Abstract
Tumour cell–derived heat shock proteins (HSPs) are used as vaccines for immunotherapy of cancer patients. However, it is proposed that the peptide chaperoned on HSPs, not HSPs themselves, elicited a potent immune response. Given that HSPs are highly expressed by most myeloma cells and vital to myeloma cell survival, we reasoned that HSPs themselves might be an ideal myeloma antigen. In the present study, we explored the feasibility of targeting HSPs themselves for treating multiple myeloma. We identified and chose HLA-A*0201-binding peptides from human HSPB1 (HSP27) and HSP90AA1 (HSP90), and confirmed their immunogenicity in HLA-A*0201 transgenic mice. Dendritic cells pulsed with HSPB1 and HSP90AA1 peptides were used to stimulate peripheral blood mononuclear cells from healthy volunteers and myeloma patients to generate HSP peptide-specific cytotoxic T lymphocytes (CTLs). HSP peptide-specific CTLs efficiently lysed HLA-A*0201+ myeloma cells (established cell lines and primary plasma cells) but not HLA-A*0201− myeloma cells in vitro, indicating that myeloma cells naturally express HSP peptides in the context of major histocompatibility complex class I molecules. More importantly, HSP peptide-specific CTLs effectively reduced tumour burden in the xenograft mouse model of myeloma. Our study clearly demonstrated that HSPs might be novel tumour antigens for immunotherapy of myeloma.
Keywords: Multiple myeloma, Heat shock proteins, Vaccine, Immunotherapy
1. Introduction
Multiple myeloma (MM) is the second most common haematological malignancy (Nalesnik, 2013). Despite the application of novel agents and advances in stem cell transplantation, which have increased the response rates and prolonged overall survival (Mellqvist et al., 2013), MM still remains largely incurable (Sellner et al., 2013). Adoptive cellular immunotherapy is one of the promising methods for treating MM and its success depends on the identification of ideal myeloma antigens (Tyler et al., 2013).Myeloma antigens were divided into tumour-specific antigen (TSA) and tumour-associated antigens (TAA), based on their expression spectrum. MM TSA are only expressed in MM cells while MM TAA are lowly expressed, or undetectable in normal cells and highly expressed in tumour cells. It has been considered that myeloma TSA, such as idiotype proteins, the monoclonal immunoglobulin (Ig) secreted by myeloma cells (Hong et al., 2012), carrying unique antigenic determinants and the sole MM TSA identified to date, are appealing immunotherapy targets, but failed to elicit potent anti-myeloma responses in clinical trials, partly due to its weak immunogenicity (Rollig et al., 2011).
However, some myeloma TAA including MAGE-3(Pellat-Deceunynck et al., 2000)and NY-ESO-1(van Baren N et al., 1999)are only expressed in a small percentages of MM patients, and thus cannot provide shared immunotherapy for various patients with MM. Other myeloma TAA, such as MUC-1, are considered not crucial to the proliferation of MM cells and would be downregulated by MM cells, which could escape the immune surveillance (Treon et al., 1999). Consequently, a key point for an effective cellular immunotherapy is to find antigens with strong immunogenicity that show wide expression in most myeloma patients and are vital to myeloma cell growth or survival.
HSPs may belong to this group of antigens. HSPs are a large family of proteins including several different subfamilies, termed as HSPB, HSP70, and HSPC (HSP90), and play a pivotal role in the maintenance of protein homeostasis by supporting the folding protein structures and preventing both newly synthesized polypeptide chains and assembled subunits from aggregating into nonfunctional structures (De Maio A, 1999; Tosi et al., 2013).Myeloma cells synthesize and secret a large amount of monoclonal proteins and some of the monoclonal proteins inevitably become unfolded or mis-folded, which are lethal to myeloma cells (Tosi et al., 2013). HSPs convert the unfolding or mis-folding proteins to a “folding-competent” state and are subsequently refolded to maintain live MM cells (Endemann et al., 2007). In addition, a large quantity of proteins known to be critical in driving MM cell survival and proliferation, including many oncoproteins, such as IKKα and IKKβ in NF-κB signalling, are also chaperoned by HSPs to correctly perform their function (Qu et al., 2012). Hence, HSPs are indispensable in maintaining MM cell survival and downregulation or inhibition of HSP90AA1, HSPB1 and HSPA13 expression results in significant apoptosis in myeloma cells (Chauhan et al., 2003; Assimon et al., 2013; Lin et al., 2013). Furthermore, HSPs are abundantly and preferentially expressed in MM cells and are 2- to 10-fold higher than their normal counterparts (Chauhan et al., 2002; De Vos J et al., 2002; Chauhan et al., 2003). Of the large number of HSPs previously studied, over-expression of HSP RNA and HSPs proteins has been repeatedly found in MM cell lines such as U266, RPMI 8226, ARH-77, LP-1and the primary neoplastic plasma cells from all MM patient samples (Duus et al., 2006; Cervantes-Gomez et al., 2011). Studies observed that HSPs in an activated, high-affinity conformation, as contained in tumour cells, were obviously different from that latent, uncomplexed state in normal cells (Kamal et al., 2003) and allowed a selective targeting of the molecules in cancer cells. Owing to the wide expression in MM cells and the key roles for MM cell growth and survival, HSPs might be ideal targets for immunotherapy of MM.
We previously demonstrated that pooled gp96, a member of the HSPC family, could effectively protect mice from MM challenge and also could treat mice with established myeloma (Qian et al., 2009). However, some authors thought that it is not HSPs themselves but the peptide chaperoned by HSPs that elicit peptide-specific anti-cancer immunity. The feasibility of HSPs themselves as myeloma immunotherapy target has not been fully investigated. Based on the wide HSP expression in most myeloma patients and that they are vital to myeloma cell growth, it is rational to propose that targeting HSPs might break the immune tolerance and induce an anti-myeloma immune response. Notably, some self antigens, such as XBP1(Bae et al., 2011a), CD138(Bae et al., 2011b) and DKK1(Qian et al., 2007), when considered as TAA, have succeeded in breaking immune tolerance and eliciting the antitumour immunity in haematological tumours with the help of professional antigen-presenting cells, which are capable of effectively stimulating rest T cells (Schreurs et al., 2000).
The aim of this study was to investigate the feasibility of human HSP as a MM immunotherapy target. Our study showed that two peptides derived from human HSPB1 and HSP90AA1 could induce peptide-specific CTLs that possess selective cytolysis to myeloma cells in a major histocompatibility complex (MHC) class-I-restricted mode. These results provide a rationale for HSP-based immunotherapy in MM.
2.Materials and methods
2.1 Myeloma cell lines and primary myeloma cells
Human myeloma cell lines used included U266, RPMI-8226, ARH77 and LP-1. All of the cell lines were preserved in our laboratory. Primary MM cells were donated by patients. All of the patients and healthy volunteers had signed informed consents. The study was approved by the Ethics Committee of Changzheng Hospital. All of the cell lines and primary cells were maintained in RPMI 1640 medium (Gibco-Life Technologies, Beijing, China) supplemented with 10% fetal bovine serum (FBS) (Gibco-Life Technologies, New York, NY USA). Primary myeloma cells were isolated from bone marrow aspirates from MM patients by density centrifugation and anti-human CD138 antibody-coated magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA). The clinical characteristics of patients with myeloma are listed in Table I. Aliquots of purified myeloma cells were used for experiments.
