Abstract
DNA damage is a cause of age related pathologies, including osteoarthritis (OA). Excision repair cross complementation group 1 (ERCC1) is an endonuclease required for DNA damage repair. In this study we investigated the function of ERCC1 in chondrocytes and its association with the pathophysiology of OA. ERCC1 expression in normal and osteoarthritic cartilage was assessed, as were changes in ERCC1 expression in chondrocytes under catabolic stress. Inhibiting ERCC1 in chondrocytes under interleukin-1β stimulation using small interfering RNA (siRNA) was also evaluated. Finally, cellular senescence and apoptosis were examined in relation to ERCC1 function. ERCC1 expression was decreased in OA cartilage and increased within 4 h of exposure to interleukin (IL)-1β, but decreased after 12 h. The inhibition of ERCC1 by siRNA increased the expression of matrix metallopeptidase 13 and decreased collagen type II. ERCC1 inhibition also increased the number of apoptotic and senescent cells. The inhibition of ERCC1 in chondrocytes increased their expression of OA related proteins, apoptosis, cellular senescence, and hypertrophic-like changes which suggest that ERCC1 is critical for protecting human chondrocytes (HCs) from catabolic stresses and provides insights into the pathophysiology of OA and a potential target for its treatment.
Keywords: ERCC1, osteoarthritis, apoptosis, senescence, interleukin (IL)-1β
Osteoarthritis (OA) is an age-related degenerative joint disorder characterized by the loss of tissue cellularity and extracellular matrix (ECM) damage.1 The homeostasis of anabolic and catabolic reactions at the molecular level plays a critical role in maintaining the integrity of articular cartilage (AC).2 Chondrocytes are the only resident cells found in AC, and are responsible for both the synthesis and the abundant turnover of the ECM3; however, the precise pathogenic process of OA has yet to be elucidated.
DNA damage is considered a major cause of age related pathologies.4–6 The stability of the genome is maintained by an intricate system of DNA repair, damage tolerance, and checkpoints that monitor DNA to counteract potential damage.7 Time-dependent accumulation of DNA damage in cells is associated with gradual functional declines which result in the phenotypic changes associated with aging.5 In particular, recent studies have demonstrated that DNA damage is significantly increased in the chondrocytes of aged human cartilage tissue, the accumulation of which impairs the ability of chondrocytes to remain functional and maintain tissue homeostasis8; therefore, DNA damage has been linked with the development of OA.9 Accumulating evidence indicates that chondrocyte apoptosis and cellular senescence are also associated with OA.9–11 Several biochemical pathways can contribute to cartilage degeneration, the disruption of which can result in the loss of joint function. The proinflammatory cytokine interleukin (IL)-1β has been implicated as a causative factor of OA which decreases the anabolic activities of chondrocytes and induces DNA damage, leading to the apoptosis of chondrocytes.12
Syndromes of advanced aging have been studied with the aim of elucidating the relationship between aging and DNA changes. Together, the excision repair cross complementation group 1 (ERCC1) and xeroderma pigmentosum complementation group F (XPF) form an endonuclease that is required for numerous DNA repair mechanisms.13–15 XPF encodes one subunit of the endonuclease while ERCC1 serves as the essential binding partner that enables repair of damaged DNA.16
OA is a consequence of “premature aging” of the chondrocytes which compromises the integrity of the joint tissue.8 Since the inhibition of ERCC1 causes a premature onset of aging-related changes, and considering its role in DNA repair, we hypothesized that ERCC1 could play a protective role in human chondrocytes (HCs) by preventing genomic alterations that ultimately cause cartilage degeneration. In the present study, we investigated the roles of ERCC1 in HCs and in the pathophysiology of OA. Our observations suggest that ERCC1 protects HCs from catabolic stress, and may thus prevent the development of OA.
MATERIALS AND METHODS
Experimental OA Models in Mice
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Ten-week-old C57BL/6J mice were used. The surgical OA model was induced in the right knee joint by destabilization of the medial meniscus (DMM).17 Mice were sacrificed 8 weeks after surgery. The knee joints (n = 6) were harvested and fixed in 10% neutral buffered formalin, decalcified with 10% formic acid, and embedded in paraffin. Sagittal histological sections were obtained, stained with toluidine blue and immunostained for ERCC1. Articular cartilage damage was scored by two observers blinded to the sample identities using a scoring system previously reported by Glasson et al.18 RNA was extracted from murine AC (n = 4), and ERCC1 expression was analyzed by quantitative real-time polymerase chain reaction (RT-PCR) as described below.
