Abstract
The capillary wall is the chief barrier to tissue entry of therapeutic nanoparticles, thereby dictating their efficacy. Collagen fibers are an important component of capillary walls, affecting leakiness in healthy or tumor vasculature. Using a computational model along with in vivo systems, we compared how collagen structure affects the diffusion flux of a 1 nm chemotherapeutic molecule (doxorubicin [DOX]) and an 80 nm chemotherapy-loaded pegylated liposome (DOX-PLD) in tumor vasculature. We found a direct correlation between the collagen content around a tumor vessel to the permeability of that vessel permeability to DOX-PLD, indicating that collagen content may offer a biophysical marker of extravasation potential of liposomal drug formulations. Our results also suggested that while pharmacokinetics determined the delivery of DOX and DOX-PLD to the same tumor phenotype, collagen content determined the extravasation of DOX-PLD to different tumor phenotypes. Transport physics may provide a deeper view into how nanotherapeutics cross biological barriers, possibly helping explain the balance between biological and physical aspects of drug delivery.
Keywords: extravasation, biological barrier, liposome, doxorubicin, computational diffusion model
Introduction
Interest in biomedical nanoparticle and microparticle applications is growing, especially for imaging and drug delivery [1–3]. Therapeutic particles bring several advantages to drug delivery, including the Enhanced Permeability and Retention (EPR) effect, which is important for tumor treatment [4]. It is assumed that EPR is related to the fact that particles of a certain size preferentially accumulate in the tumor environment [5]. An increase in the number of particles within tumors allows the delivery of more therapeutic payload, e.g. chemotherapeutic drugs. Drug carriers, like liposomes or other particulates, are distributed throughout tissues by convective transport within the vasculature tree. Outside of the vessel walls, concentration gradients frequently drive the diffusive transport of a therapeutic payload released passively into surrounding tissues, such as the tumor microenvironment [6]. The importance of transport physics goes beyond drug delivery: the physical laws and principles that define the behavior of matter are essential for understanding the initiation and progression of cancer at all size scales [7]. The complex nature of biology creates many transport barriers at different scales, demanding multiscale approaches to solve the riddles of oncophysical transport [8].
Capillary walls and the surrounding tissues form a dense and crowded medium, impede the diffusion of therapeutics, and are among the major physical barriers to drug delivery. Diffusion can be tissue-specific, and as in the case of tumors - diffusion also depends on drug properties [9]. Therefore, pharmacokinetic aspects – especially profiles of drug concentration in plasma – have direct relation to drug extravasation, because concentration in plasma controls drug gradients across vessel wall. Also, the endothelial cells that tile the vascular wall and separate the blood flow from the tissues contain transporter proteins that function as molecular pumps, fluxing out drug molecules [10, 11]. On the other hand, endothelial cells may engulf and endocytose particles carrying a large amount of drug molecules inside [12], or they may even transcytose particulates, actively transporting them across the endothelium [13]. Sometimes, capillaries develop fenestrations: openings through the capillary walls that lack endothelial cells and are covered by collagenous diaphragms [14]. Studies show that the diaphragms have a sieving function, allowing the mass exchange of small molecules like water or proteins [15, 16].
Drugs and particles that penetrate intact capillary walls or fenestrations encounter a basal membrane, where the major constituent is type-IV collagen [17]. Physical aspects of transport have an important place in the oncological context, including the role of collagen in the transport of therapeutics [18]. The ability of collagen to modulate vessel permeability, and drug permeability within tumors in general, was noticed previously [19–21], and even related to serum biomarkers [22]. Different theoretical analyses and computational techniques were applied to model diffusion across capillary walls [23–25]. How the particle size and collagen properties may modulate diffusion flux has not been explored, however, and remains an unanswered question.
Here, by combining a diffusion model and in vivo studies, we analyzed the impact of the structure of the collagen sleeve on the diffusive mass transport of the small molecule doxorubicin (DOX) and the 80-nm pegylated liposome (PLD), which together represent Doxil™ loaded with DOX inside.
Materials and methods
Cells
The 4T1, murine breast cancer, and 3LL, murine lung cancer, cells were kindly provided by Dr. Isaiah J. Fidler (University of Texas MD Anderson Cancer Center, Houston, TX). The cells were maintained in minimal essential medium supplemented with 10% fetal bovine serum and supplements, as described previously [26].
Mice
Female Balb/C and C57/BL6 mice were maintained in animal facilities at Houston Methodist Research Institute approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health. Houston.
Tracers
The area of tumor tissue perfused by blood was evaluated by imaging of a lysine-fixable 70kDa fluorescein dextran tracer (Molecular Probes, Inc. Eugene, OR) 1 hour after i.v. injection.
Establishment of experimental tumors
To produce tumors, 4T1 and 3LL cells were harvested from subconfluent cultures. A tumor-cell suspension (1 × 105 cells/100 µl) was injected subcutaneously into Balb/C or C57/BL6 mice, respectively.
Therapy and Necropsy
Starting 10 days after the inoculation with the tumor cells, the mice were intravenously injected with 6mg/kg of PLD (Doxoves™-Liposomal Doxorubicin HCl, FormuMax Scientific Inc., Palo Alto, CA) and the mice were sacrificed 24 hours after the injection. Then, the tumors were excised, frozen, and stocked to evaluate endothelial cells, basement membranes, and the accumulation of PLD in the tumors.
Immunofluorescent staining of endothelial cells (CD31), basement membranes (type-IV collagen), proliferating cells (Ki67), and imaging of PLD in the tumors
The frozen sections were fixed in cold acetone for 15 minutes. After a protein block, immunofluorescent staining of the endothelial cells or basement membrane was performed using antibodies to CD31 (BD Biosciences, San Jose, CA), type-IV collagen (Abcam, Cambridge, MA), or Ki67 (Abcam), respectively [26]. The sections were then incubated with the corresponding fluorochrome-labeled secondary antibodies (Jackson Immunoresearch). The images were captured using a confocal laser-scanning microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) and analyzed using the built-in image-analysis software [26]. The ruby-red fluorescence of anthracyclines in the tumor tissue was also imaged using confocal microscopy at the excitation and emission wavelengths of 488 nm and 590 nm, respectively [27, 28]. The imaged fluorescence of DOX originates mostly from free DOX, because the solid state of DOX inside the PLD strongly quenches the fluorescence of liposomal DOX [29].