Table I.
Clinical Characteristics of the multiple myeloma patients from whom primary myeloma cells were isolated
| Patient | Age (years) |
Sex | HLA-A *0201 |
M-protein | D-S Stagea |
ISSb stage |
|---|---|---|---|---|---|---|
| 1 | 45 | Male | + | IgA λ | III A | II |
| 2 | 56 | Male | + | IgG λ | III A | III |
| 3 | 61 | Male | + | κ | III B | III |
| 4 | 62 | Female | + | λ | III B | II |
| 5 | 54 | Female | − | IgA λ | III A | II |
| 6 | 58 | Female | + | IgG λ | III A | II |
| 7 | 66 | Male | + | λ | III B | III |
| 8 | 67 | Female | − | IgG λ | III B | III |
Durie-Salmon stage;
International Staging System
2.2 Animals
HLA-A*0201-transgenic (HLA-A2.1-tg) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) (Tangri et al., 2001; Alexander et al., 2002). The mice were maintained at MD Anderson Cancer Center Animal Facility, and the animal studies were approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. Furthermore, 4- to 6-week-old male non-obese diabetic severe combined immunodeficiency (NOD/SCID) mice were obtained from Shanghai SLAC Laboratory Animal CO. LTD and maintained under pathogen-free conditions. All of the animal studies were conducted in accordance with protocols approved by the Animal Research Committee of the Second Military Medical University.
2.3 Immunohistochemistry examination
Immunohistochemistry examination was performed in Changzheng Hospital according to previously described methods (Jaiswal et al., 2013). Tissues were fixed in 10% neutral-buffered formalin at 4°C overnight and rinsed three times in phosphate-buffered saline (PBS) for 5 min and infused with 30% sucrose solution at 4°C overnight or until the tissue had sunk. The tissues were frozen in OCT (Tissue-Tek, Chicago, IL, USA) by liquid nitrogen. Frozen tissue was stored at −80°C until use. Frozen 5-µm sections from frozen tissues were mounted onto saline-coated glass slides (Dako, Glostrup, Denmark) and stored at –80°C until use. The Dako EnVision+ System-horseradish peroxidase (HRP) (DAB) (Dako) was used to stain the frozen sections according to the manufacturer’s instructions. The sections were heated in the microwave in sodium citrate buffer (pH6) for antigen retrieval. For protein detection sections were incubated with 10 µg/ml of anti-HSP mouse monoclonal antibody or 1:100 dilutions of anti-HSP rabbit polyclonal antibodies (Abcam, Cambridge, UK) in 1% bovine serum albumin (BSA)-PBS for 2 h at room temperature. After washing, sections were incubated with HRP-labelled secondary antibodies. The chromogen 3,3’-diaminobenzidine was used as substrate for the EnVision +HRP-system according to the manufacturer’s instructions. The sections were counterstained with Meyer’s haematoxylin and mounted in Faramount aqueous mounting medium (both from Dako). The immunostaining was evaluated by light microscopy (Carl Zeiss AG, Oberkochen, Germany) and a high resolution camera (Canon G10; Canon, Tokyo, Japan).
2.4 HSP peptide-T2 cell binding assay
The sequences of HSP were reviewed for peptides that could potentially bind to HLA-A*0201 using a peptide binding database (http://www-bimas.cit.nih.gov/molbio/hla_bind/) and the SYFPEITHI system (http://www.syfpeithi.de/bin/MHCServer.dll/EpitopePrediction.htm). Peptides that were used in our in vivo examination were synthesized in the Peptide Synthesis Facility of the MD Anderson Cancer Center. Peptides that were used for in vitro investigation were synthesized by the China Botai Biochemical Company (Zhanjiang, China). The purity of the synthetic peptides was confirmed to be more than 98% by reverse-phase high-performance liquid chromatography and mass spectrometry. The synthetic peptides were dissolved in dimethyl sulfoxide (Sigma, St. Louis, MO, USA) or PBS (Gibco-Life Technologies, Beijing, China) according to the peptides’ different chemical qualities and stored at –20°C until use. The sequences of the peptides were listed in table II. Peptide binding to HLA-A*0201 molecules was measured using the T2 cell line (kindly provided by Dr. Guangyan Zhou, Immunology Department of Shanghai Jiao Tong University School of Medicine) according to a previously described protocol. Briefly, T2 cells were incubated overnight with 3 µg/mL β2-microglobulin (Sigma) and 100µg peptides, followed by washing and incubation with fluorescein isothiocyanate (FITC)-labelled anti-HLA-A*0201 monoclonal antibody (mAb) BB7.2 (BD Pharmingen, San Jose, CA, USA). After washing, the cells were analysed for their levels of HLA-A*0201 expression by flow cytometry. HLA-A*0201 expression was quantified according to the formula [(mean fluorescence with peptide − mean fluorescence without peptide)/mean fluorescence without peptide] × 100.
Table II.
Binding of HSP peptides to human HLA-A*0201
| HSP | Position | Sequence | Score Ic |
Score IId |
|---|---|---|---|---|
| HSPB1 | aa27 | RLFDQAFGL | 1879 | 23 |
| HSP90AA1α | aa362 | KLYVRRVFI | 642 | 22 |
| aa670 | ALLSSGFSL | 458 | 26 | |
| HSPA13 | aa393 | LLLLDVAPL | 309 | 30 |
| Flu matrix | aa58 | GILGFVFTL | 550 | 67 |
| HIV pol | aa476 | ILKEPVHGV | 39 | 34 |
HSP, heat shock protein
NIH BIMAS (http://www-bimas.cit.nih.gov/molbio/hla_bind/)
2.5 In vivo immunogenicity of HSP peptides
For immunization, peptides were diluted in PBS at room temperature, mixed, and emulsified with an equal volume of incomplete Freund’s adjuvant (Sigma). Groups of three HLA-A2.1-tg mice were subcutaneously immunized at the tail base with 100 µL of peptide emulsion containing 100 µg of peptide. Two weeks after the immunization, mice were euthanized, and their splenocytes were isolated for in vitro studies.