Immunohistochemistry
Immunohistochemistry was performed to detect ERCC1 expression in the AC. A rabbit anti-mouse ERCC1 polyclonal antibody (Cell Signaling, Danvers, MA) was used at a 1:100 dilution and incubated at 4°C overnight. The following day, Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:200; Molecular Probes, Carlsbad, CA) was used at room temperature for 2 h to detect ERCC1 expression. After staining, ERCC1 expression in the cartilage was analyzed using Northern Eclipse software (Empix Imaging, Inc., Mississauga, ON, CANADA).
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from murine AC with QIAshredder homogenizers and the RNeasy Mini kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s protocol. One microgram of RNA was used for random hexamer-primed cDNA synthesis using a SuperScript II preamplification system (Invitrogen, Carlsbad, CA).
The converted cDNA samples were amplified in triplicate by RT PCR (using the ABI Prism 7700 sequence detection system) in a final volume of 25µl using SYBR Green Master Mix reagent. Melting curve analysis was performed using Dissociation Curves software (Applied Biosystems, Foster City, CA) to ensure that only a single product was amplified. Specificity of the reactions was confirmed by 2.5% agarose gel electrophoresis. Results were obtained using ABI Prism 7700 sequence detection software and evaluated using Excel (Microsoft, Redmond, WA). The primer pairs used for this study, were designed based on the sequences in the GenBank database, are as follows: ERCC1: 5′-GGAAGGACGAGGAAAGTCGG-3′ (sense) and 5′-AATAAGGGCTTGACCCCTGC-3′ (antisense).
Culture of HCs
Normal human knee articular chondrocytes (NHAC-kn (CC-2550)) were purchased from Lonza (Walkersville, MD) which are human primary chondrocytes isolated from normal donor knee joints that had no visible abnormalities. These chondrocytes were cultured and re-differentiated to express their marker profile according to the manufacturer’s protocol.19,20 Chondrocytes were used for experiments immediately after re-differentiation.
Catabolic Stress
Twenty-four hours after culture, adherent cells were subjected to 10 ng/ml of IL-1β (R&D Systems, Minneapolis, MN) to promote catabolic stress. After 2–48h of IL-1β stimulation, the change in ERCC1 expression was examined by immunoblotting.
ERCC1 Inhibition
The expression of ERCC1 was suppressed by small interfering RNA (siRNA) in HCs. The sense strand sequence of the RNA duplex was as follows: ERCC1, 5′-GACUGCACAUUGAUCCUCGCCUGGA-3′ (Invitrogen). The siRNA was delivered into the normal chondrocytes by Lipofectamine 2000 transfection according to the manufacturer’s instructions (Invitrogen). Twenty-four hours after transfection, the cells were treated with 10 ng/ml IL-1β and then, 24 h after treatment with IL-1β, TUNEL or Senescence Associated-β-galactosidase staining, or protein extraction was performed.
TUNEL Assay
Apoptosis was evaluated in cultured cells using the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Penzberg, Germany), for TUNEL detection following the manufacturer’s protocol. To visualize the nuclei, the cultures were incubated with DAPI for 10min. Fluorescence was visualized using a Leica DMIRB microscope equipped with a Retiga camera (QImaging). The number of cells that were TUNEL-positive was calculated and then averaged across 15 fields from each of 3 replica platings for 4 independent experiments. The percentage of apoptotic cells was calculated as TUNEL-positive cells/total cells × 100.
Senescence Associated-β-Galactosidase (SA-β-gal) Assay
Cellular senescence was evaluated in cultured cells using a Senescence β-Galactosidase Staining Kit (Cell Signaling Technology, Danvers, MA) following the manufacturer’s protocol. Images were captured using a Leica DMIRB microscope equipped with a Retiga camera (QImaging). The number of SA-β-gal-positive cells was calculated and averaged across 15 fields from each of 3 replica platings for 4 independent experiments. The percentage of senescent cells was calculated as SA-β-gal-positive cells/total cells × 100.