Imaging analysis
The ImageJ program was used to analyze the tissue samples [30], using the adequate RGB channels as 8-bit images, which were treated with background subtraction and automatic thresholding using the triangle method. The results presented in this paper were calculated from five replicate measurements. The whole images were used in the analysis. The numbers of collagen and vessel entities after automatic triangle thresholding were calculated using a standard particle-analysis procedure. To avoid misinterpreting monocytes as vessels in the CD31 staining, the minimal area was set to 250 µm2. The colocalization between collagen and vessels was calculated by using ImajeJ/Fiji program (coloc2 plug-in) [31], where M1 coefficient denotes the overlap of vessels by collagen and M2 – the overlap of collagen by vessels. The extravasation of the dextran tracer and DOX was calculated as a function of the distance in terms of pixels (after automatic thresholding). In the case of dextran, the distance was calculated to the nearest vessel, whereas in the case of DOX, the distance was calculated to the centers of red-cell nuclei. The count of cells (DOX-affected and proliferating cells stained with Ki67) and their statistics were calculated by using stained nuclei centers.
Transport model
We applied a recently developed, hierarchical, multiscale diffusion modeling technique [32–35], which has an advantage over other approaches because it accounts for the material microstructure and the physico-chemical characteristics of the diffusing molecules, including the size of the molecules or particles. See Supplementary Material for details, and Figures S1–S5 and Table S1.
Results
To examine the role of collagen in vascular permeability, we used a mouse model to analyze the type-IV collagen content and its co-localization with blood vessels and the effects of collagen on extravasation and tumor-cell proliferation. The in vivo study was followed by a computational analysis of the permeability of the collagen network to DOX and PLD particles, in order to interpret the role of collagen in modulating transport between the vessel and tumor microenvironments.
Collagen content and localization
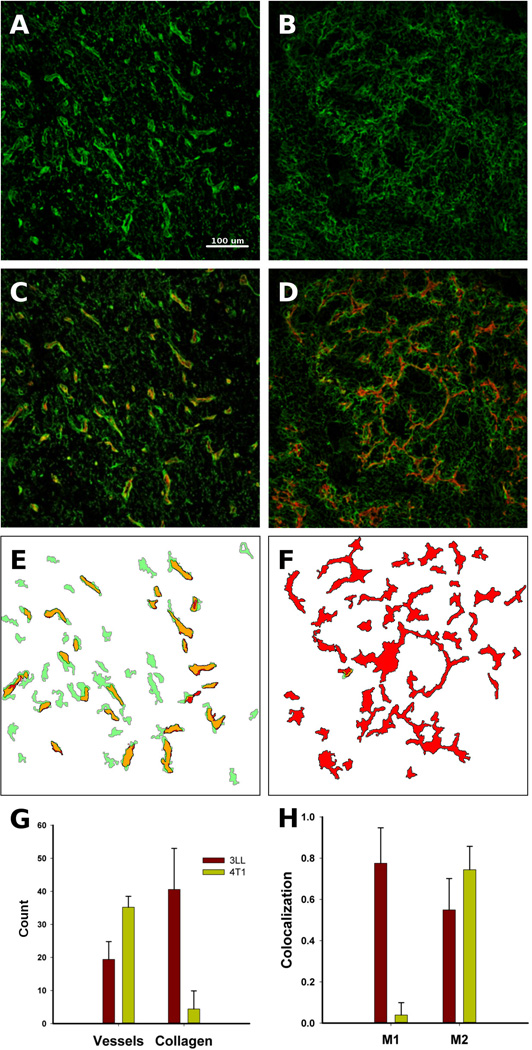
The content of type-IV collagen (hereafter referred to as collagen) in 3LL and 4T1 tumors was quantified in in vivo. The representative images of stained collagen show different structures, with 3LL tumors possessing brighter collagen nodules as compared to 4T1 tumors (Figure 1A–B) and indicating larger collagen content. The majority of the collagen nodules visually overlapped with the red-stained capillaries in 3LL tumors, but not in 4T1 tumors (Figure 1C–D). Using imaging analysis, we outlined each vessel and zone of high collagen content in the 3LL and 4T1 tumors, revealing that the stained vessels (red) and collagen (green) overlapped substantially in the 3LL tumors (yellow zones; Figure 1E–F). In contrast to the 3LL tumors, the 4T1 tumors did not show the same overlap. The main reason for the different results could be the automatic segmentation analysis of the tissue images: the lack of intensity of the stained collagen did not allow collagen segments to be identified. The 4T1 tumors showed more vessels and less vessel wall collagen content than the 3LL tumors (Figure 1G), which may explain the large amount of vessel and collagen co-localization observed in the images of the 3LL tumors but not in those of the 4T1 tumors (Figure 1H). Therefore, the vessels of the 4T1 tumors lacked collagen compared with the 3LL tumors.
Figure 1.
In vivo collagen features in 3LL and 4T1 tumors. 3LL tumors contained thick type IV collagen (green) structures (A), which were missing in 4T1 tumors (B). The visual co-localization of vessels (red) and collagen was stronger in the 3LL tumors (C) than in the 4T1 tumors (D). The image analysis revealed substantially more structural collagen/vessel overlap (yellow) in the 3LL tumors (E) than in the 4T1 tumors (F). Quantitative analysis showed more vessels in the 4T1 tumors than in the 3LL tumors, but the collagen content in the 4T1 tumors was dramatically lower (G). The co-localization of vessels and collagen was expressed in both tumors, but the 3LL tumors have substantially more vessels covered by collagen; M1 and M2 are Mender’s coefficients indicating vessel overlap with collagen (M1) and collagen overlap by vessel (H).