2.6 Dendritic cell (DC) culture, CTL generation and rapid expansion
Monocyte-derived mature DCs were generated from peripheral blood mononuclear cells (PBMCs) according to a previous protocol (Qian et al., 2005). Briefly, PBMCs were allowed to adhere to culture flasks for 2 h, and the non-adherent cells were collected and cryopreserved for future use. The adherent cells were cultured in RPMI-1640 medium supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF; 10 ng/mL) and interleukin 4 (IL4; 10 ng/mL, both from R&D Systems, Minneapolis, MN, USA) in the presence of 10% FBS, with further addition of cytokines every other day. After 5 days of culture, DCs were induced to mature by the addition of tumour necrosis factor-α (TNF-α; 10 ng/mL; Xiamen Amoytop Biotech CO., Xiamen, China ) for 48 h. HSP-specific T cells were generated from PBMCs of HLA-A*0201+ healthy volunteers and patients with myeloma by repeated stimulations of autologous T cells with HSP peptide-loaded mature DCs. Briefly, the non-adherent PBMCs (2 × 106/ml; used as T cell population) were co-cultured with mature DCs (2 × 105/ml) (irradiated at 30Gy) in 50-ml tissue-culture flasks at 37°C in 5% CO2 for 7 – 10 days in RPMI-1640 medium supplemented with 10% pooled human serum (T cell medium). The mature DCs had been pre-incubated with HSP peptides at a final concentration of 50 µg/ml at 37°C for 2 h. After culturing, the T cells were collected and restimulated with HSP peptide-pulsed autologous mature DCs every week, and the cultures were fed every 5 days with fresh medium containing recombinant IL2 (20 iu/ml), IL7 (5 ng/ml) and IL15 (5 ng/ml) (all from eBioscience Inc., San Diego, CA, USA). The induction of HSP-specific T cells was monitored weekly using a T cell proliferation assay and HSP peptide-HLA-A*2 tetramer staining (synthesized by Epigen Biotech, Beijing, China). T cell lines were established after 3 to 4 cycles of in vitro stimulation and selection. The T cell lines were expanded in T cell medium containing IL2 (100 iu/ml), IL7 (5 ng/ml), and IL15 (5 ng/ml) for 2 weeks and then subjected to functional tests.
2.7 Cell proliferation assay by CFSE labelling
T cells (5 × 104/100 µl/well) were harvested, washed twice in PBS, and seeded into 96-well U-bottomed tissue-culture plates (Corning Glassworks, Corning, NY, USA) in T cell medium. A 5 mM carboxyfluorescein succinimidyl ester (CFSE; eBioscience Inc) stock solution in dimethyl sulfoxide was added to the CTLs to achieve a final concentration of 5 µM and incubated for 10 min at 37°C in 5% CO2 and protected from light. Various numbers of autologous mature DCs loaded with or without HSP peptides were added to the plates and cultured for 4 days at 37°C in 5% CO2. After incubation, five volumes of ice-cold PBS with 2% FBS (PBS-FBS) were added to the cells to quench the reaction. CFSE-labelled CTLs were harvested and examined on day 4 to determine the cells’ proliferation in response to peptide. In another examination, CFSE-labelled HSP-specific CTLs (2 × 106 cells/mL) were coincubated with U266, RPMI-8226 (2 × 105 cells/mL), or media alone at 37°C in 5% CO2 and humidified air. CFSE-labelled CTLs were harvested and examined on day 4 to determine cells proliferation in response to peptide or U266 stimulation. The results were expressed as the mean counts per minute of triplicate cultures.
2.8 Immunophenotyping and intracellular cytokine staining of CTLs
Mouse anti-human mAbs against CD45RO, CD69, interferon-γ (IFN-γ), IL4, and TNF-β (all from eBioscience Inc.); Fas ligand (FasL) (Serotec Ltd., Oxford, UK), perforin (Bioligand Inc., San Diego, CA,USA) and CD138, conjugated to FITC, phycoerythrin (PE), peridinin-chlorophyll protein complex (PerCP), or allophycocyanin (APC) were added to cell pellets, incubated for 30 min on ice and washed before analysis. Intracellular cytokine or granzyme B staining was performed using a Cytofix/Cytoperm kit (eBioscience Inc.) according to the manufacturer’s recommendations. The cells were first stained with cell surface-specific mAbs and then fixed, permeabilized and stained with cytokine or granzyme B-specific mAbs. The PanToxiLux (OncoImmunin Inc. Gaithersburg, MD, USA) assay was used to determine if granzyme B activity are involved in the killing of tumour cells in this assay according to the manufacturer’s recommendations. The CD107a assay was set up with minor modifications to evaluate the functional activity of HSP-specific CTLs (Betts et al., 2003). Primary CD138+ MM cells derived from bone marrow mononuclear cells were isolated from HLA-A*0201+ myeloma patients using CD138+ immunomagnetic selection technology per the manufacturer’s instructions. The HSP-specific CTLs were coincubated with HLA-A*0201+/CD138+ MM cells at a ratio of 1 CTL: 10 CD138+ MM cells in a 96-well round-bottomed plate. At the same time, mouse anti-human CD107a- APC mAb was added to each well. After 1 h incubation, Brefeldin A (BD Biosciences, Shanghai, China) and Monensin (BD Biosciences) were added to the cells and incubated for an additional 4 h, after which the cells were harvested, washed, stained with mouse anti-human CD8-PE mAb and for 30 min at 4°C, washed again, and analysed by flow cytometry. The expression of CD107a by the CD8+ T cells was determined as a measure of the degranulation of the HSP-specific CTLs in response to MM cells.
2.9 Cytotoxicity assay
To test the cytotoxicity of the CTLs, we used two methods: lactate dehydrogenase (LDH)or 51Cr-release assays. Target cells included autologous DCs or T2 cells loaded with or without peptides, myeloma cell lines, allogenitic primary myeloma cells isolated from patients and autologous PBMCs. The HSPB1-and HSP90-specific T cell lines were derived from different patients and healthy volunteers (Table III). The HSP-specific T cell lines from the same origin were used to test their cytotoxicity on allogenitic primary myeloma cells and established cell lines. In 51Cr-release assay, target cells were incubated with 3.7 MBq of 51Cr-sodium chromate for 1 h, washed extensively, seeded (1 × 104 cells/well) into 96-well U-bottomed plates in T-cell medium. For the LDH release assay, the target cells were seeded (1 × 104 cells/well) into 96-well U-bottom plates in T cell medium and then co-cultured for 4 h with various amounts of T cells. All assays were performed in triplicates. The results were expressed as the mean percentage of LDH or 51Cr release, calculated as follows: [(sample counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100%. Spontaneous release was less than 20% of the maximum. To determine whether the cytolytic activity was restricted by MHC class I or II molecules, 20µg/mL mAbs against HLA-ABC (W6/32) or HLA-A*0201 (BB7.2; both from Serotec Ltd.), HLA-DR (B8.12.2; Immunotech, Marseilles, France), or isotypic controls (Immunotech) were added to the cultures at the start of the assay.
Table III.