Sodium Dodecyl Sulfate (SDS)–Polyacrylamide Gel Electrophoresis and Immunoblotting
Chondrocytes were lysed and SDS–polyacrylamide gel electrophoresis and immunoblotting were performed as reported previously.20,21 The following antibodies were used: rabbit anti-human ERCC1, rabbit anti-human p16, rabbit anti-human cleaved poly (ADP-ribose) polymerase (PARP; all from Cell Signaling), rabbit anti-human matrix metallopeptidase (MMP)13, rabbit anti-human IL-6 (both from Abcam, Cambridge, England), mouse anti-human collagen type II, alpha 1 (COL2A1; Millipore, Billerica, MA), HRP-conjugated goat anti-rabbit IgG, and HRP-conjugated goat anti-mouse IgG (both from Cell Signaling). The intensities of the bands were quantified using ImageJ software (National Institutes of Health). Values were normalized relative to β-actin expression.
Statistical Analysis
The data were expressed as the mean ± standard deviation (SD). The Mann–Whitney U test was used for direct comparisons between two groups. The one-way ANOVA or the Kruskal–Wallis test was applied for multiple comparisons between independent groups, and the repeated measure ANOVA test was applied for multiple comparisons between dependent groups. Pair-wise, multiple comparisons were performed using the Tukey–Kramer or Scheffe’s post-hoc test. Data analyses were performed using PASW Statistics 21 (SPSS, Chicago, IL). Statistical significance was accepted at p < 0.05.
RESULTS
ERCC1 Expression in the AC From an Experimental Mouse Model of OA
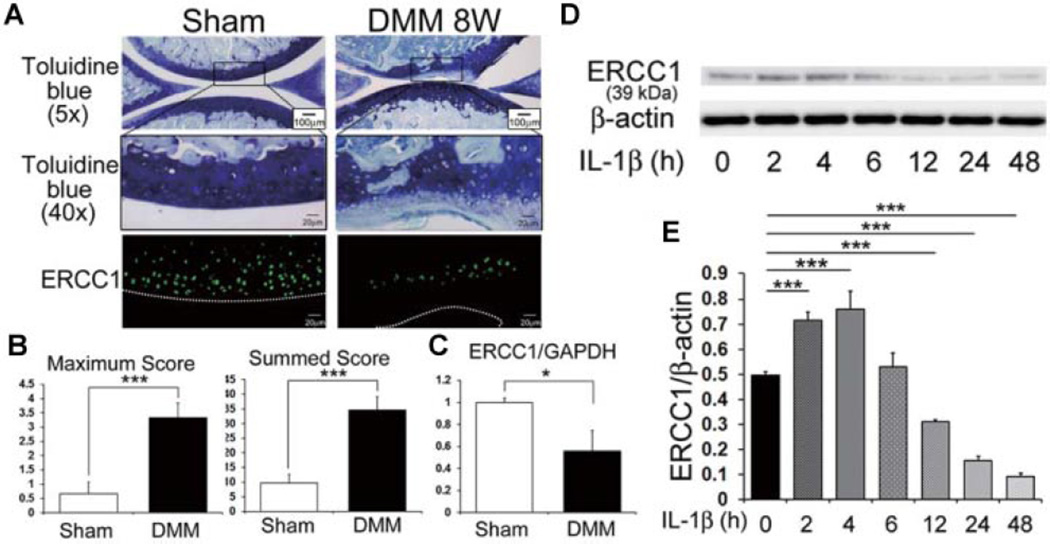
AC was examined 8 weeks after DMM surgery. Toluidine blue staining was performed to assay glycosaminoglycan content (OA pathology). Immunohistochemical analysis of the OA AC demonstrated a reduction of ERCC1 positive cells compared with the control cartilage that underwent the sham surgery (Fig. 1A). The maximum and summed OA scores in the DMM injured mice were significantly increased compared to the control mice that underwent sham surgeries. (Note that higher scores reflect an increase in abnormal AC pathology; ***P = 0.001 for maximum and summed score, respectively; Fig. 1B.) Quantitative RT-PCR showed a significant reduction of ERCC1 expression in the AC that was tested 8 weeks after DMM surgery when compared to the sham group (*P = 0.029; Fig. 1C).
Figure 1.
Expression of ERCC1 in murine AC and HC. (A) Representative image of murine cartilage from normal and OA induced knee joints 8 weeks after DMM surgery, stained with toluidine blue. Boxed areas in toluidine blue stained images (scale bar = 100 m) are shown in higher magnification (scale bar = 20µm) and immunostained for ERCC1 (green). (B) Graph indicating the maximum OA and summed OA scores. Summed OA scores were calculated from all four quadrants and eight sections from each knee. Error bars indicate the SD (***p < 0.001). (C) Quantitative RT-PCR to measure ERCC1 expression in murine cartilage from normal and OA induced knees. Error bars indicate the SD (*p < 0.05). (D) Shown is a representative image of an immunoblot to measure the protein level of ERCC1 in HC under IL-1β stress at each time point (hours, h). (E) Quantification of the expression ratios of ERCC1 to β-actin. Error bars indicate the SD (***p < 0.001).
