Abstract
Besides the two nuclear estrogen receptors (ESR1/ESR2), the G protein-coupled estrogen receptor (GPER) was described in the human testis but little is known about testicular GPER during development or male infertility. We performed an immunohistochemical analysis using human and rhesus monkey testicular samples. The results obtained in adult primate testes showed GPER in interstitial and vascular cells as well as in the smooth muscle-like peritubular cells, which build the wall of seminiferous tubules. Expression of GPER was also found in cultured human testicular peritubular cells (HPTCs) by Western blotting and RT-PCR/sequencing. Furthermore, as seen in time-lapse videos of cultured cells, addition of a specific GPER agonist (G1) significantly reduced the numbers of HTPCs within 24 h. A GPER antagonist (G15) prevented this action, implying a role for GPER related to the control of cell proliferation or cell death of peritubular cells. Peritubular cell functions and their phenotype change, for example, during postnatal development and in cases of male infertility. The study of non-human primate samples revealed that GPER in peritubular cells was detectable only from the time of puberty onwards, while in samples from infantile and prepubertal monkeys only interstitial cells showed immunopositive staining. In testicular biopsies of men with mixed atrophy a reduction or loss of immunoreactive GPER was found in peritubular cells surrounding those tubules, in which spermatogenesis was impaired. In other cases of impaired spermatogenesis, namely when the tubular wall was fibrotically remodeled, a complete loss of GPER was seen. Thus, the observed inverse relation between the state of fertility and GPER expression by peritubular cells implies that the regulation of primate testicular peritubular cells by estrogens is mediated by GPER in both, health and disease.
Keywords: cell culture, development, sex hormone receptor, seminiferous tubules, infertility
INTRODUCTION
There is increasing evidence for a crucial role of the “female” sex hormone estrogen in spermatogenesis and male fertility (reviewed in Hess, 2003; Carreau and Hess, 2010; Joseph et al., 2011). Estrogen actions are mediated by three distinct estrogen receptors. All of them are known to be expressed in the human testis and are physiologically activated by estradiol (E2). The two nuclear estrogen receptors (ESR1 and ESR2) act primarily as transcription factors (Hess et al., 2011; reviewed in Heldring et al., 2007; Stanisic et al., 2010), whereas the more recently discovered membrane-associated G protein-coupled estrogen receptor (GPER or formerly known as GPR-30) mediates predominantly rapid non-genomic effects (Rago et al., 2011; Hess et al., 2011). Nevertheless each of the three estrogen receptors is able to mediate rapid signaling events and induce changes in gene transcription (Prossnitz et al., 2008).
In normal adult human and rat testes, GPER was reported in somatic (Sertoli and Leydig cells; Lucas et al., 2010; Rago et al., 2011) and germ cells (spermatogonia and spermatocytes; Chevalier et al., 2012). It was also described in germ cell tumors (Franco et al., 2011) and in rat tumor Leydig cells (R2C) (Chimento et al., 2013). It appears that the three estrogen receptors are expressed in the human testis in a partially overlapping and cell-type-specific pattern (Prossnitz et al., 2008), but this remains to be fully elucidated. Similarly, the specific functions of the three different estrogen receptors within the testis remain to be established. An artificial, specific ligand of GPER, G1, which does not activate the other ESRs if used in concentrations below 10 μM (Bologa et al., 2006), has emerged as a useful tool, together with G15, a specific antagonist (Filardo and Thomas, 2012). Recent studies showed that activation of GPER through this specific ligand can lead to both, proliferation and apoptosis depending on the particular tissue (Prossnitz et al., 2008; Prossnitz and Barton 2014). In Leydig tumor cells, G1 mediates an antiproliferative and even pro-apoptotic effect, suggesting that G1 may have potential as a therapeutic cancer agent (Chimento et al., 2013).
Functions and cellular composition of the testis change during development and during formation of male infertility (Welter et al., 2013; Schell et al., 2010; Adam et al., 2011, 2012; Holm et al., 2003), but the underlying endocrine mechanisms are unclear. Our primary goal was to examine GPER expression in postnatal testicular development and in male infertility. Therefore our study utilized human testicular biopsies as well as samples from a translational non-human primate model, the rhesus monkey.
MATERIAL AND METHODS
Human and monkey samples
Testicular biopsies were obtained from men with normal and impaired spermatogenesis, and had been used in a previous study (Welter et al., 2013). Biopsies were arranged into groups, depending on their morphohistological appearance: The first group examined showed normal spermatogenesis (n = 4); the second group included patients with mixed atrophy (MA; n = 5); the third group included patients suffering from Sertoli cell only syndrome (SCO; n = 5); and the last group showed germ cell arrest (GA; n = 2). The local ethical committee approved this human study and all participants granted written informed consent.