Origin of HSP-specific T cell lines
| Origin | Age (years) |
Sex | HLA-A *0201 |
M- protein |
D-S stagea |
ISS b stage |
Designed assay |
|---|---|---|---|---|---|---|---|
| HV1 | 29 | Male | + | − | − | − | 51Cr-release (HSPB1) Xenograft model |
| HV2 | 31 | Male | + | − | − | − | 51Cr-release(aa670) Xenograft model |
| HV3 | 35 | Female | + | − | − | − | LDH release(HSPB1) |
| Pt1 | 52 | Male | + | IgA λ | IIA | I | 51Cr-release(aa670) |
| Pt2 | 64 | Male | + | IgA λ | IIIA | II | LDH release(HSPB1) |
| Pt3 | 58 | Female | + | IgG λ | IIA | II | LDH release(aa670) |
| Pt4 | 56 | Male | + | λ | IIA | II | LDH release(aa670) |
| Pt5 | 60 | Female | + | IgG λ | IIIA | II | LDH release(HSPB1) |
HV, healthy volunteer; Pt, Patient; LDH, lactate dehydrogenase
Durie-Salmon stage;
International Staging System
2.10 Xenograft model of myeloma
We used an established myeloma xenograft mouse model (LeBlanc et al., 2002). Human myeloma cell lines were mixed with Matrigel (BD Biosciences, Shanghai, China), as previously described (Qian et al., 2005), and were then subcutaneously injected into the left flank of each mouse (200 µl/mouse, containing 2 × 107 U266 cells). Tumours were measured at their greatest length and width, and the tumour area was calculated as the tumour width × tumour length (five mice per group). CTLs or unstimulated T cells (107 cells per injection), which served as a control, were injected intravenously, together with IL2 (10,000 units/day, for 4 consecutive days) when the tumour area reached 4 mm2, and survival was monitored. Mice were humanely euthanized when the tumour area reached 225 mm2.
2.11 Statistical analysis
Unless otherwise indicated, mean and standard deviation (SD) are shown. To assess the tumour growth curves, tumour areaes were calculated as mean ± SEM. Repeated-measure analysis of variance was used to determine the statistical significance of the differences in the rate of tumour generation between the experimental groups. For other analyses, a Student’s t test was used to determine significance. Moreover, in animal experiments, because of differences in the rate of MM progression between the cohorts of mice, the data were normalized.
3. Results
3.1 HSPs were highly expressed in myeloma cells
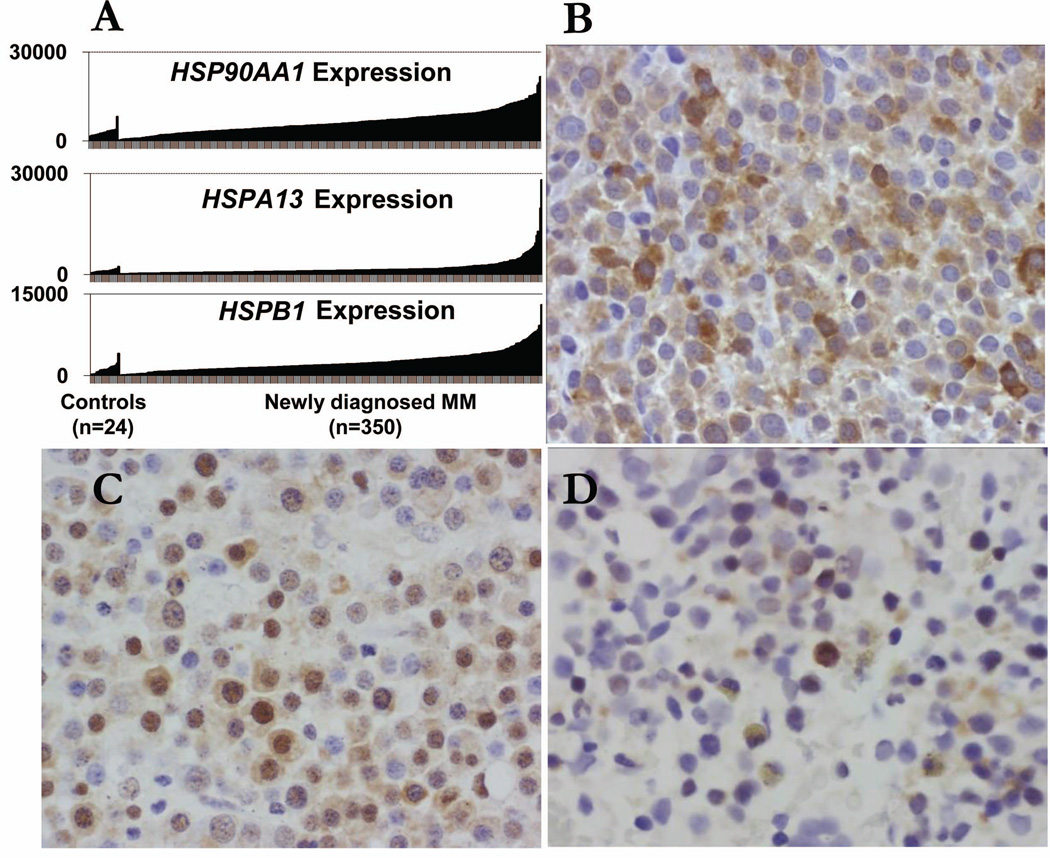
We analysed the gene expression patterns of HSP in MM cells in the public databases using Cancer Outlier Profile Analysis (COPA) for 350 MM patients and 24 healthy volunteers in University of Arkansas for Medical Sciences’ database (Available from: www.oncomine.com), and found that HSP mRNA were commonly and highly expressed by purified CD138+ primary MM cells compared with normal CD138+ plasma cells (Fig 1A). Immunohistochemical staining of MM bone marrow biopsies for HSP90AA1 (Fig 1B), HSPA13 (Fig 1C) and HSPB1 (Fig 1D) showed that only plasma cells were positively stained. As normal haematopoietic cells expressed low basal levels of HSP, they were stained negative for HSP90AA1, HSPA13 and HSPB1, indicating that HSPs were MM.
Figure 1. Expression of HSP genes and proteins in normal and myeloma plasma cells.
(A) HSP expression is elevated in most myeloma tumour cells. Levels of HSP90AA1, HSPA13, and HSPB1 expression were assessed by gene array in bone marrow CD138+ plasma cells from 24 healthy controls and 350 newly diagnosed myeloma patients. Each vertical bar represents an individual sample. Immunohistochemical staining of MM bone marrow biopsies for HSP90AA1 (B), HSPA13 (C) and HSPB1 (D) expression in bone marrow CD138+ plasma cells, which show that only myeloma plasma cells but no normal haematopoietic cells were positively stained (original magnification × 400).
3.2 Binding affinity and immunogenicity of HSP peptides
After comparing the predictive binding scores, we identified and selected four peptides that could potentially bind to HLA-A*0201 molecules: two peptides (aa362 and aa670) derived from HSP90AA1α, one (hp27) from HSPB1, and one (hp70) from HSPA13 (Table II). We chose the HSP90AA1α-derived (aa670) and HSPB1-derived (hp27) peptides for the following experiments on the basis of their high binding affinity (Fig 2A) and stability (Fig 2B), as measured by peptide-T2 binding assay. Peptides from the influenza virus matrix protein (Flu-matrix) 27 and human immunodeficiency virus (HIV) type 1 reverse transcriptase (HIV-pol) were used as positive controls. To examine whether the peptides were able to immunize HLA-A2.1-tg mice, we subcutaneously injected mice with 100 µg of peptides aa670 and hp27 or the Flu-matrix peptide (Tangri et al., 2001). Two weeks after the immunization, mice were euthanized and their splenocytes were collected, restimulated with the immunizing peptides for 5 days, and subjected to analyses. The in vivo immunization successfully generated peptide-specific T cells, detected as CFSE proliferation ( Fig 2C) IFN-γ-expressing (Fig 2D) and peptide (aa670 or hp27)-specific (Fig 2E) CD8+ T cells. Furthermore, the splenocytes displayed strong cytolytic activity against peptide-pulsed, but not unpulsed, murine DCs (Fig 2F). Peptides aa670 or hp27 appeared to be as immunogenic as the Flu-matrix peptide in immunizing mice. These results indicate that human HSP peptides were able to induce a strong peptide-specific CTL response in HLA-A2.1-tg mice in vivo.