ERCC1 Expression in HCs Under Catabolic Stress
The protein expression level of ERCC1 was measured by immunoblotting when the chondrocytes were put under IL-1β catabolic stress in vitro (Fig. 1D). ERCC1 expression was significantly increased in the first 4 h following the initiation of IL-1β catabolic stress (***P < 0.001 at 2 and 4 h relative to the time zero), but decreased after 12 h to almost undetectable levels at 48 h after treatment (***P < 0.001 at 12, 24, and 48 h relative to the time zero; Fig. 1E).
Inhibition of ERCC1 Increased Osteoarthritic Associated Changes in HCs
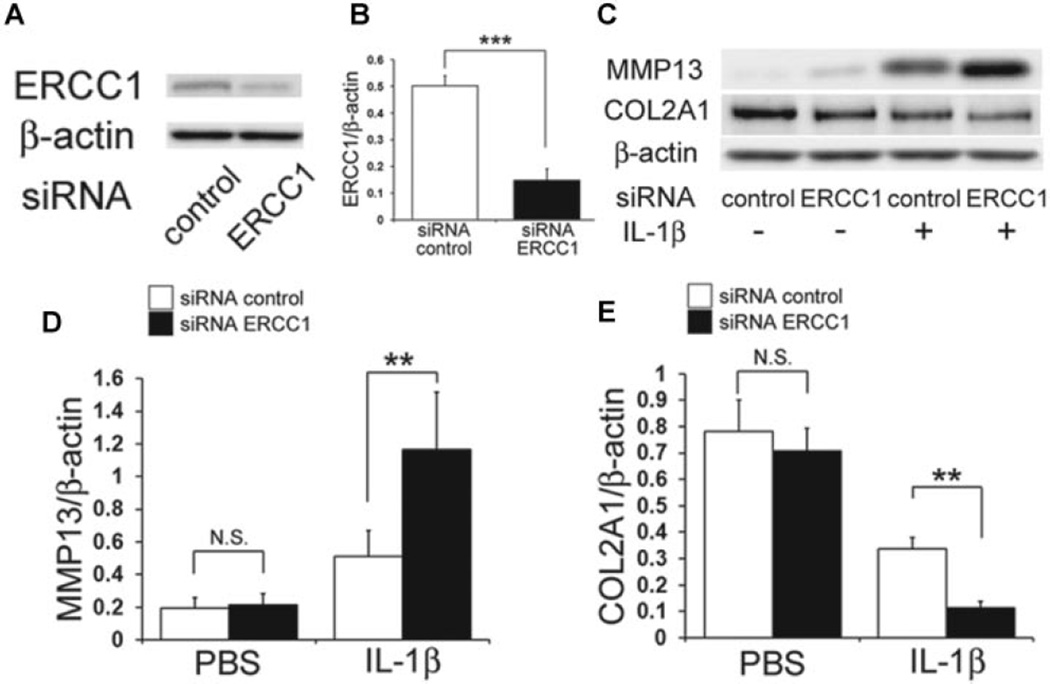
The down regulation of ERCC1 protein levels using siRNA in HCs was confirmed by immunoblotting (Fig. 2A). The mean expression level of ERCC1 in siRNA ERCC1 treated chondrocytes was reduced by 73% compared to the control level (***P < 0.001; Fig. 2B). Immunoblotting was next used to examine the effect that ERCC1 inhibition had on IL-1β-induced OA associated changes in HCs (Fig. 2C). There was no significant difference in the expression levels of MMP13 and COL2A1 between the siRNA ERCC1 and the control siRNA (P = 0.999: MMP13, P = 0.604: COL2A1; Fig. 2C). In contrast, under IL-1β stimulation, the inhibition of ERCC1 significantly increased MMP13 expression (**P = 0.003; Fig. 2D) and decreased COL2A1 expression (**P = 0.007; Fig. 2E).
Figure 2.
Effect of ERCC1 inhibition on HC under IL-1β stimulation. (A) Shown is a representative image of an immunoblot to measure the protein level of ERCC1 after transfection of control siRNA or ERCC1 siRNA. (B) Plotted is the ratio of ERCC1 to β-actin. Error bars indicate the SD (***p < 0.001). (C) Shown is a representative image of an immunoblot to measure the protein levels of MMP13 and COL2A1 after ERCC1 inhibition and stimulation with IL-1β. (D,E) Quantification of the expression ratio of MMP13 (D) or COL2A1 (E) to β-actin. Error bars indicate the SD. **p < 0.01, not significant (NS).
