dxf045. [DOI] [PubMed] [Google Scholar]
- Kuroda E, Ho V, Ruschmann J, Antignano F, Hamilton M, Rauh MJ, et al. SHIP represses the generation of IL-3-induced M2 macrophages by inhibiting IL-4 production from basophils. J Immunol. 2009;183:3652–60. doi: 10.4049/jimmunol.0900864. [DOI] [PubMed] [Google Scholar]
- Liu J, Copland DA, Horie S, Wu WK, Chen M, Xu Y, et al. Myeloid cells expressing VEGF and arginase-1 following uptake of damaged retinal pigment epithelium suggests potential mechanism that drives the onset of choroidal angiogenesis in mice. PLoS One. 2013;8:e72935. doi: 10.1371/journal.pone.0072935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattarollo SR, Rahimpour A, Choyce A, Godfrey DI, Leggatt GR, Frazer IH. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J Immunol. 2010;184:1242–50. doi: 10.4049/jimmunol.0902191. [DOI] [PubMed] [Google Scholar]
- Ming XF, Rajapakse AG, Yepuri G, Xiong Y, Carvas JM, Ruffieux J, et al. Arginase II Promotes Macrophage Inflammatory Responses Through Mitochondrial Reactive Oxygen Species, Contributing to Insulin Resistance and Atherogenesis. J Am Heart Assoc. 2012;1:e000992. doi: 10.1161/JAHA.112.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D, Kassianos AJ, Tran LS, Bergot AS, Gosmann C, Hofmann J, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Invest Dermatol. 2013;133:2686–94. doi: 10.1038/jid.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z, Muleme HM, Liu D, Jia P, Okwor IB, Kuriakose SM, et al. Parasite-derived arginase influences secondary anti-Leishmania immunity by regulating programmed cell death-1-mediated CD4+ T cell exhaustion. J Immunol. 2013;190:3380–9. doi: 10.4049/jimmunol.1202537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–7. [PubMed] [Google Scholar]
- Narayan S, Choyce A, Linedale R, Saunders NA, Dahler A, Chan E, et al. Epithelial expression of human papillomavirus type 16 E7 protein results in peripheral CD8 T-cell suppression mediated by CD4+CD25+ T cells. Eur J Immunol. 2009;39:481–90. doi: 10.1002/eji.200838527. [DOI] [PubMed] [Google Scholar]
- North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L911–20. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–6s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Peng HB, Spiecker M, Liao JK. Inducible nitric oxide: an autoregulatory feedback inhibitor of vascular inflammation. J Immunol. 1998;161:1970–6. [PubMed] [Google Scholar]
- Pinto NB, Morais TC, Carvalho KM, Silva CR, Andrade GM, Brito GA, et al. Topical anti-inflammatory potential of Physalin E from Physalis angulata on experimental dermatitis in mice. Phytomedicine. 2010;17:740–3. doi: 10.1016/j.phymed.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Pistoor FH, Bos JD, Kapsenberg ML, Das PK. Effects of contact allergens on human Langerhans cells in skin organ culture: migration, modulation of cell surface molecules, and early expression of interleukin-1 beta protein. Lab Invest. 1996;74:422–36. [PubMed] [Google Scholar]
- Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuepbach-Mallepell S, Philippe V, Bruggen MC, Watanabe H, Roques S, Baldeschi C, et al. Antagonistic effect of the inflammasome on thymic stromal lymphopoietin expression in the skin. J Allergy Clin Immunol. 2013;132:1348–57. doi: 10.1016/j.jaci.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–97. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoermer KA, Burrack A, Oko L, Montgomery SA, Borst LB, Gill RG, et al. Genetic ablation of arginase 1 in macrophages and neutrophils enhances clearance of an arthritogenic alphavirus. J Immunol. 2012;189:4047–59. doi: 10.4049/jimmunol.1201240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 transcription is regulated by human papillomavirus 16 E6 and E7 oncoproteins: evidence of a corepressor/coactivator exchange. Cancer Res. 2007;67:3976–85. doi: 10.1158/0008-5472.CAN-06-4273. [DOI] [PubMed] [Google Scholar]
- Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343–53. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Ogino K, Takemoto K, Hamanishi S, Wang DH, Takigawa T, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L17–24. doi: 10.1152/ajplung.00216.2009. [DOI] [PubMed] [Google Scholar]
- Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–38. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]
- Traidl C, Jugert F, Krieg T, Merk H, Hunzelmann N. Inhibition of allergic contact dermatitis to DNCB but not to oxazolone in interleukin-4-deficient mice. J Invest Dermatol. 1999;112:476–82. doi: 10.1046/j.1523-1747.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Trimble CL, Frazer IH. Development of therapeutic HPV vaccines. Lancet Oncol. 2009;10:975–80. doi: 10.1016/S1470-2045(09)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Fujisawa H, Zhuang L, Freed I, Howell BG, Shahid S, et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol. 2000;165:6783–90. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, et al. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–51. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, et al. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol. 2009;175:891–902. doi: 10.2353/ajpath.2009.081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohlnhofer D, Brand K, Schipek K, Pogatsa-Murray G, Schomig A, Neumann FJ. Adhesion of monocyte very late antigen-4 to endothelial vascular cell adhesion molecule-1 induces interleukin-1beta-dependent expression of interleukin-6 in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:353–9. doi: 10.1161/01.atv.20.2.353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.