Extravasation in the microenvironment
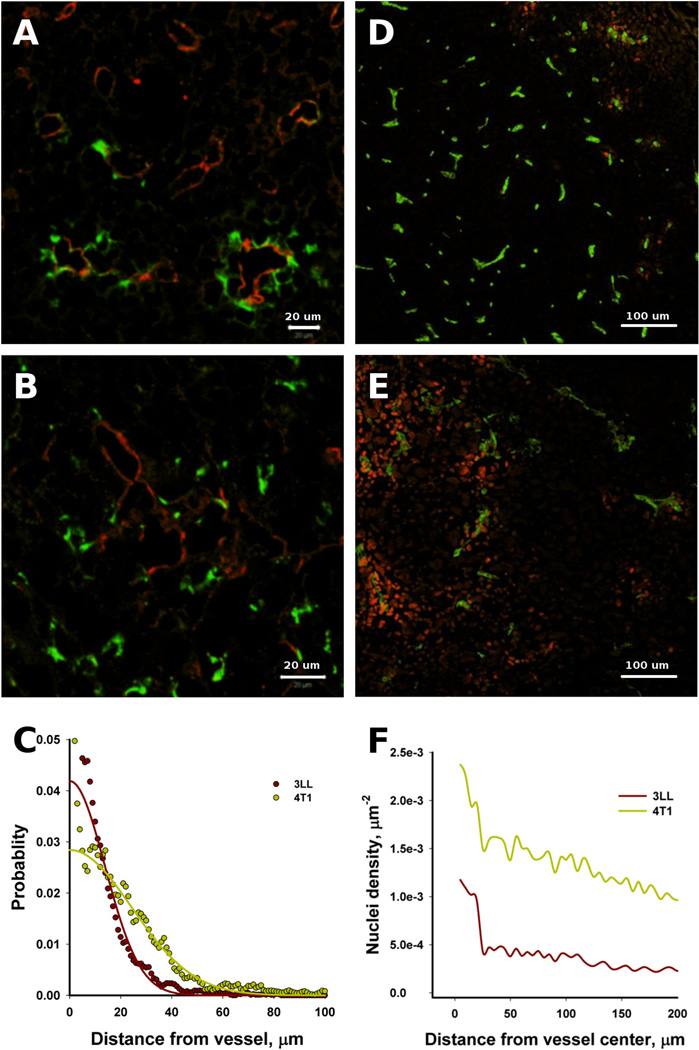
FITC-tagged dextran was used as a tracer to evaluate the extravasation of small molecules into the tumor microenvironment. Our imaging data showed that the tracer extravasated in both tumor types (Figure 2). The extravasation of tracer in the 3LL tumors was localized around vessels and showed a decaying intensity with increasing distance from the vessels (Figure 2A); whereas in the 4T1 tumors, the extravasation was more scattered (Figure 2B). Interestingly, many of the vessels in the 3LL tumors did not display visual extravasations of the tracer, meaning that the leakiness of the 3LL vasculature was heterogeneous, a characteristic feature of tumor vasculature [36]. The normalized tracer extravasation from the vessel wall was calculated from the imaging data (Figure 2C). Within 1 hour and within the detection limit, the tracer extravasated up to 40 and 60 µm from the vessel in the 3LL and 4T1 tumors, respectively. Based on the half-width of the stained areas (Figure 2C), the characteristic distance of extravasation was approximately 20 and 30 µm for the 3LL and 4T1 tumors, respectively.
Figure 2.
The extravasation differences of the tracer (left) and the PLD (right) in the 3LL and 4T1 tumors within 1 h and 24 hours after the tracer and PLD administration, respectively. The tracer (tagged dextran; green) extravasated from capillaries (red) in the 3LL (A) and 4T1 (B) tumors. The tracer concentration field appeared slightly farther from the vessels in the 4T1 tumors than in 3LL tumors (C). The PLD (red) extravasation from the vessels (green) was negligible in the 3LL tumors (D), but substantial in the 4T1 tumors (E). The 4T1 tumors contained a larger number of nuclei affected by DOX than the 3LL tumors (F).
While the 3LL and 4T1 tumors showed different, but comparable, extravasation patterns of dextran tracers, the extravasation of PLD was very different: the 3LL tumors did not show any substantial concentration field around the vessels (Figure 2D), whereas the 4T1 tumors showed a very distinct concentration of red fluorescence (DOX) around the vessels (Figure 2E). The density of DOX-containing nuclei was quantified and showed that the 4T1 tumors had substantially more DOX-stained nuclei around the vessels (Figure 2F). Taking into account that only free DOX can be imaged [29], we can conclude that our results indicate free DOX delivered and released from PLD.