Postmortem testicular samples were obtained from rhesus monkeys (Macaca mulatta) through the ONPRC Tissue Distribution Program and were identical to ones used in previous postnatal developmental studies (Adam et al., 2012; Frungieri et al., 2000). The study included 7 immature animals (ages: 6, 7, 120 and 281 days; 1, 2, and 3 years), as well as three young adults (ages: 5, 5 and 6 years); Before use, testes were fixed in Bouin’s solution, embedded in paraffin and cut into 5-μm thick slices.
Immunohistochemistry
For immunohistochemistry anti-GPER antiserum (1:500; affinity isolated, polyclonal rabbit anti-human GPER; Sigma Prestige Antibodies, St. Louis, USA) was used, as previously described (Schell et al., 2010). Controls included rabbit IgG (2 μg/ml; Millipore, Billerica, USA) instead of the primary antibody. Samples were examined with Zeiss microscope (Oberkochen, Germany), pictures were taken by a camera (Visitron Systems, Puchheim, Germany) and evaluated with SPOT image software (SPOT Imaging Solutions, Burroughs, USA).
Cell culture studies
Testicular biopsies were used to isolate and to culture HTPCs, as previously described (Albrecht et al., 2006; Schell et al., 2010, Adam et al., 2011; 2012; Spinnler et al., 2010). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM; PAA GmbH, Cölbe, Germany) supplemented with 10 % fetal bovine serum (FBS; PAA GmbH, Cölbe, Germany) and 1 % Penicillin/Streptomycin in a humidified atmosphere (37° C, 5 % CO2). When required confluence was reached, cells were starved for at least 24 h to synchronize the cell cycle before use. G1 and G15 (TOCRIS, Bioscience, Bristol, UK) were dissolved in dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, USA) therefore DMSO at a final concentration of 0.01 ‰ served as control. Concentration of G1/G15 at 1 μM was chosen based on previous reports (Prossnitz et al., 2008; Chimento et al., 2013).
RT-PCR
Total RNA was extracted from cells of three different donors and RT-PCR was performed as previously described (Schell et al., 2010). Primer3web software (http://primer3.wi.mit.edu) was used to design the following GPER primer: Forward: 5′-AGG GAC AAG CTG AGG CTG TA-3′ and reverse: 5′-GAA CCT CAC ATC CGA CTG CT-3′. RNA instead of cDNA served as negative control. Agarose gel (2 %) electrophoresis and sequence analysis (GATC, Freiburg, Germany) should corroborate GPER transcript.
Western blotting
Western blotting experiments with cultured HTPCs (10 μg total protein) were performed as previously described (Schell et al., 2010). Membranes were incubated overnight with polyclonal rabbit anti-human GPER (1:2000, Sigma Prestige Antibodies, St. Loius, USA), i.e. the same antibody as used for immunohistochemistry. Experiments included a total of 14 different samples, and the results were normalized to GAPDH (Cell Biolabs Inc., San Diego, USA) serving as loading control.
Live cell video imaging
HTPCs were cultured in a glass bottomed 35 mm3 culture dish (Ibidi; Martinsried, Germany) for at least 24 h to allow adhesion before they were starved for 24 h. Then 1μM G1 was added while in the control culture dish DMSO at 0.01 ‰ was applied. In some experiments 1 μM G15 was added 30 minutes prior to stimulation with 1 μM G1. The samples were put in a gas incubation (The Brick, Ibidi; Martinsried, Germany) and heating system (HT200, Ibidi; Martinsried, Germany) to assure the right condition for cell growth (5 % CO2, 37° C, 89 l/h, 95 % relative humidity, 2 % O2). Pictures were taken (ProgRes MF, Jenoptik, Jena, Germany) every 10 min with a transmitted light microscope (Zeiss, Axiovert 135) at 20x magnification. A time-lapse series was created using Micro-Manager 1.3 Microscopy Software (Vale Lab, San Francisco, USA). Movie sequences were produced with iMovie 9.0.3 (Apple Inc., Cupertino, USA). Experiments were repeated 4 times using cells from different donors.