Inhibition of ERCC1 Increased Apoptosis in AC Stimulated With IL-1β
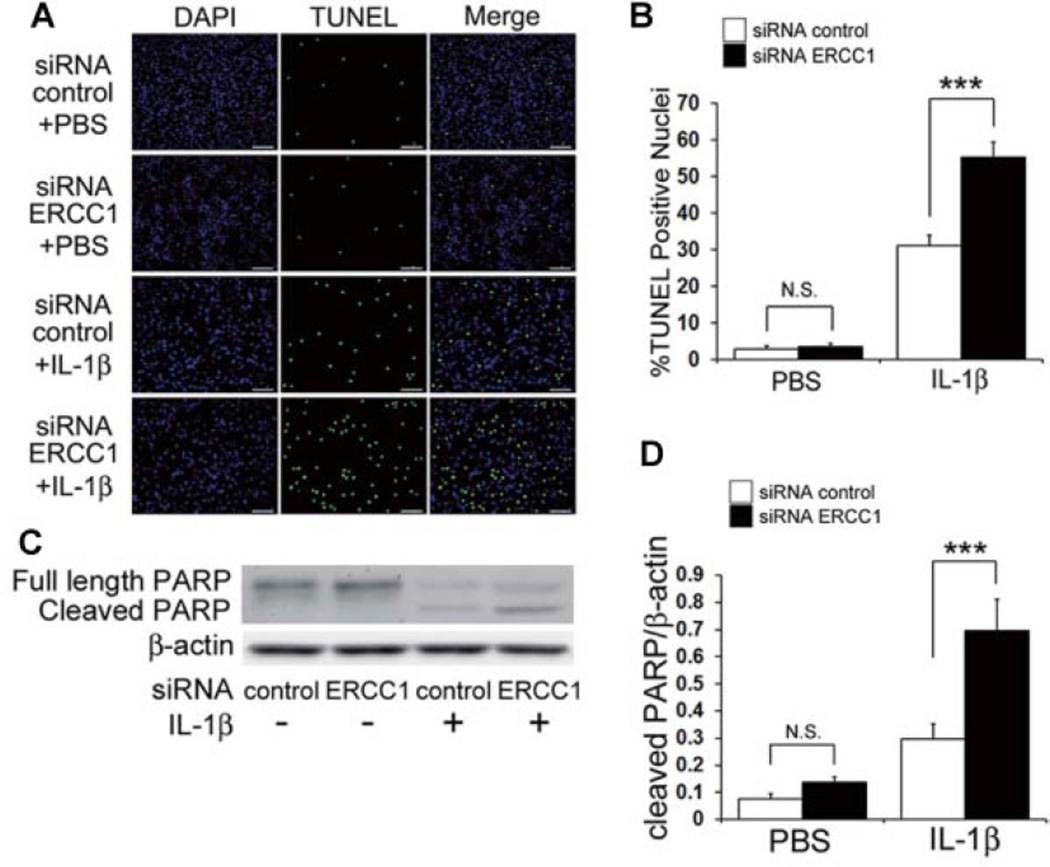
Since it has been reported that increased cell apoptosis is a potential cause of OA,11,12 the effect that ERCC1 inhibition has on HC apoptosis was examined by TUNEL staining (Fig. 3A). IL-1β treatment increased the percentage of TUNEL-positive cells from 2.8% to 31.1% of the total number of cells (***P < 0.001). In addition, siRNA ERCC1 transfected cells further increased the percentage of TUNEL-positive cells to 55.2% of the total number of cells (***P < 0.001; Fig. 3B). A significant increased amount of cleaved PARP, a marker for apoptosis, was demonstrated by immunoblotting which further confirmed that ERCC1 inhibition of chondrocytes under IL-1β stimulation increased apoptosis (Fig. 3C,D).
Figure 3.
Effect of ERCC1 inhibition on cell death in HC under IL-1β stimulation. (A) Representative images of TUNEL staining after inhibition of ERCC1 under IL-1β stimulation. HC were immunostained for TUNEL (green) and DAPI (blue). Scale bar = 100µm. (B) Quantification of %TUNEL positive cells was calculated as the percent of cells (DAPI) expressing TUNEL (green). Error bars indicate the SD. ***p <0.001. (C) Representative image of an immunoblot to measure the protein level of full length and cleaved PARP after inhibition of ERCC1 when stimulated with IL-1β. (D) Quantification of the expression ratios of cleaved PARP to β-actin was calculated. Error bars indicate the SD. ***p < 0.001, not significant (NS).