Cell proliferation
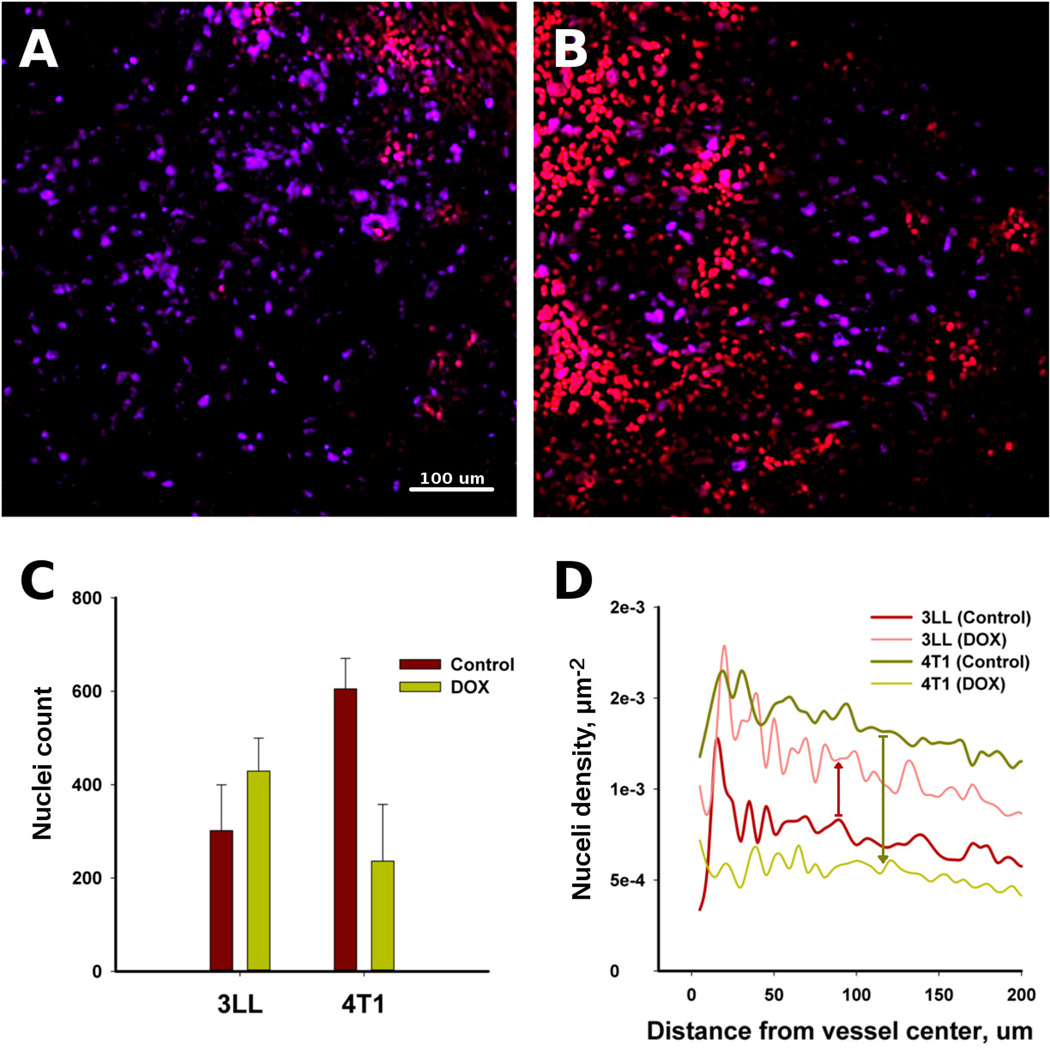
Proliferating cell nuclei were imaged and quantified by immunofluorescence staining of the tumor sections with Ki67 antibody to estimate the effects of DOX extravasation and collagen content around the vessels. Figure 3A shows proliferating 3LL cells, which are positively stained with Ki67 antibody (purple) with minor red nuclei containing DOX; proliferation was dominant throughout all the imaged regions. The 4T1 tumors showed zones of proliferation that do not contain DOX (red nuclei) (Figure 3B). The average number of proliferating cells decreased substantially in the 4T1 tumors compared to controls without treatment; but in the 3LL tumors, the average number of proliferating cells actually increased slightly compared with the controls (Figure 3C). Also, the correlation analysis shows a decreased proliferating cell density around vessels in the 4T1 tumors (Figure 3D).
Figure 3.
Cell proliferation in the 3LL and 4T1 tumors was a function of DOX extravasation. The 3LL sample (A) shows that proliferating cells (purple) dominate the imaging area, while proliferation is absent where DOX extravasated (red) in the 4T1 tumor (B). The count of proliferating cells in the 3LL and 4T1 tumors (C) and the proliferating cell density around the vessels as a function of distance (D).
Calculated permeability to PLD and DOX
To investigate how the collagen sleeve alone may modulate mass transport through the capillary wall, we developed a collagen-sleeve model that mimics the collagen surrounding capillaries [17, 37–39]. We studied the diffusion transport of PLD and DOX, which differ in size and physico-chemical properties, in response to different structures of collagen-fiber mesh.
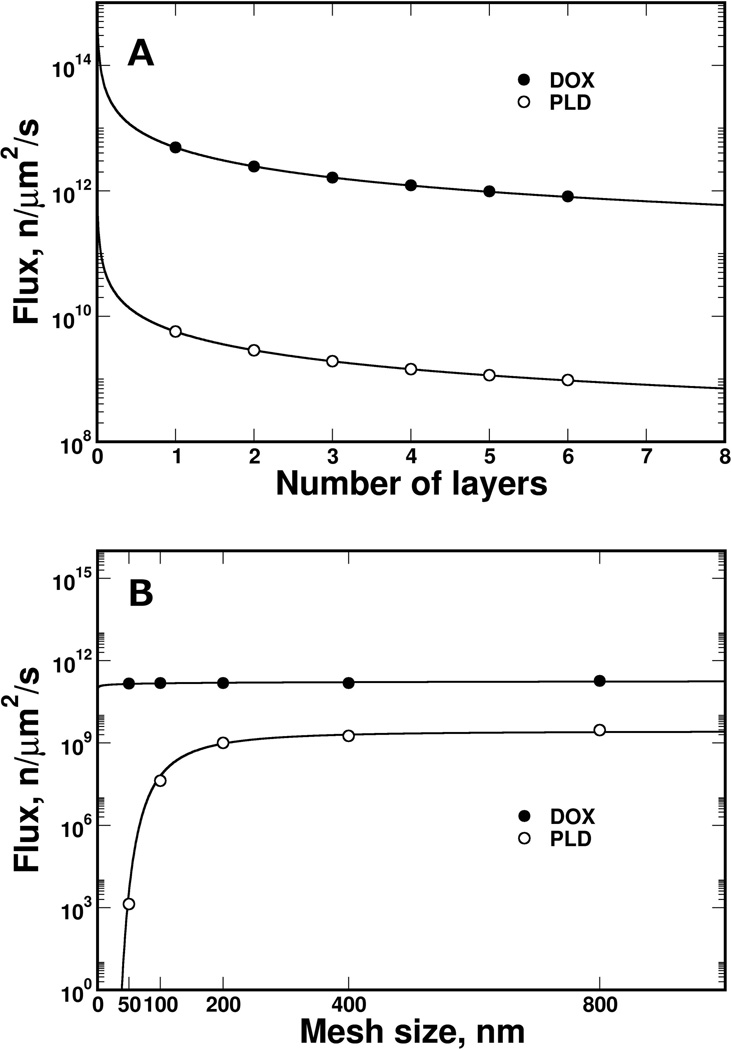
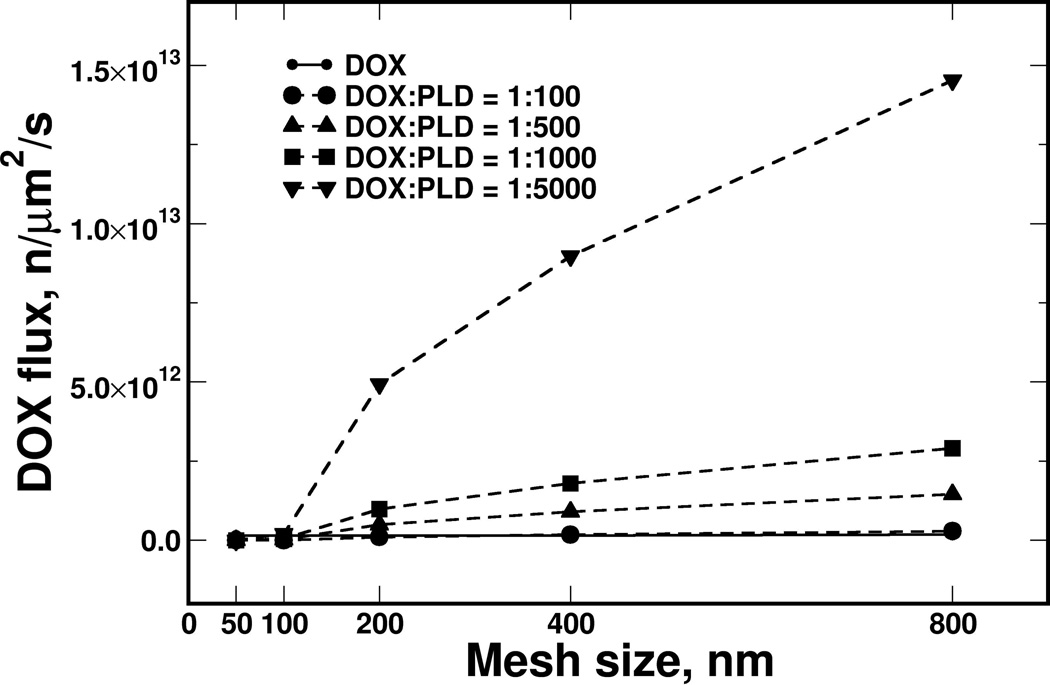
Different collagen sleeve thicknesses were modeled by changing the number of collagen-fiber layers (from 1 to 6; each layer was 10 nm thick [37]), covering the physiological thickness of collagen in a capillary wall [39]. Increasing the number of fiber layers reduced the PLD flux much faster than the DOX flux (Figure 4A). Therefore, PLD met stronger resistance than DOX diffusing through the fiber mesh, because of the difference in particle size (PLD and DOX have