Electronic cell counter for determining cell number and their viability (CASY)
HTPCs were counted in an automated cell counting device (CASY; Schärfe System GmbH, Reutlingen, Germany) prior to seeding and after stimulation with G1 for 24 h. Equal numbers of cells (ranging from 5000 to 10000 per cm2) from the same donor were seeded on 60-mm dishes. The cells were serum-starved for 24 h before addition of G1 at 1 μM for 24 h or DMSO at 0.01 ‰ as control. After the treatment, cells were trypsinized (PAA GmbH, Cölbe, Germany) and centrifuged before resuspended in PBS. A 1:500 dilution in a special formula (CasyTon; Innovatis, Reutlingen, Germany) of the cell suspension was used for the measurement. CASY technology uses a low voltage field to assess the integrity of the plasma membrane and thus concludes about cell viability.
Data analysis
Results are expressed as the mean values + SEM. Analysis was performed using Prism 4.0a (GraphPad Software Inc., San Diego, USA). Student’s t-test was used to compare two groups. A probability value of p < 0.05 was considered statistically significant.
RESULTS
GPER expression in human testicular biopsies of adult men with normal spermatogenesis
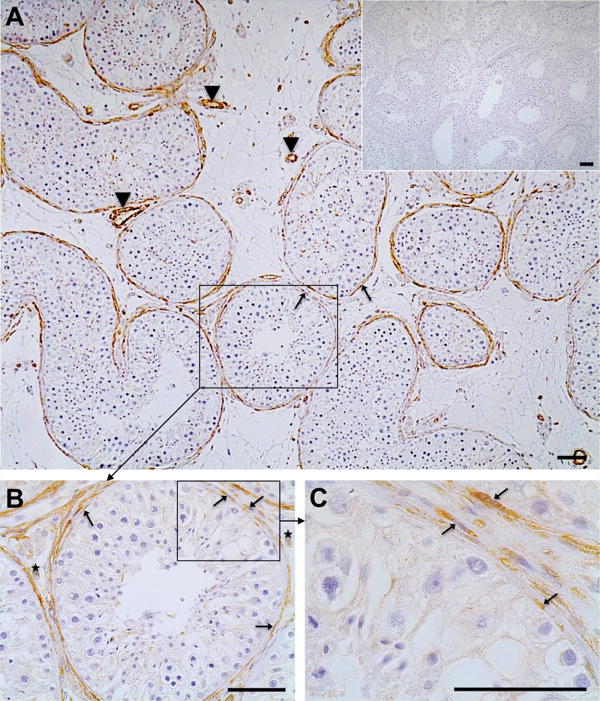
Immunoreactive GPER was predominantly located in cells forming the wall of the seminiferous tubules (arrows, Fig.1 A–C) as well as in smooth muscle cells of blood vessels (arrowheads). Some GPER-positive cells scattered in the interstitial region (asterisks, Fig. 1 B) were also observed. No labeling was evident in control sections when the primary antibody was substituted by IgG (Inset, Fig. 1 A). The staining results were identical in all four testicular samples examined.
Figure 1. Immunohistochemistry of GPER in the human testis (A–C).
In the human testes smooth muscle-like peritubular cells (arrows; A–C) and smooth muscle cells of blood vessels (arrowheads; A, C) express GPER. Few interstitial cells (asterisks, B) also stain for GPER. Negative control (A, inset) was performed with IgG instead of the antibody. Haematoxylin was used to stain nuclei. Scale bars: 50 μm.
GPER in HTPCs: Expression and functional assays
Cultured HTPCs from three different patients were studied by RT-PCR/sequencing and Western blotting. Results allowed us to identify a 120 bp GPER transcript (Fig. 2 A) showing 100 % homology to the human sequence (data not shown). The protein bands detected by Western blotting (Fig. 2 B; using the same antibody as for immunohistochemistry) correspond well with the estimated molecular weight of GPR30 (42 kDA).
Figure 2. GPER-expression in isolated human testicular peritubular cells.
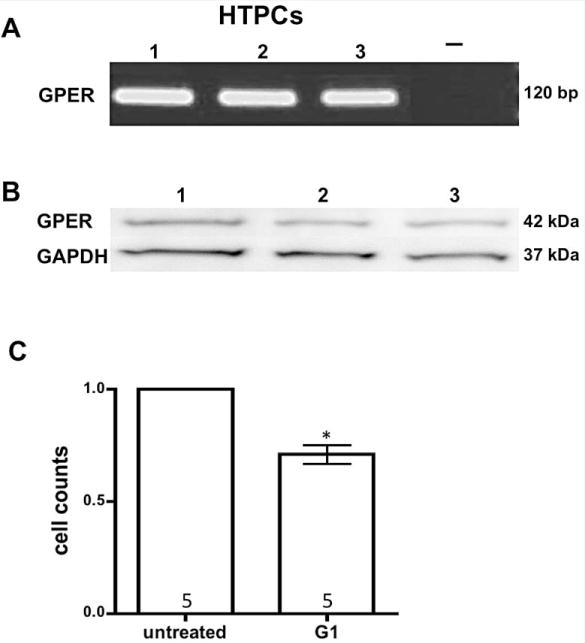
Result of a representative RT-PCR experiment (A) using HTPCs of three different donors (1–3). A single band at the expected size of 120 bp is depicted, which upon sequencing was found to correspond to human GPER. Negative control (−) including RNA instead of cDNA showed no band.