Inhibition of ERCC1 Enhanced Cellular Senescence in HCs
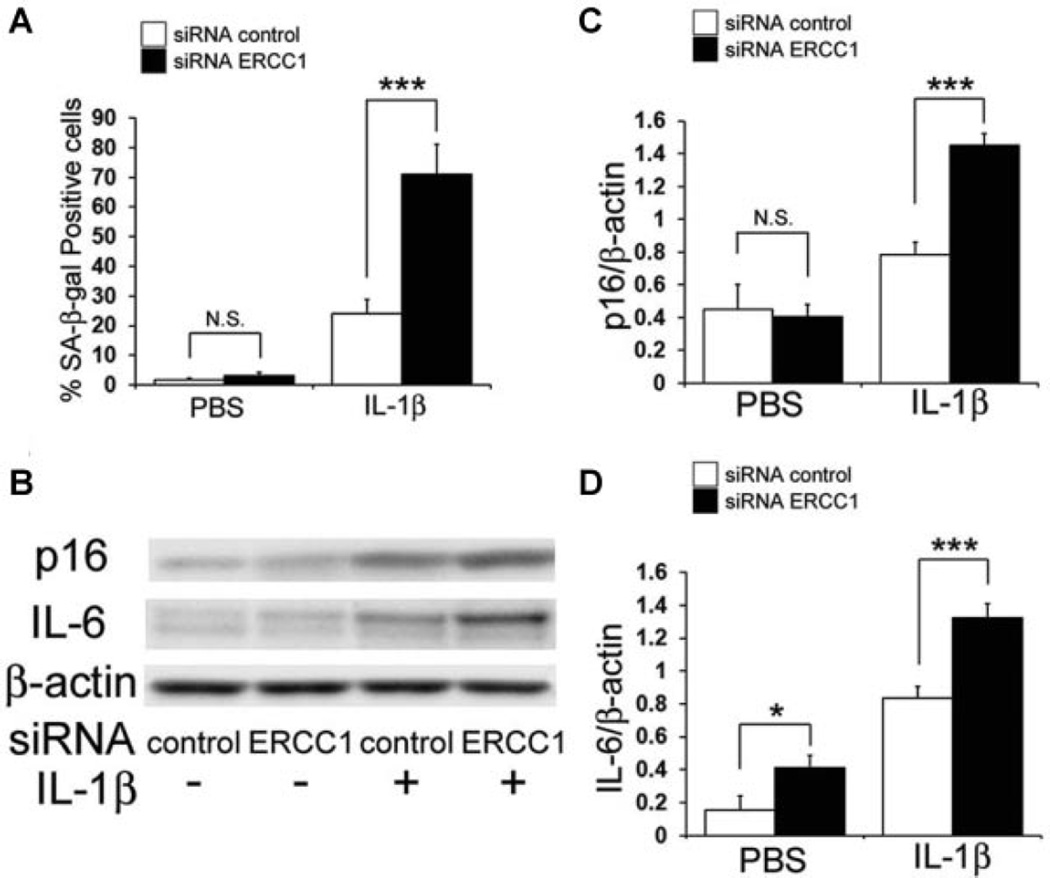
It has been reported that cellular senescence is also associated with OA,22,23 and the effect that ERCC1 inhibition has on HC senescence was examined by β-galactosidase staining. IL-1β treatment increased the percentage of β-galactosidase-positive cells from 1.8% to 24.2% of the total number of cells (P < 0.001). In addition, the transfection of siRNA for ERCC1 further increased the percentage of β-galactosidase-positive cells to 71.0% of the total number of cells (P < 0.001; Fig. 4A). It has also been reported that the activation of p16 through the reactive oxygen species (ROS) pathway has been implicated in causing DNA damage and/or senescence in aging cells.24 Moreover, IL-6 is known to initiate inflammatory responses associated with many age-related phenomena including OA.25 An increase in the expressions of these senescence associated proteins, also supports that inhibiting ERCC1 increases chondrocyte senescence (Fig. 4B–D). An increase in the expressions of senescence associated proteins, p16 and IL-6 also confirmed that ERCC1 inhibition increased chondrocyte senescence (Fig. 4B–D).