sizes of 80 nm and 1 nm, respectively). Furthermore, the influence of the collagen fiber density was calculated for a three-layer sleeve with mesh openings ranging from 50 to 800 nm in size (Figure 4B). Transport of DOX molecules was not affected substantially by increasing the collagen-fiber density, mostly because the DOX molecular size is substantially smaller than any opening in the mesh, and consequently there was a lack of resistance from the collagen sleeve. On other hand, the calculated PLD flux was sharply decreased by shrinking the mesh opening below 200 nm, and the flux was diminished below an opening size of 100 nm - as the size of the openings approached the size of PLD. The ratios of the PLD particle size to the mesh opening size were 1:2.5, 1.25, and 0.62 for mesh sizes of 200, 100, and 50 nm, respectively.
Figure 4.
The calculated flux of DOX and PLD particles through the collagen sleeve. The PLD diffusive flux was reduced faster by increasing the collagen thickness with a collagen-mesh size of 400 nm (A), while mesh size had limited impact on DOX (B). The model revealed a small effect of collagen mesh size on DOX flux, while PLD flux was diminished abruptly when the mesh size approached the size of PLD (below 200 nm). Flux is presented on a logarithmic scale.
Each PLD contains a large amount of DOX, similar to Doxil™. Based on our volumetric estimates, a Doxil™ liposome could contain DOX in a ratio of approximately 1:4000 or more, which is the same order of magnitude as the 1:10,000 ratio reported previously [40]. Using different DOX/PLD ratios, we calculated the DOX flux for different mesh sizes and compared the results to those calculated for free DOX flux. Figure 5 shows that for a very tight collagen mesh with a 50–100 nm openings, the packing of DOX did not affect the DOX flux, so free DOX would be more efficient. However, the DOX packing inside liposomes became advantageous for a collagen mesh with an opening of 200 nm or larger. The strongest effect was found for the liposomes with the highest DOX packing density, suggesting that it could potentially determine the therapeutic properties of such particles.
Figure 5.
The effect of DOX loading into PLD on DOX flux through a collagen mesh with the same DOX and PLD particle concentrations.