Western blot (B) of GPER was performed with 10 μg of total proteins extracted from 3 different HTPCs (1–3) showing a band of 42 kDa. The membrane was stripped and reprobed for GAPDH (37 kDA) as a loading control.
Analysis of cell number and their viability using CASY (C) revealed a significant lower cell count in G1 stimulated HTPCs after 24 h compared to vehicle treated cells. n = 5; Student’s t-test, p < 0.05.
Since GPER activation by G1 was reported to inhibit proliferation and/or to induce apoptosis, we used G1 at the concentration of 1 μM in HTPCs to explore whether this receptor is functional. CASY measurements revealed that exposure of HTPCs to 1 μM G1 for 24 h reduced the numbers of cells significantly (p < 0.05) in 5 independent experiments (Fig. 2 C).
To study the cellular basis for this result, video imaging of HTPCs were performed for 24 h. Normally HTPCs proliferate at a slow pace and this can be witnessed. The analysis of time-lapse videos showed that in the presence of 1 μM G1, HTPCs did however not proliferate (Fig. 3 A, B). Instead, stimulated cells changed their morphology; they became rounded and detached from the plate within 24 hours. This may indicate that some cells die (Fig. 3 B). In contrast, addition of G15 30 min prior to G1 completely suppressed the effect of G1 (Fig. 3 C, D) and mitotic divisions continued to occur.
Figure 3. Results of time lapse video monitoring (A–D).
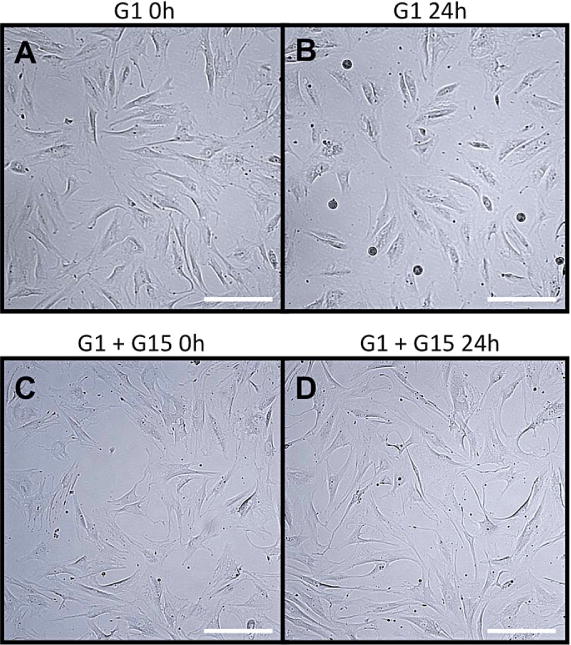
Example view taken from video imaging of HTPCs over 24 h. Box A shows HTPCs prior addition of G1 (1 μM). Box B shows the same area after 24 h. Box C shows HTPCs exposed to G1 and G15 before and box D 24 h later. Note the differences in cell density as well as rounded and detached cells from the plate in B. Scale bars: 50 μm.
Age-dependent GPER expression in the testes of rhesus monkeys
As observed for the adult human testis, GPER was expressed by peritubular cells surrounding the seminiferous tubules in the adult (5–6 years) monkey (arrows, Fig. 4 A, and enlargement of the boxed area) and was localized in vascular smooth muscle cells of testicular blood vessels (arrowheads, Fig. 4 A, including enlargement of the framed area). Some cells in the interstitial region were also GPER-positive (asterisks, enlargement of the framed area of Fig. A). Staining with the GPER antibody was specific since no labeling became evident when the primary antibody was replaced by IgG (Inset of Fig. 4 A).
Figure 4. GPER expression in peritubular cells of the monkey testis begins with puberty.