Figure 4.
Effect of ERCC1 inhibition on cellular senescence in HC under IL-1β stimulation. (A) Plotted is the average of SA-β-gal-positive cells. Error bars indicate the SD (***p < 0.001). (B) Shown is a representative image of an immunoblot measuring the expression levels of p16 and IL-6 in HC. Quantification of the expression ratios of p16(C) and IL-6 (D) to β-actin were calculated. Error bars indicate the SD. *p < 0.05, ***p < 0.001, not significant (NS).
DISCUSSION
Osteoarthritis is a disease of the diarthrodial joints in which cartilage tissue degenerates in an age-dependent manner and different molecular pathways have been implicated in the development of OA; however, the roles that angiogenesis, cellular senescence and apoptosis play in its development remain unclear. Emerging theories implicate impaired DNA monitoring and repair mechanisms coupled with DNA damage as a potential link between cellular and molecular pathway dysfunction and the ultimate phenotypic changes observed in OA. DNA damage may initiate apoptosis and senescence of chondrocytes, which causes AC degeneration. ERCC1-XPF is an endonuclease required for the repair of damaged DNA.13 ERCC1 deficiency has been shown to accelerate the onset of age related phenotypic changes26; therefore, the goal of this study was to investigate the role of ERCC1 in the development of OA.
Immunohistochemical staining indicated that ERCC1 expression was decreased in the OA lesions of our OA mouse model. ERCC1 expression was higher in areas with less severe OA degeneration versus those with more severe degeneration; moreover, the chondrogenic differentiation capacity of muscle derived stem cells isolated from Ercc1 deficient mice was reduced when compared with cells isolated from WT mice,26 which provides evidence that ERCC1 plays a role in maintaining normal chondrogenesis.
ERCC1 expression varied depending on the time the cells were subjected to IL-1β stimulation where they increased expression after 4 h and decreased expression after 12 h of stimulation. This implies that chondrocytes subjected to catabolic stresses increase ERCC1 expression initially and begin to decrease expression under long term stress. These results suggest that ERCC1 expression increases as an adaptive response to protect the cells from stress during the development of OA, and the failure of this response may lead to further progression of the degenerative process.
Catabolic stress was applied to a series of chondrocytes with concurrent ERCC1 inhibition which revealed that ERCC1 inhibition in HCs under the influence of IL-1β increased MMP13 expressions and decreased COL2A1 expressions, suggesting the inhibition of ERCC1 enhanced the manifestation of IL-1β induced OA associated changes in HCs. To examine the mechanism of ERCC1 regulation in the OA associated changes in HCs, we investigated whether ERCC1 modulates apoptosis and cellular senescence. Although the inhibition of ERCC1 did not significantly affect apoptosis without IL-1β stimulation, ERCC1 inhibition increased apoptosis with concomitant IL-1β stimulation, suggesting that ERCC1 is necessary for the prevention of apoptosis of HCs. Numerous other studies have also shown the importance of ERCC1 in regulating cell survival when cells are subjected to cellular stresses such as oxidative stress and DNA damage,27–30 further supporting the notion that ERCC1 protects the cells from stresses that would initiate apoptosis. Recent studies have indicated that senescence is another cellular phenomena associated with the development OA.22,23 Cellular responses to DNA damage are the most prominent steps in the initiation of senescence, and these responses are characterized by the induction of cell cycle arrest.31 Cellular senescence was investigated while inhibiting ERCC1 in cultured HC while they were subjected to IL-1β stimulation, which increased the expression of cellular senescence associated proteins and the cellular senescence marker p16, indicating that ERCC1 prevented the initiation of cellular senescence. These results imply that ERCC1 plays an important role in preventing OA by inhibiting chondrocytic cellular senescence. IL-6 is secreted by senescent cells and is known to initiate inflammatory responses associated with many age-related phenomena including cardiac hypertrophy, osteoporosis, neurodegenerative disease, and cancer.32–35 The up-regulation of IL-6 has also been associated with OA in aged mice, while the inhibition of IL-6 halted AC destruction in this animal model.25 In our study, the inhibition of ERCC1 significantly increased the level of IL-6 expression under IL-1β induced catabolic stress which suggests that inhibiting ERCC1 enables the development of OA changes via the increased expression of IL-6.