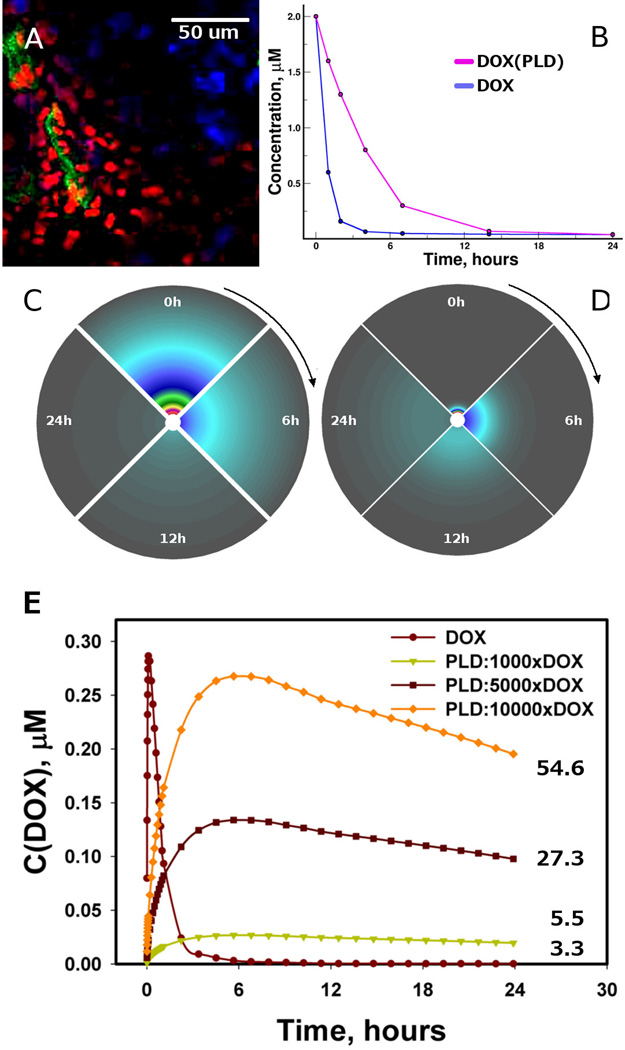
We created a diffusion model containing a source (capillary), vessel wall (represented by the collagen sleeve), and a cellular microenvironment surrounding the capillary. But it is important to note that this model is focused to the collagen role and does not seek to explain the actual extravasation in the presence of endothelium or other biological factors. Figure 6A shows a DOX concentration field developed around a capillary in a 4T1 tumor within 24 hours after treatment with PLD, where DOX intensity decreases with increasing distance from a vessel (Figure S5, Supplementary Material). In view of collagen as a physical barrier in drug transport, the models suggest that DOX can penetrate the collagen sleeve, or any other dense environment, more efficiently than PLD. Therefore, we calculated PLD and DOX extravasation in a phenomenological fashion using their concentration profiles in plasma, similar to those found in a other study [41], where the same DOX concentration was injected in two different formulations (pure DOX and PLD), and found that concentration of DOX released from PLD was higher than that of free DOX over 24 hours (Figure 6B). The concentrations of both PLD and DOX inside the tumor microenvironment increased and then diminished over 24 hours in our models (Figure 6C and D). The concentration of the larger and more slowly diffusing PLD particle changed slower and persisted longer in the microenvironment, while the concentration gradient of DOX developed quickly and was rapidly cleared. The overall concentration behavior was very similar to that in a previous study [42] comparing the DOX concentrations after treating a tumor with pure DOX and liposomal DOX: the concentration of the pure DOX in the tumor was much lower than that of the liposomal formulation. The longer persistence of PLD in the plasma resulted in a more sustained DOX concentration in tumor microenvironments that can synergize with DOX loading in PLD particles and is reflected in the calculated average concentration of DOX (Figure 6E).
Figure 6.
The concentration fields of DOX and PLD around the capillary. (A) DOX concentration (red) around capillaries (green) in a 4T1 tumor; some proliferating nuclei staining in purple with Ki67. By using experimental concentration profiles in plasma (B), the concentration fields for DOX (C) and PLD (D) were calculated, with the vessel being a source surrounded by a 100 µm-thick microenvironment medium. (E) The calculated profiles of average DOX concentration in tissue domain over time; the adjacent numbers show the area under the curve in µM*hours.
Discussion
We have attempted to establish a physical linkage between vessel wall collagen and drug extravasation. In order to understand some fundamental properties of collagen type-IV in basement membrane, we have developed computational models of collagen sleeve that surround capillaries in tumor microenvironment. The models did not use any parameters fitting, and rely merely on laws of physical and chemistry, and known physiological properties, like: the thickness of collagen fibers, the thickness of collagen sleeve around capillaries, dimensions of therapeutic substances, plasma concentration profiles of PLD or DOX, and their diffusivities. The results from collagen models revealed that a collagen sleeve alone does not function as a substantial barrier to DOX, which also could be extrapolated to other small molecules. However, vessel wall collagen may be a barrier to PLD carrying DOX, especially if PLD has to extravasate through dense and thick collagen layers. Those characteristics are crucial in further discussion of our results.
Our quantified in vivo results indicated that 3LL and 4T1 tumors are different in the contents of collagen type-IV, which is the major constituent is type-IV collagen [17]. Moreover, the colocalization of collagen and capillaries was observed by finding yellow zones (Figure 1), which indicates the co-localization of red and green fluorescence [43, 44]. McDonald and colleagues showed similar co-localization of vessels and collagen [44]. Their study found that tumor vessels – in contrast to healthy vessels – had separated layers of collagen, endothelial cells, and pericytes. The results of the same study suggested that collagen content might be different in different vascular segments, leading to differences in how tightly the collagen in basement membrane and endothelial cells adhere to one another. Therefore, the imaging results illustrated in Figure 1 could indicate different phonotypic states of vessels and associated collagen in the 3LL and 4T1 tumors. In our experiment no noticeable PLD extravasation was observed in healthy organs (Figure S6, Supplementary Material). That supports lower systemic toxicity finding for liposomal formulation of DOX [40], and suggests that collagen type-IV could be very dynamic property of tumor microenvironment and deserves deeper research spanning beyond the scope of this study.