Immunohistochemistry of GPER of adult rhesus monkeys (A) showed strong and robust GPER expression in the peritubular compartment (arrows). Smooth muscle cells of blood vessels (arrowheads) served as positive intrinsic control. GPER staining of the peritubular wall was not found in samples from prepubertal rhesus monkeys (B). Only some interstitial cells (asterisks) and blood vessels (arrowheads) were stained. A sample of peripubertal rhesus monkey (C) shows strong staining of interstitial cells (asterisks), incipient GPER expression of the peritubular wall of the seminiferous tubules (arrows) and constantly stained blood vessels (arrowheads). Negative control (A, inset) was performed with IgG. Haematoxylin was used to counterstain nuclei. Scale bars: 50 μm.
GPER expression in testicular samples of sexually immature rhesus monkeys was strikingly different. Generally, peritubular cells were not, or only occasionally, GPER-immunopositive. In detail, in testicular biopsies of new born (days 6–7, data not shown) or infantile monkeys (one year old; Fig. 4 B), GPER was solely expressed by vascular smooth muscle cells cell (arrowheads) and in rare cases found in some interstitial cells (asterisks). In prepubertal rhesus monkeys (2–3 years old) interstitial cells markedly stained immunopositive for GPER (asterisks, Fig. 4 C). Individual GPER-positive peritubular cells became evident in the seminiferous tubules (arrows, Fig. 4 C) while vascular smooth muscle cells constantly stained GPER-immunopositive, independent from age, hence serving as a positive intrinsic control (Fig. 4 A–C, arrowheads).
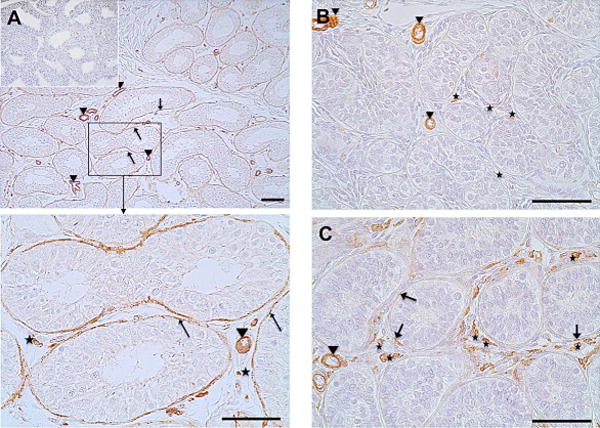
Reduction / Loss of GPER expression in testicular biopsies of infertile men
The onset of peritubular GPER-expression around puberty implies a role linked to testicular function/fertility. Therefore, we examined samples of infertile men with different forms of impaired spermatogenesis, namely SCO, GA and MA (Fig. 5). In contrast to testicular biopsies from men with normal spermatogenesis showing regular peritubular GPER expression, samples from infertile men exhibited a reduced or even deficient GPER immunostaining. An almost complete loss of seminiferous tubular GPER protein was observed in SCO patients (Fig. 5 A; arrows may indicate residual GPER), while in patients with GA more peritubular cells remained GPER-immunopositive (arrows, Fig. 5 B). GPER staining was mostly retained in unaffected tubules of MA patients (Fig. 5 C, arrows, including enlargement of the framed area) while hyalinized tubules (asteriks) were always associated with a complete demise of GPER. Again, vascular smooth cells constantly stained immunopositive (arrowheads, Fig. 5).
Figure 5. Peritubular GPER expression is reduced or lost in infertile men.
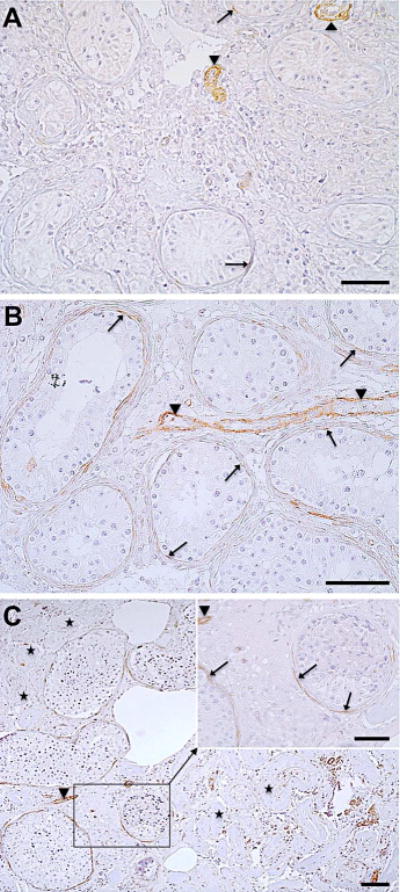
Seminiferous tubules of patients with SCO (A; n = 5) were associated with an almost complete loss of peritubular GPER while in GA patients (B; n = 2) some peritubular cells remained GPER-immunopositive (arrows). In contrast, GPER staining was partly retained in healthy tubules of MA patients (arrows, enlargement of the framed area of Fig. 5 C; n = 5) while hyalinized tubules (asteriks) were always associated with a complete loss of GPER. Vascular smooth cells constantly stained immunopositive (arrowheads) independent from infertility state. Scale bars: 50 μm.