In summary, this study demonstrated that decreased levels of ERCC1 were associated with an increased severity of OA degeneration in the knees of an OA mouse model. Furthermore, the inhibition of ERCC1 enhanced the manifestation of IL-1β induced OA changes in HCs, including increased apoptosis and senescence. These observations suggest that ERCC1 plays an important role in protecting HC from catabolic stress that correlates with the pathogenesis of OA. Further study of ERCC1 function in chondrocytes will provide novel insights into the pathophysiology of OA and could lead to new therapeutic approaches for the treatment of OA.
ACKNOWLEDGEMENTS
The authors are grateful for the technical advice provided by Jessica Tebbets, and James Cummins and Bria King for their editorial assistance. This work was funded in part by the Henry J. Mankin Endowed Chair at the University of Pittsburgh and the William F. and Jean W. Donaldson Endowed Chair at the Children’s Hospital of Pittsburgh. We also thank Dr. Paul D. Robbins and Dr. Laura J, Niedernhofer (Department of Metabolism and Aging, The Scripps Research Institute, FL) for their expert advice regarding Ercc1. This study was also supported in part by a NIH grant awarded to PR. Robbins (PI) and J. Huard (PI, Project 3) (PO1 AG043376).
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Vignon E, Arlot M, Meunier P, et al. Quantitative histological changes in osteoarthritic hip cartilage-morphometric analysis of 29 osteoarthritic and 26 normal human femoral heads. Clin Orthop Relat Res. 1974;103:269–278. [PubMed] [Google Scholar]
- 2.Loeser RF, Carlson CS, Del Carlo M, et al. Detection of nitrotyrosine in aging and osteoarthritic cartilage: correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 4.Hoeijmakers JHJ. DNA damage, aging, and cancer (vol 361, pg 1475 2009) N Engl J Med. 2009;361:1914–1914. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Vijg J. The role of DNA damage and repair in aging: new approaches to an old problem. Mech Ageing Dev. 2008;129:498–502. doi: 10.1016/j.mad.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 8.Aigner T, Richter W. OA in 2011 age-related OA—a concept emerging from infancy? Nat Rev Rheumatol. 8:70–72. doi: 10.1038/nrrheum.2011.206. [DOI] [PubMed] [Google Scholar]
- 9.Rose J, Soder S, Skhirtladze C, et al. DNA damage, discoordinated gene expression and cellular senescence in osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2012;20:1020–1028. doi: 10.1016/j.joca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Kim HA, Lee YJ, Seong SC, et al. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27:455–462. [PubMed] [Google Scholar]
- 11.Blanco FJ, Guitian R, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis—a possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Armada MJ, Carames B, Lires-Dean M, et al. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad A, Robinson AR, Duensing A, et al. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–5092. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niedernhofer LJ, Odijk H, Budzowska M, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sijbers AM, de Laat WL, Ariza RR, et al. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 16.Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst) 2011;10:781–791. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Glasson SS, Chambers MG, Van Den Berg WB, et al. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 18:S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki H, Takayama K, Matsushita T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 20.Takayama K, Ishida K, Matsushita T, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki H, Takayama K, Matsushita T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 22.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A:106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 23.Price JS, Waters JG, Darrah C, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 25.Ryu JH, Yang S, Shin Y, et al. Interleukin-6 plays an essential role in hypoxia-inducible factor 2 alpha-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2011;63:2732–2743. doi: 10.1002/art.30451. [DOI] [PubMed] [Google Scholar]
- 26.Lavasani M, Robinson AR, Lu A, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 3:608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hlavin EM, Smeaton MB, Miller PS. Initiation of DNA interstrand cross-link repair in mammalian cells. Environ Mol Mutagen. 51:604–624. doi: 10.1002/em.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunkern TR, Kaina B. Cell proliferation and DNA breaks are involved in ultraviolet light-induced apoptosis in nucleotide excision repair-deficient Chinese hamster cells. Mol Biol Cell. 2002;13:348–361. doi: 10.1091/mbc.01-05-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niedernhofer LJ, Garinis GA, Raams A, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 30.Osawa T, Davies D, Hartley JA. Mechanism of cell death resulting from DNA interstrand cross-linking in mammalian cells. Cell Death Dis. 2:e187. doi: 10.1038/cddis.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passos JF, Nelson G, Wang C, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 33.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurdi M, Randon J, Cerutti C, et al. Increased expression of IL-6 and LIF in the hypertrophied left ventricle of TGR(mRen2)27 and SHR rats. Mol Cell Biochem. 2005;269:95–101. doi: 10.1007/s11010-005-3085-1. [DOI] [PubMed] [Google Scholar]