Figure 2. Binding affinity and in vivo immunogenicity of HSP peptides.
Peptide binding assay showing (A) affinity and (B) stability (fluorescence index) of HLA-A*0201-binding peptides from human HSP. HIV-pol and Flu-matrix peptides were used as positive controls. In these studies, T2 cells were incubated with100 µg/ml peptides overnight, or with 100 µg/ml peptides for different time points, and analysed as detailed in Materials and methods. We identified and selected four peptides that could potentially bind to HLA-A*0201 molecules: two peptides (aa362 and aa670) derived from HSP90AA1α, one (hp27) from HSPB1, and one (hp70) from HSPA13. We chose the HSP90AA1α-derived (aa670) and HSPB1-derived (hp27) peptides for the following experiments on the basis of their high binding affinity (Figure 2A). Also shown are (C) T cell proliferation detected by CFSE-labelled assay, (D) Intracellular staining for CD8+ IFN-γ–expressing T cells, (E) HLA-A*0201-peptide-tetramer staining showing HSP peptide-specific CD8+ T cells, and (F) Cytotoxicity of splenocytes of mice immunized with Flu-matrix peptide (Flu), hp27, or aa670 peptides. Splenocytes were re-stimulated with (+Peptide) or without (-Peptide) the immunizing peptides for 5 days before analysis. Representative results of 3 independent experiments are shown. *P < 0.05; **P < 0.01.
3.3 Generation of HSP peptide-specific CTL lines
We first examined whether HSP peptide-specific CTL precursors were present in patients with MM. Using hp27- or aa670-HLA-A*0201 tetramers to stain T cells of patients with myeloma and a healthy volunteer., we showed that HSP peptide-specific CD8+ T cells were detected, although at low frequencies, in three patients with myeloma, whereas the frequency of such T cells was lower in the healthy volunteer (Fig 3A). To generate HSP peptide-specific T cells from HLA-A*0201+ healthy volunteers and patients with myeloma, autologous mature DCs pulsed with peptides were used as antigen-presenting cells. Three to 4 rounds of in vitro stimulation using peptide-pulsed DCs, increased the frequency of peptide-specific CD8+ T cells, from 3% to 4% of peptide-specific T cells at the second stimulation to 13% to 15% at the fourth stimulation (Fig 3B). T cell lines were obtained and proliferated in response to autologous DCs pulsed, but not unpulsed, with the HSP peptide aa670 or hp27 (P < 0.01, compared with unpulsed DCs; Fig 3D). The same results were also obtained with the CFSE-labelling assay, which measured T cell proliferation (Fig 3C).
Figure 3. Circulating and stimulated HSP-specific T cells in healthy volunteers and patients with MM.
(A) HLA-A*0201-peptide-tetramer staining showing the presence and percentages of HSP peptide-specific CD8+ T cells in the blood of 3 HLA-A*0201+ patients (pt1-pt3) with multiple myeloma (MM) and a healthy volunteer (HV). (B) HLA-A*0201 peptide-tetramer staining showing the representative frequency of HSP peptide-specific CD8+ T cells in cultures during in vitro stimulations. Similar results were obtained with T-cell lines from other MM patients or healthy volunteers. Also shown are proliferative responses measured by (C) CFSE dilution assay or (D) MTT assay of T cell lines specific for hp27 or aa670 generated from a HLA-A*0201+ healthy volunteers in response to unpulsed dendritic cells (DCs) or DCs pulsed with hp27 or aa670 peptides. In D, different ratios of T cell:DC were used. *P < 0.05; **P < 0.01.
3.4 HSP-specific CTLs lysed HLA-A*0201+ but not HLA-A*0201− myeloma cells
The standard 4-h 51Cr-release and LDH release assays were used to examine the cytotoxicity of the T cells. The primary myeloma cells isolated from the bone marrow of MM patients could not survive more than 48 h in vitro, indicating that all of the primary myeloma cells examined in the cytotoxicity assay are allogeneic. As exemplified by the results obtained with T cell lines specific for hp27 or aa670, generated from HLA-A*0201+ patients with myeloma and healthy volunteers, the T cells specifically lysed autologous primary myeloma cells, HLA-A*0201+ allogeneic primary myeloma cells and HLA-A*0201+ myeloma cell lines U266 and ARH-77 cells but not HLA-A*0201− primary MM cells or MM cell lines RPMI-8226 and LP-1. No killing was observed against HLA-A*0201+ autologous PBMCs (Fig 4). These results suggest that the T cell lines recognized the HSP peptides that were naturally processed and presented in the context of HLA-A*0201 molecules on myeloma cells.
Figure 4. Cytotoxicity of HSP peptide-specific CTL lines against myeloma cells.
Shown are percentages of cytotoxicity detected by 51Cr-release (51Cr, upper panels) or lactate dehydrogenase (LDH) release (LDH; lower panels) assays of hp27- and aa670-specific T cell lines from HLA-A*0201+ myeloma patients and healthy volunteers. The primary myeloma cells isolated from the bone marrow of multiple myeloma (MM) patients could not survive more than 48 h in vitro, indicating that all of the primary myeloma cells examined in the cytotoxicity assay were allogeneic. The HSPB1- and HSP90AA1-specific T cell lines were derived from different patients and healthy volunteers, details can be found in Table III. The HSP-specific T cell lines from the same origin were used to test their cytotoxicity not only on primary myeloma cells but also the established cell lines. In brief, T cell lines examined in the LDH release of HSPB1 and HSP90AA1 on MM cell lines, and the 51Cr-release of HSPB1 and HSP90AA1 were all derived from 2 healthy volunteers and 1 patient. The allogeneic CTL cell lines examined in the LDH release of HSPB1 and HSP90AA1 on primary MM cells and the 51Cr-release of HSPB1 and HSP90AA1 were all derived from 2 patients and 1 healthy volunteer. Target cells include myeloma cell lines and allogeneic primary myeloma cells from patients. HLA-A*0201+ autologous peripheral blood mononuclear cells (PBMCs) were used as controls. MM patients 1, 2, 3, 4, 6 and 7 were HLA-A*0201+, and patients 5 and 8 were HLA-A*0201−. Different effector:target (E:T) cell ratios were used. *P < 0.05; **P < 0.01.