DISCUSSION
Testicular estrogen receptors are emerging as indispensable regulators of male fertility. This study shows for the first time that human and non-human primate testicular peritubular cells express GPER in vivo and in vitro and that this expression is associated with the process of spermatogenesis, and hence with male fertility in vivo. Thus, in rhesus monkeys peritubular cells express GPER only upon the onset of puberty, and in man GPER expression is lost in case of idiopathic infertility.
Expression by testicular vascular smooth muscle-cells was evident in all samples and is in line with previous reports showing localization in non-testicular vasculature (Barton, 2012; Meyer et al., 2009). These and other data suggest that estrogens via GPER can regulate the vascular tone (Prabhushankar et al., 2014) and vascular function (Haas et al., 2009). Furthermore, the positive staining of testicular vascular cells in our study provided an ideal intrinsic positive control and excludes technical problems. It supports the unexpected results related to peritubular cells. The specificity of the antiserum was further substantiated by results of Western blotting experiments using human granulosa cells, recently reported to express GPER (Pavlik et al., 2011; results not shown). To our knowledge GPER expression by the smooth-muscle-like peritubular cells of the testis has not been reported in previous studies using different antibodies and immunohistochemistry. Surprisingly the antiserum, which we used in our study, did not stain germ cells or Sertoli cells. Chevalier et al. (2012) reported Sertoli and germ cells to be immunoreactive while Rago et al. (2011) detected GPER solely in somatic but not germ cells. These discrepancies may be related in part to the different antibodies used. Since GPER may reside in membranes of intracellular organelles, the nucleus and/or the plasma membrane (Filardo & Thomas, 2012) the accessibility of the antibody to these compartments is a further factor that may be involved. In our study immunoreactive interstitial cells were also found in both, the adult and the immature testis, and may represent Leydig cells and/or immune cells.
To further test the unexpected localization of GPER and the specificity of the antiserum, we used HTPCs (see Albrecht et al., 2006; Schell et al., 2010, Adam et al., 2011, 2012: Mayerhofer 2013; Flenkenthaler et al., 2014). The expression of GPER by adult human peritubular cells was confirmed by sequence analysis of the PCR product. Furthermore, the same antiserum we used for immunohistochemistry detected a 42-kDa band corresponding to the one described for GPER (Chimento et al., 2013). HPTCs also responded selectively to the non-steroideal agonist G1 and the results showed that this receptor is involved in reducing proliferation and altering morphology of these cells in vitro. G15 specifically blocked the inhibitory effects of G1 on cell proliferation, hence a functional GPER exists in HTPCs.
Clearly, signaling and down-stream consequences of GPER activation in HTPCs remain to be further studied. Yet, its antiproliferative effect is in agreement with actions of GPER observed in endothelial and vascular smooth muscle cells promoting cell cycle retardation and attenuating proliferation (Meyer et al., 2009; Barton, 2012; Holm et al., 2011; Li et al., 2013). In Leydig tumor (Chimento et al., 2013) and ovarian cancer cells (Wang et al., 2013), GPER activation by G1 has even been linked to apoptosis.
Testicular peritubular cells in man and non-human primates are known to form a multi-layered compartment between the germinal epithelium and the interstitial compartment (Maekawa et al., 1996; Mayerhofer et al., 2013). The cells of this compartment undergo changes and differentiate at puberty, evidenced by the expression of smooth muscle actin (SMA; Schlatt et al., 1993). They also appear to loose at least part of their differentiation state (i.e. SMA and other smooth muscle markers) in cases of idiopathic male infertility (Schell et al., 2010; Welter et al., 2013). The results of the present study reveal that GPER-expression by these cells follows the same pattern. Expression of GPER can only be found in adult peritubular cells if spermatogenesis in the respective tubule is active. This can be concluded from studies using MA samples, in which normal tubules co-exist with ones containing impaired spermatogenesis. The use of this type of samples rules out technical problems (e.g. unequal fixation or embedding conditions).