In view of collagen transport properties obtained from physical models and collagen content from in vivo studies, the logical correlates were found between collagen content in basement membranes of capillaries and drug extravasations. The extravasation of the tracer in the 3LL and 4T1 tumors correlated with the respective collagen contents, suggesting that vascular collagen could modulate the transport of small molecules to limited amount. However, the DOX extravasation using PLD had a strong negative correlation with the collagen content in the 3LL and 4T1 tumors: the extravasation occurred from vessels that were not tightly covered by collagen type-IV (Figure S7, Supplementary Material). Other studies have shown that the extravasation of liposomes is limited and heterogeneous, forming perivascular clusters that do not move significantly and could be observed for up to 1 week [45]. Collagen, as the major component of the extracellular matrix, substantially limits the diffusion of PLD because of the liposome size [18]. Moreover, tumor cells can have a very limited role in internalizing PLD [46]. Altogether, PLD displays impeded extravasation into the tumor microenvironment beyond the premises of vessels, as calculated by our models and supported by our and other experimental results. As a consequence of an absence of thick collagen bundles around the vessels, the decrease in the proliferation of the 4T1 cells may be related to the large amount of extravasated DOX loaded inside PLD. In the case of the 3LL tumors, the presence of a thick collagen coating around the vessels was correlated with extremely limited or absent extravasation of DOX loaded inside PLD, which did not affect cell proliferation.
The collagen type-IV content around vessels by itself could potentially be a biophysical marker predicting drug extravasation and therapeutic efficacy. Because passive transport, i.e. diffusion, obeys the laws of physics - whether in vitro or within biological tissues - the models help to decouple the roles of physical and biologic transport mechanisms and are supported by in vivo results. The use of PLD prolonged the circulation time of DOX, facilitated DOX extravasation, and decreased the tumor-cell proliferation rate in the 4T1 tumors. Despite the 3LL tumors have more collagen, the tracer permeability was similar in the 3LL and 4T1 tumors (although slightly more extravasation of the tracer was found in the 4T1 tumors). This is in agreement with the model results and suggests that collagen may have a small effect on the extravasation of small molecules, because small molecules should diffuse relatively easily through capillary collagen.
Therefore, differences in DOX extravasation might be related to endothelium as a barrier to PLD before even reaching collagen itself. The role of collagen in angiogenesis has been shown to the degree that the vessel collagen sleeve remains after anti-vascular therapy and may serve as a scaffold to revascularize tissues [47]. Moreover, the disruption of contact between endothelial cells and the vessel basement membrane can lead to endothelial apoptosis [44]. On the other hand, fenestrations are structures of the basement membrane, where collagen plays a major role too. It is widely accepted that fenestrations may help to deliver therapeutic payloads [48–50]. Furthermore, the plasma concentration kinetics may play an important role, favoring long-circulating PLD, as in the example presented in Figure 6. The results show that even at small 1:1000 DOX packing inside PLD, the calculated area under the curve (AUC) is larger than using free DOX. The drug packing in PLD revealed strong effects to drug delivery in our transport models, even if PLD extravasation is limited comparing to free drug. The PLD concentration in plasma may be lower by several orders of magnitude than that of the free therapeutic molecule, but still that can possess a similar or higher therapeutic efficacy with respect to the free molecule formulation, since each liposome may carry many molecules of payload. However, according to our models, the advantages of PLD will diminish and smaller molecules would penetrate the collagen sleeve more efficiently, if collagen mesh size gets comparable to the size of PLD or gets smaller.
The correlation between the collagen content around tumor vessels and the permeability of a liposomal formulation of doxorubicin was found, indicating that collagen content could be a biophysical marker useful to predict drug extravasation potential. Experiments and model synergistically show that collagen sleeve may modulate vessel permeability to therapeutic particles by size exclusion. Our results show differences in DOX extravasation among tumor phenotypes when PLD formulation is used. This can be explained by differences in capillary collagen content: larger amount of collagen associated with vessels correlates with poorer DOX extravasation into tumor microenvironment. The differences in pharmacokinetics of DOX and PLD suggest the benefits of using PLD in the presence of collagen as a barrier for drug delivery.
To the best of our knowledge, biophysical markers that utilize the EPR effect and assist in the prediction of the therapeutic efficacy have not been reported to date. The analysis of nanotherapeutics transport from systemic circulation to tumor microenvironment provides additional framework to improve cancer therapy. The differences in the collagen content and structure - as the marker of therapeutics extravasation – could be taken in account for planning the therapeutic strategy for patients. But further studies focused on evaluating the potential of biophysical markers are needed to help in personalizing nanotherapeutic for cancer therapy.
Supplementary Material
Acknowledgments
This project was partially supported by the Houston Methodist Research Institute, and also by grant OI 174028 of the Serbian Ministry of Education and Science. The authors also acknowledge partial support from the following funding sources: the National Institute of Health (U54CA143837 – M.F., K.Y., U54CA151668 - M.F.), the Ernest Cockrell Jr. Distinguished Endowed Chair (M.F.), and the US Department of Defense (W81XWH-09-1-0212) (M.F.). The authors acknowledge the Texas Advanced Computing Center (TACC) at The University of Texas at Austin for providing HPC resources that contributed to the results reported in this paper.
Footnotes
Disclosure of any potential Conflicts of Interest: M.F. serves on the Board of Directors of NanoMedical Systems, Inc., ArrowHead Research Corporation, and Leonardo Biosystems, and discloses potential financial interest in the companies. All other authors declare no competing financial interests.