3.5 Immunophenotype and intracellular cytokine profiles of the CTLs
Representative experiments of surface expression of activation markers and intracellular cytokine staining for IFN-γ and IL4 in the T cell lines are shown. Upon restimulation with DCs pulsed with the peptides, the T cells expressed higher levels of CD45RO and CD69 (Fig 5A) as compared with unstimulated T cells, indicating that the CTLs were activated memory effector cells (Bae et al., 2012). Furthermore, a high proportion (> 90%) of the CTLs expressed IFN-γ whereas IL4-expressing T cells were very few (3–4%). Taken together, these data suggest that the T cell lines were type-1 memory effector CD8+ T cells (Fig 5B). To determine MHC restriction of the T-cell–mediated cytotoxicity, we evaluated the inhibitory effects of anti-MHC mAbs. As shown in Fig 5C, mAbs against MHC-I or HLA-A*0201 significantly inhibited (70%–80% inhibition) T-cell–mediated cytotoxicity against peptide-pulsed T2 cells (P<0.05 compared with medium control). No inhibitory effect was observed with mAb against MHC-II and isotype control IgG. The results indicate that the cytotoxicity was attributed to MHC class I and, more specifically, HLA-A*0201-restricted CD8+ CTLs.
Figure 5. Phenotype and cytokine expression profiles of HSP peptide-specific CTL lines.
Flow cytometry analysis showing the expression of (A) surface CD45RO and CD69; (B) intracellular staining of interleukin 4 (IL4) and γ-interferon (IFN-γ) in unstimulated T cells and HSP peptide-specific CTL lines one week after the fourth cycle of peptide stimulation. The results are representative of three independent experiments. (C) Inhibition of the T-cell lines–mediated cytotoxicity against peptide-pulsed T2 cells by mAbs against MHC class I (aMHC-I), MHC class II (aMHC-II), or HLA-A*0201 (aHLA-A2). Isotypic IgG was used as control. An effector-target (E/T) ratio of 10:1 was used in panels C. Results of 4 independent experiments are shown. Error bars show standard deviation. Similar results were obtained with other T-cell lines from blood donors and patients with myeloma. *P < 0.05. CTL, cytotoxic T lymphocytes
3.6 Cytotoxicity of CTLs was mediated by perforin/granzyme B but not by Fas-FasL pathways
Flow cytometry analysis was used to examine the expression of granzyme B, perforin, FasL, and CD107a, an antigen transiently present on the cell surface after release of cytotoxic granules, by the T cell lines. As shown in Fig 6A, it seems that the T cell lines killed their target cells via the perforin/granzyme pathways because the T cells expressed high levels of perforin but not FasL. The restimulated T cells also secreted increased amounts of TNF-β. Myeloma cells did not express granzyme B but granzyme B could be detected in myeloma cells after coculture with CTLs (Fig 6B), indicating that CTLs released granzyme B into their target cells. In line with these findings, HSP-specific CTL lines displayed significantly increased CD107a expression upon stimulation with HLA-A*0201+ U266 but not HLA-A*0201− LP-1 cells (Fig 6C). In contrast, unstimulated or stimulated (with myeloma cell lines U266 or LP-1) CD8+ T cells from the same donors showed minimal expression of CD107a.
Figure 6. Cytolytic machinery of HSP peptide-specific CTL lines.
Flow cytometry analysis showing the expression of (A) perforin, FasL, tumour necrosis factor (TNF)-β by the cytotoxic T lymphocytes (CTL) lines, (B) granzyme B by target cells labelled with fluorescent dye, TFL4, alone or after 4-h coculture with effector CTLs at two different E:T ratios, and (C) CD107a by the CTL lines and unstimulated T cells from the same donor, before and after restimulation with myeloma cell lines LP-1 (HLA-A*0201−) or U266 (HLA-A*0201+). Similar results were obtained with other T cell lines derived from other MM patients or healthy volunteers. **P < 0.01.
3.7 HSP-specific CTLs suppress myeloma growth in vivo
Because the HSP-specific CTLs were highly cytotoxic to MM cells in vitro, we next examined the efficacy of the CTLs on established MM in vivo. While all 5 mice injected with control T cells died of MM by week 8 (Fig 7A), 4 out of 5 mice that received aa670-specific CTLs showed a dramatic decrease in tumour size (Fig 7B). Treatment with aa670-specific CTLs significantly prolonged the survival of myeloma-bearing mice (Fig 7C; P < 0.01, compared with control mice).
Figure 7. In vivo anti-myeloma effects of HSP peptide-specific CTL lines.
Non-obese diabetic/severe combined immunodeficiency mice (5 per group) were injected subcutaneously with 2 × 107 U266 cells on day 0. When tumour areas reached 4 mm2, aa670-specific CTLs (1 × 107) or unstimulated T cells from the same donors were injected intravenously into myeloma-bearing mice together with subcutaneous injection of interleukin 2 at 10,000 iu/mouse. Tumour area was measured twice every week. Shown are tumour sizes in mice receiving injection of (A) control T cells or (B) HSP peptide-specific CTLs and (C) mouse survival data.
4. Discussion
In the present study, we demonstrated that the RNA and protein levels of HSPs expressed on most of the primary myeloma cells were dramatically higher than that expressed on normal cells, in line with other results (Duus et al., 2006; Cervantes-Gomez et al., 2011). Of the large number of HSP previously studied, RNA and protein over-expression of HSPs had been repeatedly found in all of the MM cell lines used in our investigations, such as U266, RPMI 8226, ARH-77, LP-1and the primary myeloma cells from all MM patient’s samples (Duus et al., 2006; Cervantes-Gomez et al., 2011), strongly suggesting that HSPs were myeloma TAA. Hence, it was possible to use HSPs as a selective target in myeloma cells. To determine whether HSPs could serve as ideal TAA in MM, we identified and synthesized four HSP peptides after searching the HSP sequences for HLA-A*0201 binding motifs. Subsequently, we selected two peptides, aa670 and aa27, for further studies because of their high binding affinity and binding stability in the T2 binding assay. After immunization of HLA-A*0201-tg mice with the peptides, splenocytes isolated from the immunized mice contained large numbers of peptide-specific T lymphocytes, which were peptide-tetramer-positive, and IFN-γ-secreting CD8+ T lymphocytes. These T cells were able to kill autologous DCs pulsed with HSPs, but not unpulsed DCs, which confirmed the immunogenicity of the peptides. The detection of HSPs peptide-specific CTL in the blood of MM patients and healthy volunteers indicated that the peptides could be naturally presented by DCs and be recognized by CD8+ T cells, further demonstrating the antigenicity of the peptides.
A longstanding goal of cancer immunotherapy is the generation of an adequate amount of tumour-specific CD8+ T cells that are capable of effectively clearing the tumour cells. DCs are highly immune-stimulatory antigen-presenting cells capable of breaking immune tolerance and activating resting T cells (Hart, 1997). In the present study, with the help of DCs, we indeed harvested adequate amount of HSP specific CTL. The HSPs specific CTLs demonstrated by peptide/MHC tetramer staining increased with the repeated stimulation by up to a 14%–16% after the fourth stimulation.