Taken together, the results from this study suggest specific roles of estrogens via GPER in both, the human and non-human primate testis, which are related to the functions of peritubular cells (Mayerhofer, 2013; Nurmio et al., 2012). Thus the smooth muscle-like cellular phenotype of peritubular cells (Schell et al., 2010; Welter et al., 2013), and their secretory phenotype (Flenkenthaler et al., 2014) may be regulated by estrogen and GPER. Human peritubular cells produce, for example, glial cell line derived neurotrophic factor (GDNF), which is essential for spermatogonial stem cells (Spinnler et al., 2010) and thus may directly contribute to the spermatogonial stem cell niche. A plethora of further secreted factors was revealed by a recent proteomic study (Flenkenthaler et al., 2014), which could play important roles in the paracrine regulation of spermatogenesis and other testicular functions. Additional studies are now required to tackle this issue and to study the possible role of GPER in testicular peritubular smooth muscle-like cells.
Acknowledgments
We gratefully acknowledge A. Tiefenbacher for her skilled technical procedures.
Grant information: Supported by Friedrich-Baur Stiftung and in parts by DFG MA1080/21-1 and NIH grants AG036670 and OD011092.
Footnotes
Disclosure statement: The authors have nothing to disclose. Part of this work was done to fulfill the requirements for Dr. med. thesis of F.S. at Ludwig Maximilian University.
Author’s contribution: A.M. conceived of the study and together with H.W. supervised the experiments. F.S. performed the majority of the experiments and analyzed the data, H.W. performed part of the video imaging studies. S.F., H.W. and A.M. drafted the paper. JU.S. and FM.K. contributed the human testis tissue, HF.U. contributed the rhesus monkey testis tissue and they provided important conceptual input. All authors finalized and approved the manuscript.
References
- Adam M, Schwarzer JU, Köhn FM, Strauss L, Poutanen M, Mayerhofer A. Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: possible role of decorin in male infertility by interfering with growth factor signaling. Hum Reprod. 2011;26:2613–2625. doi: 10.1093/humrep/der245. [DOI] [PubMed] [Google Scholar]
- Adam M, Urbanski HF, Garyfallou VT, Welsch U, Köhn FM, Ullrich Schwarzer J, Strauss L, Poutanen M, Mayerhofer A. High levels of the extracellular matrix proteoglycan decorin are associated with inhibition of testicular function. Int J Androl. 2012;35:550–561. doi: 10.1111/j.1365-2605.2011.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht M, Rämsch R, Köhn FM, Schwarzer JU, Mayerhofer A. Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocrinol Metab. 2006;91:1956–1960. doi: 10.1210/jc.2005-2169. [DOI] [PubMed] [Google Scholar]
- Barton M. Position paper: The membrane estrogen receptor GPER–Clues and questions. Steroids. 2012;77:935–942. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Vega A, Bouskine A, Siddeek B, Michiels JF, Chevallier D, Fénichel P. GPR30, the non-classical membrane G protein related estrogen receptor, is overexpressed in human seminoma and promotes seminoma cell proliferation. PLoS One. 2012;7:e34672. doi: 10.1371/journal.pone.0034672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento A, Casaburi I, Bartucci M, Patrizii M, Dattilo R, Avena P, Andò S, Pezzi V, Sirianni R. Selective GPER activation decreases proliferation and activates apoptosis in tumor Leydig cells. Cell Death Dis. 2013;4:e747. doi: 10.1038/cddis.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenkenthaler F, Windschüttl S, Fröhlich T, Schwarzer JU, Mayerhofer A, Arnold GJ. Secretome Analysis of Testicular Peritubular Cells: A Window into the Human Testicular Microenvironment and the Spermatogonial Stem Cell Niche in Man. J Proteome Res. 2014;13(3):1259–69. doi: 10.1021/pr400769z. 2014 Mar 7. [DOI] [PubMed] [Google Scholar]
- Franco R, Boscia F, Gigantino V, Marra L, Esposito F, Ferrara D, Pariante P, Botti G, Caraglia M, Minucci S, Chieffi P. GPR30 is overexpressed in post-puberal testicular germ cell tumors. Cancer Biol Ther. 2011;11:609–613. doi: 10.4161/cbt.11.6.14672. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Urbanski HF, Höhne-Zell B, Mayerhofer A. Neuronal elements in the testis of the rhesus monkey: ontogeny, characterization and relationship to testicular cells. Neuroendocrinology. 2000;71:43–50. doi: 10.1159/000054519. [DOI] [PubMed] [Google Scholar]
- Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod. 2011;84:207–217. doi: 10.1095/biolreprod.110.087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damja-novic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Holm M, Rajpert-De Meyts E, Andersson AM, Skakkebaek NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J Pathol. 2003;199:378–386. doi: 10.1002/path.1309. [DOI] [PubMed] [Google Scholar]
- Holm A, Baldetorp B, Olde B, Leeb-Lundberg LM, Nilsson BO. The GPER1 agonist G-1 attenuates endothelial cell proliferation by inhibiting DNA synthesis and accumulating cells in the S and G2 phases of the cell cycle. J Vasc Res. 2011;48:327–335. doi: 10.1159/000322578. [DOI] [PubMed] [Google Scholar]
- Hess RA. Estrogen in the adult male reproductive tract: A review. Repro Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. J Androl. 2011;32:600–613. doi: 10.2164/jandrol.110.012872. [DOI] [PubMed] [Google Scholar]
- Li F, Yu X, Szynkarski CK, Meng C, Zhou B, Barhoumi R, White RE, Heaps CL, Stallone JN, Han G. Activation of GPER Induces Differentiation and Inhibition of Coronary Artery Smooth Muscle Cell Proliferation. PLoS One. 2013;8(6):e64771. doi: 10.1371/journal.pone.0064771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas TF, Royer C, Siu ER, Lazari MF, Porto CS. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biol Reprod. 2010;83:307–317. doi: 10.1095/biolreprod.110.084160. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol. 1996;59:1–13. doi: 10.1679/aohc.59.1. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A. Human testicular peritubular cells: more than meets the eye. Reproduction. 2013;145:R107–116. doi: 10.1530/REP-12-0497. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmio M, Kallio J, Adam M, Mayerhofer A, Toppari J, Jahnukainen K. Peritubular myoid cells have a role in postnatal testicular growth. Spermatogenesis. 2012;2:79–87. doi: 10.4161/spmg.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlik R, Wypior G, Hecht S, Papadopoulos P, Kupka M, Thaler C, Wiest I, Pestka A, Friese K, Jeschke U. Induction of G protein-coupled estrogen receptor (GPER) and nuclear steroid hormone receptors by gonadotropins in human granulosa cells. Histochem Cell Biol. 2011;136(3):289–99. doi: 10.1007/s00418-011-0846-7. [DOI] [PubMed] [Google Scholar]
- Prabhushankar R, Krueger C, Manrique C. Membrane estrogen receptors: their role in blood pressure regulation and cardiovascular disease. Curr Hypertens Rep. 2014;16:408. doi: 10.1007/s11906-013-0408-6. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. Estrogen Biology: New Insights into GPER Function and Clinical Opportunities. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.02.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rago V, Romeo F, Giordano F, Maggiolini M, Carpino A. Identification of the estrogen receptor GPER in neoplastic and non-neoplastic human testes. Reprod Biol Endocrinol. 2011;9:135. doi: 10.1186/1477-7827-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell C, Albrecht M, Spillner S, Mayer C, Kunz L, Köhn FM, Schwarzer U, Mayerhofer A. 15-Deoxy-delta 12–14-prostaglandin-J2 induces hypertrophy and loss of contractility in human testicular peritubular cells: implications for human male fertility. Endocrinology. 2010;151:1257–1268. doi: 10.1210/en.2009-1325. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Weinbauer GF, Arslan M, Nieschlag E. Appearance of alpha-smooth muscle actin in peritubular cells of monkey testes is induced by androgens, modulated by follicle-stimulating hormone, and maintained after hormonal withdrawal. J Androl. 1993;14:340–350. [PubMed] [Google Scholar]
- Spinnler K, Köhn FM, Schwarzer U, Mayerhofer A. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum Reprod. 2010;25:2181–2187. doi: 10.1093/humrep/deq170. [DOI] [PubMed] [Google Scholar]
- Stanisić V, Lonard DM, O’Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 2010;181:153–176. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- Wang C, Lv X, He C, Hua G, Tsai MY, Davis JS. The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 2013;4:e869. doi: 10.1038/cddis.2013.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter H, Kampfer C, Lauf S, Feil R, Schwarzer JU, Köhn FM, Mayerhofer A. Partial loss of contractile marker proteins in human testicular peritubular cells in infertility patients. Andrology. 2013;1:318–324. doi: 10.1111/j.2047-2927.2012.00030.x. [DOI] [PubMed] [Google Scholar]