References
- 1.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine: Nanotechnology, Biology and Medicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 3.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the epr effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 6.Yuan F. Seminars in Radiation Oncology. Elsevier; 1998. Transvascular drug delivery in solid tumors. [DOI] [PubMed] [Google Scholar]
- 7.Michor F, Liphardt J, Ferrari M, Widom J. What does physics have to do with cancer? Nat Rev Cancer. 2011;11:657–670. doi: 10.1038/nrc3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari M. Frontiers in cancer nanomedicine: Directing mass transport through biological barriers. Trends Biotechnol. 2010;28:181–188. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 10.Sharom FJ. Abc multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 12.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simionescu M, Popov D, Sima A. Endothelial transcytosis in health and disease. Cell Tissue Res. 2009;335:27–40. doi: 10.1007/s00441-008-0688-3. [DOI] [PubMed] [Google Scholar]
- 14.Schechter J. Ultrastructural changes in the capillary bed of human pituitary tumors. The American journal of pathology. 1972;67:109. [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 16.Bearer EL, Orci L. Endothelial fenestral diaphragms: A quick-freeze, deep-etch study. The Journal of cell biology. 1985;100:418–428. doi: 10.1083/jcb.100.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yurchenco PD, Ruben GC. Basement membrane structure in situ: Evidence for lateral associations in the type iv collagen network. The Journal of cell biology. 1987;105:2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanujan S, Pluen A, Mckee T, Brown E, Boucher Y, Jain R. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophys J. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert A, Godeau G, Miskulin M, Moati F. Mechanism of action of collagenase on the permeability of the blood-brain barrier. Neurochem Res. 1977;2:449–455. doi: 10.1007/BF00965468. [DOI] [PubMed] [Google Scholar]
- 20.Utoguchi N, Mizuguchi H, Dantakean A, Makimoto H, Wakai Y, Tsutsumi Y, et al. Effect of tumour cell-conditioned medium on endothelial macromolecular permeability and its correlation with collagen. Br J Cancer. 1996;73:24. doi: 10.1038/bjc.1996.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 22.Yokoi K, Tanei T, Godin B, Ven AVD, Hanibuchi M, Matsunoki A, et al. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 24.Bassingthwaighte JB, Chan ISJ, Wang C. Computationally efficient algorithms for convection-permeation-diffusion models for blood-tissue exchange. Ann Biomed Eng. 1992;20:687–725. doi: 10.1007/BF02368613. [DOI] [PubMed] [Google Scholar]
- 25.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin g, f (ab′) 2, and fab in tumors. Cancer Res. 1989;49:5656–5663. [PubMed] [Google Scholar]
- 26.Yokoi K, Godin B, Oborn CJ, Alexander JF, Liu X, Fidler IJ, et al. Porous silicon nanocarriers for dual targeting tumor associated endothelial cells and macrophages in stroma of orthotopic human pancreatic cancers. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaage J, Donovan D, Uster P, Working P. Tumour uptake of doxorubicin in polyethylene glycol-coated liposomes and therapeutic effect against a xenografted human pancreatic carcinoma. Br J Cancer. 1997;75:482. doi: 10.1038/bjc.1997.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erikson A, Tufto I, Bjønnum AB, Bruland ØS, Davies CL. The impact of enzymatic degradation on the uptake of differently sized therapeutic molecules. Anticancer Res. 2008;28:3557–3566. [PubMed] [Google Scholar]
- 29.Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1995;1233:134–144. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 30.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with imagej. Biophotonics international. 2004;11:36–42. [Google Scholar]
- 31.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: An open-source platform for biological-image analysis. Nat Meth. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziemys A, Kojic M, Milosevic M, Kojic N, Hussain F, Ferrari M, et al. Hierarchical modeling of diffusive transport through nanochannels by coupling molecular dynamics with finite element method. J Comput Phys. 2011;230:5722–5731. [Google Scholar]
- 33.Kojic M, Milosevic M, Kojic N, Ferrari M, Ziemys A. On diffusion in nanospace. Journal of the Serbian Society for Computational Mechanics. 2011;5:104–118. doi: 10.24874/jsscm.2017.11.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kojic M, Ziemys A, Milosevic M, Isailovic V, Kojic N, Rosic M, et al. Transport in biological systems. Journal of the Serbian Society for Computational Mechanics/Vol. 2011;5:101–128. doi: 10.24874/jsscm.2017.11.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojic M, Milosevic M, Kojic N, Kim K, Ferrari M, Ziemys A. A multiscale md-fe model of diffusion in composite media with internal surface interaction based on numerical homogenization procedure. Computer Methods in Applied Mechanics and Engineering. 2014;269:123–138. doi: 10.1016/j.cma.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 37.Timpl R, Wiedemann H, Delden V, Furthmayr H, Kuhn K. A network model for the organization of type iv collagen molecules in basement membranes. Eur J Biochem. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Rest M, Garrone R. Collagen family of proteins. The FASEB journal. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 39.Laurie G, Leblond C, Martin G. Localization of type iv collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. The Journal of cell biology. 1982;95:340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barenholz YC. Doxil®—the first fda-approved nano-drug: Lessons learned. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Junping W, Maitani Y, Takayama K, Nagai T. In vivo evaluation of doxorubicin carried with long circulating and remote loading proliposome. Int J Pharm. 2000;203:61–69. doi: 10.1016/s0378-5173(00)00410-5. [DOI] [PubMed] [Google Scholar]
- 42.Laginha KM, Verwoert S, Charrois GJ, Allen TM. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11:6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- 43.Cretu A, Roth JM, Caunt M, Akalu A, Policarpio D, Formenti S, et al. Disruption of endothelial cell interactions with the novel hu177 cryptic collagen epitope inhibits angiogenesis. Clin Cancer Res. 2007;13:3068–3078. doi: 10.1158/1078-0432.CCR-06-2342. [DOI] [PubMed] [Google Scholar]
- 44.Baluk P, Morikawa S, Haskell A, Mancuso M, Mcdonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. The American journal of pathology. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Mirovascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 46.Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Size-dependent extravasation and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. Int J Pharm. 1999;190:49–56. doi: 10.1016/s0378-5173(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 47.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (vegf) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. The American journal of pathology. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nature Reviews Clinical Oncology. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis ME. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.