Both the productions of HSP-specific CTLs and the “quality” of the CTLs are critical. The high quality of CTLs are of activated/memory T cells demonstrated by the phenotype, and of the potent anti-myloma cytotoxicity as well as of the expressed cytokines mainly IFN-γ. The CTL subsets have been identified according to their immune phenotype examined by flow cytometry. Previously, subsets of T lymphocytes were classified according to the expression of CD45RA and CD45RO on the surface of T lymphocytes (Albrecht et al., 2011). Recently, this definition has been abandoned because CD45RA expression is regained in the late CTL (Hufford et al., 2011). Nowadays, the cytotoxic cells have been sub-classified based on the expression of CD45RO and CD69 (Bordoni et al., 2012). Our results displayed an altered T lymphocyte phenotype including an increased proportion of activated/memory (CD69+/CD45RO+) T lymphocytes
Furthermore, the activated/memory (CD69+/CD45RO+) CTLs behaved with strong cytolytic activity against HSP peptide-pulsed T2 cells, autologous DCs, HLA-A*0201+ myeloma cell lines and primary myeloma cells from HLA-A*0201+ patients, but not HLA-A*0201− patients. More importantly, these CTLs could significantly reduce tumour burden and prolong the survival of myeloma-bearing mice indicating that HSP-specific CTLs might be promising effector cells for immunotherapy of MM.
CTLs recognize target cells via T cell receptors through activating two major cytotoxic mechanisms. One is the fast-acting, perforin-mediated pathway and the other is the slower acting, FasL-mediated pathway. The perforin/granzyme B system plays an important role in anti-tumour immunity and it has been associated with a favorable prognosis in different types of cancers (Vermijlen et al., 2001). In the present study, HSP-specific CTLs seemed to lyse the target cells mainly via the perforin-mediated pathway because the T lymphocytes expressed high levels of perforin but not FasL, and granzyme B could be detected in target cells after coculture with CTLs. To further confirm the possible mechanism responsible for the cytotoxicity of these CTLs, degranulation of HSP-CTLs was analysed. The level of CD107a, a surface antigen transiently presented on the cell surface after release of cytotoxic granules containing perforin and granzyme B, was examined by flow cytometer. Compared with unstimulated T lymphocytes, CD107a expression on HSPB1-and HSP90AA1-CTLs was dramatically increased after restimulation with MM cells. This phenomenon could be used as further evidence for the perforin/granzyme B cytotoxic mechanisms.
It has been reported that myeloma cells resistant to chemo-agents also became resistant to Fas-mediated apoptosis (Landowski et al., 1997). Thus, pore-forming CTLs might be an option for the treatment of drug-resistant myeloma. Notably, the HSPs are over-expressed in minimal residual disease, which could be further diminished by the HSP-specific CTLs (Abdi et al., 2013).
In conclusion, our study demonstrated that HSP (peptides) could indeed be a valid target for immunotherapy of MM. Human HSP peptide-specific CTLs could be generated by stimulating T lymphocytes with DCs pulsed with the peptides. These CTLs were potent cytotoxic cells and were able to specifically and effectively lyse myeloma cells, including myeloma cell lines and the primary myeloma cells, but not normal blood cells, both in vitro and in vivo. Thus, our study might be the first to provide direct evidence to support the application of HSP-based immunotherapy in MM.
Acknowledgments
We thank Dr. Jie Yang for flow cytometry analyses. This work was supported by grants from National Natural Science Funds (Grant Nos. 30828017 and 81102284), Shanghai Science and Technology International Cooperation Key Programme (Grant No. 11410708300), and by National Cancer Institute R01 CA138402, R01 CA138398, R01 CA163881, Leukemia & Lymphoma Society translational research grants; and by the Multiple Myeloma Research Foundation.
Footnotes
Contributions: J.H. and Q.Y. initiated the work and designed the experiments. R.L., J.Q., J.H. and Q.Y wrote the manuscript. R.L., W.Z., J.Q., H.J. and H.Z performed the experiments and statistical analyses. W.F., C.Z., H.X. and J.D. provided samples and critical suggestion to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- Abdi J, Chen G, Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget. 2013;4:2186–2207. doi: 10.18632/oncotarget.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J, Frey M, Teschner D, Carbol A, Theobald M, Herr W, Distler E. IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype. Cancer Immunology Immunotherapy. 2011;60:235–248. doi: 10.1007/s00262-010-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Oseroff C, Dahlberg C, Qin M, Ishioka G, Beebe M, Fikes J, Newman M, Chesnut RW, Morton PA, Fok K, Appella E, Sette A. A decaepitope polypeptide primes for multiple CD8+ IFN-gamma and Th lymphocyte responses: evaluation of multiepitope polypeptides as a mode for vaccine delivery. Journal of Immunology. 2002;168:6189–6198. doi: 10.4049/jimmunol.168.12.6189. [DOI] [PubMed] [Google Scholar]
- Assimon VA, Gillies AT, Rauch JN, Gestwicki JE. Hsp70 protein complexes as drug targets. Current Pharmaceutical Design. 2013;19:404–417. doi: 10.2174/138161213804143699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, Munshi NC. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia. 2011a;25:1610–1619. doi: 10.1038/leu.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. British Journal of Haematology. 2011b;155:349–361. doi: 10.1111/j.1365-2141.2011.08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Smith R, Daley J, Mimura N, Tai YT, Anderson KC, Munshi NC. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clinical Cancer Research. 2012;18:4850–4860. doi: 10.1158/1078-0432.CCR-11-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of Immunological Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Bordoni V, Casetti R, Capuano G, De Stefani B, Piselli P, Gioia C, Agrati C, Martini F. A novel 8-color flow cytometry panel to study activation, maturation and senescence of CD4 and CD8 T lymphocytes in HIV-infected individuals at different stages of disease. International Journal of Immunopathology and Pharmacology. 2012;25:415–424. doi: 10.1177/039463201202500211. [DOI] [PubMed] [Google Scholar]
- Cervantes-Gomez F, Nimmanapalli R, Gandhi V. ATP analog enhances the actions of a heat shock protein 90 inhibitor in multiple myeloma cells. Journal of Pharmacology and Experimental Therapeutics. 2011;339:545–554. doi: 10.1124/jpet.111.184903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, Gupta D, Richardson P, Schlossman RL, Krett N, Chen LB, Munshi NC, Anderson KC. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, Burger R, Munshi N, Ohtake Y, Saxena S, Anderson KC. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock (Augusta, Ga.) 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- De Vos J, Thykjaer T, Tarte K, Ensslen M, Raynaud P, Requirand G, Pellet F, Pantesco V, Reme T, Jourdan M, Rossi JF, Orntoft T, Klein B. Comparison of gene expression profiling between malignant and normal plasma cells with oligonucleotide arrays. Oncogene. 2002;21:6848–6857. doi: 10.1038/sj.onc.1205868. [DOI] [PubMed] [Google Scholar]
- Duus J, Bahar HI, Venkataraman G, Ozpuyan F, Izban KF, Al-Masri H, Maududi T, Toor A, Alkan S. Analysis of expression of heat shock protein-90 (HSP90) and the effects of HSP90 inhibitor (17-AAG) in multiple myeloma. Leukemia & Lymphoma. 2006;47:1369–1378. doi: 10.1080/10428190500472123. [DOI] [PubMed] [Google Scholar]
- Endemann M, Bergmeister H, Bidmon B, Boehm M, Csaicsich D, Malaga-Dieguez L, Arbeiter K, Regele H, Herkner K, Aufricht C. Evidence for HSP-mediated cytoskeletal stabilization in mesothelial cells during acute experimental peritoneal dialysis. American Journal of Physiology-Renal Physiology. 2007;292:F47–F56. doi: 10.1152/ajprenal.00503.2005. [DOI] [PubMed] [Google Scholar]
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- Hong S, Li H, Qian J, Yang J, Lu Y, Yi Q. Optimizing dendritic cell vaccine for immunotherapy in multiple myeloma: tumour lysates are more potent tumour antigens than idiotype protein to promote anti-tumour immunity. Clinical and Experimental Immunology. 2012;170:167–177. doi: 10.1111/j.1365-2249.2012.04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. Journal of Experimental Medicine. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal MK, Agrawal V, Jaiswal YK. Lipopolysaccharide drives alternation of heat shock proteins and induces failure of blastocyst implantation in mouse. Biology of Reproduction. 2013;88:162. doi: 10.1095/biolreprod.113.108068. [DOI] [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Gleason-Guzman MC, Dalton WS. Selection for drug resistance results in resistance to Fas-mediated apoptosis. Blood. 1997;89:1854–1861. [PubMed] [Google Scholar]
- LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Research. 2002;62:4996–5000. [PubMed] [Google Scholar]
- Lin H, Kolosenko I, Bjorklund AC, Protsyuk D, Osterborg A, Grander D, Tamm KP. An activated JAK/STAT3 pathway and CD45 expression are associated with sensitivity to Hsp90 inhibitors in multiple myeloma. Experimental Cell Research. 2013;319:600–611. doi: 10.1016/j.yexcr.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Mellqvist UH, Gimsing P, Hjertner O, Lenhoff S, Laane E, Remes K, Steingrimsdottir H, Abildgaard N, Ahlberg L, Blimark C, Dahl IM, Forsberg K, Gedde-Dahl T, Gregersen H, Gruber A, Guldbrandsen N, Haukas E, Carlson K, Kvam AK, Nahi H, Lindas R, Andersen NF, Turesson I, Waage A, Westin J. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial. Blood. 2013;121:4647–4654. doi: 10.1182/blood-2012-11-464503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalesnik MA. Plasma cell tumors in transplant patients. Blood. 2013;121:1247–1249. doi: 10.1182/blood-2013-01-475947. [DOI] [PubMed] [Google Scholar]
- Pellat-Deceunynck C, Mellerin MP, Labarriere N, Jego G, Moreau-Aubry A, Harousseau JL, Jotereau F, Bataille R. The cancer germ-line genes MAGE-1, MAGE-3 and PRAME are commonly expressed by human myeloma cells. European Journal of Immunology. 2000;30:803–809. doi: 10.1002/1521-4141(200003)30:3<803::AID-IMMU803>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Qian J, Wang S, Yang J, Xie J, Lin P, Freeman ME, 3rd, Yi Q. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clinical Cancer Research. 2005;11:8808–8815. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- Qian J, Xie J, Hong S, Yang J, Zhang L, Han X, Wang M, Zhan F, Shaughnessy JD, Jr, Epstein J, Kwak LW, Yi Q. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110:1587–1594. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Hong S, Wang S, Zhang L, Sun L, Wang M, Yang J, Kwak LW, Hou J, Yi Q. Myeloma cell line-derived, pooled heat shock proteins as a universal vaccine for immunotherapy of multiple myeloma. Blood. 2009;114:3880–3889. doi: 10.1182/blood-2009-06-227355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Du J, Zhang C, Fu W, Xi H, Zou J, Hou J. Arsenic trioxide exerts antimyeloma effects by inhibiting activity in the cytoplasmic substrates of histone deacetylase 6. PLOS One. 2012;7:e32215. doi: 10.1371/journal.pone.0032215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollig C, Schmidt C, Bornhauser M, Ehninger G, Schmitz M, Auffermann-Gretzinger S. Induction of cellular immune responses in patients with stage-I multiple myeloma after vaccination with autologous idiotype-pulsed dendritic cells. Journal of Immunotherapy (Hagerstown, Md.: 1997) 2011;34:100–106. doi: 10.1097/CJI.0b013e3181facf48. [DOI] [PubMed] [Google Scholar]
- Schreurs MW, Eggert AA, de Boer AJ, Vissers JL, van Hall T, Offringa R, Figdor CG, Adema GJ. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Research. 2000;60:6995–7001. [PubMed] [Google Scholar]
- Sellner L, Heiss C, Benner A, Raab MS, Hillengass J, Hose D, Lehners N, Egerer G, Ho AD, Goldschmidt H, Neben K. Autologous retransplantation for patients with recurrent multiple myeloma: a single-center experience with 200 patients. Cancer. 2013;119:2438–2446. doi: 10.1002/cncr.28104. [DOI] [PubMed] [Google Scholar]
- Tangri S, Ishioka GY, Huang X, Sidney J, Southwood S, Fikes J, Sette A. Structural features of peptide analogs of human histocompatibility leukocyte antigen class I epitopes that are more potent and immunogenic than wild-type peptide. Journal of Experimental Medicine. 2001;194:833–846. doi: 10.1084/jem.194.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi P, Tomassetti S, Merli A, Polli V. Serum free light-chain assay for the detection and monitoring of multiple myeloma and related conditions. Therapeutic advances in hematology. 2013;4:37–41. doi: 10.1177/2040620712466863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, Raje N, Hilgers JH, Nadler L, Belch AR, Pilarski LM, Anderson KC. Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999;93:1287–1298. [PubMed] [Google Scholar]
- Tyler EM, Jungbluth AA, O'Reilly RJ, Koehne G. WT1-specific T-cell responses in high-risk multiple myeloma patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation and donor lymphocyte infusions. Blood. 2013;121:308–317. doi: 10.1182/blood-2012-06-435040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baren N, Brasseur F, Godelaine D, Hames G, Ferrant A, Lehmann F, Andre M, Ravoet C, Doyen C, Spagnoli GC, Bakkus M, Thielemans K, Boon T. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood. 1999;94:1156–1164. [PubMed] [Google Scholar]
- Vermijlen D, Froelich CJ, Luo D, Suarez-Huerta N, Robaye B, Wisse E. Perforin and granzyme B induce apoptosis in FasL-resistant colon carcinoma cells. Cancer Immunology Immunotherapy. 2001;50:212–217. doi: 10.1007/s002620100191. [DOI] [PMC free article] [PubMed] [Google Scholar]