Abstract
Hepatocellular carcinoma (HCC) was thought historically to arise from hepatocytes, but gene expression studies have suggested it can also arise from fetal progenitor cells or their adult progenitor progeny. Here we report the identification of a unique population of fetal liver progenitor cells in mice that can serve as a cell of origin in HCC development. In the transgenic model used, mice carry the Cited1-CreER™-GFP BAC transgene in which a tamoxifen-inducible Cre (CreER™) and GFP are controlled by a 190kb 5′ genomic region of Cited1, a transcriptional co-activator protein for CBP/p300. Wnt signaling is critical for regulating self-renewal of progenitor/stem cells and has been implicated in the etiology of cancers of rapidly self-renewing tissues, so we hypothesized that Wnt pathway activation in CreER™-GFP+ progenitors would result in HCC. In livers from the mouse model, transgene-expressing cells represented 4% of liver cells at E11.5 when other markers were expressed characteristic of the hepatic stem/progenitor cells that give rise to adult hepatocytes, cholangiocytes and SOX9+ periductal cells. By 26 weeks of age, >90% of Cited1-CreER™-GFP; Ctnnb1ex3(fl) mice with Wnt pathway activation developed HCC and, in some cases, hepatoblastomas (HB) and lung metastases. HCC and HB resembled their human counterparts histologically, showing activation of Wnt, Ras/Raf/MAPK and PI3K/AKT/mTOR pathways, and expressing relevant stem/progenitor cell markers. Our results show that Wnt pathway activation is sufficient for malignant transformation of these unique liver progenitor cells, offering functional support for a fetal/adult progenitor origin of some human HCC. We believe this model may offer a valuable new tool to improve understanding of the cellular etiology and biology of HCC and HB and the development of improved therapeutics for these diseases.
Introduction
Hepatocellular cancer (HCC) is the fifth most common cancer worldwide with a very high mortality rate (1). Historically, HCCs were thought to arise from hepatocytes. Interestingly, gene expression profiling of human HCCs has suggested that a subset of HCCs can also arise from a liver progenitor/stem cell (2). Molecular analyses of HCCs have identified various gene mutations and dysregulated signaling pathways in tumors, including alterations that up-regulate the Wnt/β-catenin, Ras/Raf/MEK/ERK, PI3K/mTOR and Sonic Hedgehog pathways (3). Gene mutations that activate the Wnt/β-catenin signaling pathway are observed in 50% of HCCs, and the most common of these is CTNNB1 mutations that result in stabilization of β-catenin (4). Thus, one approach for generating mouse models for HCC has been to activate the Wnt signaling pathway via Ctnnb1 mutation (5). Wnt pathway activation in adult murine hepatocytes fails to induce tumors (6–8). However, introduction of genetic alterations such as Ha-Ras or Akt mutation in adult hepatocytes in addition to Wnt pathway activation does result in HCC (9, 10). Published data therefore indicate that activation of the Wnt pathway alone is insufficient for HCC initiation, at least in hepatocytes.
Because the Wnt signaling pathway plays a critical role in regulating stem/progenitor cell self-renewal and because of the suggestion that a fetal progenitor is the cell of origin for some human HCCs, we hypothesized that activation of the Wnt pathway in a unique population of bipotential fetal liver cells that we have identified could give rise to HCC in vivo without the introduction of additional genetic events. As presented below, these fetal liver cells are characterized by their expression of the Cited1-CreER™-GFP BAC transgene (11) and express CD45, in addition to markers characteristic of hepatic stem/progenitor cells in fetal liver. They can differentiate, both in vitro and in vivo, into hepatocytes and cholangiocytes. We assessed the ability of β-catenin stabilization to transform these cells by generating mice (Tg-β-catS) that carried both the BAC transgene and a Ctnnb1 conditionally stabilized allele (Ctnnb1+/ex3(fl)) (11, 12). By 26 weeks of age, 91% of Tg-β-catS mice developed hepatocellular carcinomas, demonstrating that introduction of a stabilizing Ctnnb1 mutation into a fetal liver progenitor can result in endogenous HCCs in adult mice. Hepatoblastomas and lung metastases were also observed in mutant mice.
Materials and methods
Mouse strains
Animal work was carried out in compliance with the Institutional Animal Care and Use Committee of MD Anderson Cancer Center (Houston, Texas). Cited1- CreER™-GFP is a transgenic line carrying a BAC transgene in which expression of the Cre gene (and also a GFP reporter) is driven by a 190kb fragment 5′ of the Cited1 gene, and Cre function is inducible with tamoxifen in a dose-dependent manner (11). Ctnnb1+/ex3(fl) and ROSA26R-LacZ mouse strains were also used in the study (11–13). Ctnnb1+/fl; Cited1-CreER™-GFP embryos were generated and treated with tamoxifen (0.5mg/40g maternal body weight) at E14.5, which resulted in β-catenin stabilization in transgene-expressing cells (Tg-β-catS).
RT-PCR analysis
Conditions for RT-QPCR and primers are listed in Supplemental Methods.
Histology and immunohistochemistry (IHC)
Tissues were paraformaldehyde-fixed, paraffin-embedded and assessed by H&E or IHC. For IHC, tissue sections were deparaffinized and the antigens were retrieved by boiling for 20 minutes in citrate buffer (pH 5). Antibodies used are listed in Supplemental Methods.
Western blotting
Proteins were extracted from snap-frozen tissues and western blotting was performed by standard protocols (14). Antibodies used are listed in Supplemental Methods.
Reverse phase protein array (RPPA) analysis
Protein extracts from normal livers and liver tumors were prepared and subjected to RPPA as previously described (15).
FACS analysis
Single cell suspensions of fetal liver (E12.5 – E17.5) from Cited1-CreER™-GFP mice were prepared by homogenization of fetal livers and sorting for GFP expression using the BD FACS Aria High Speed Digital Cell Sorter. Cell suspensions from embryos with no transgene served as negative controls. The Cited1-CreER™-GFP transgene is known to be expressed in fetal kidney cells (11) and kidney suspensions from Cited1-CreER™-GFP mice were positive controls. Antibodies used and conditions for FACS analysis is provided in Supplemental Methods.
Cell culture
GFP-sorted cells from fetal liver were cultured in laminin-coated dishes for 21 days in differentiating medium (16). Cells were photomicrographed, RNA was extracted, cDNA was synthesized and semi-quantitative PCR analysis was performed as previously described (17).
X-gal staining
Cited1-CreER™-GFP; ROSA26R-LacZ embryos were treated in utero at E14.5 with tamoxifen (3mg/40g maternal body weight). Livers were dissected at two months of age and stained with X-gal as described in Supplemental Methods.
Statistical analysis
Statistical significance of the results between groups was determined by Student’s t-test. Statistical differences were considered significant if p <0.05 (*), <0.01 (**) and <0.001 (***). All data is represented as mean ± SEM.
Results
Expression of Cited1-CreER™-GFP transgene in fetal liver
Transgene expression was detectable by GFP fluorescence in E13.5 liver (Fig. S1, A,a). FACS analysis of liver cell suspensions (E11.5 – E17.5) showed robust GFP expression in 4% of fetal liver cells (denoted as CreER™-GFP+) at E11.5. This declined to 0.3–0.6% by E14.5, and by E17.5 the GFP+ cells were undetectable. GFP+ cells in fetal kidney served as a control (Table 1a). In contrast to the very low frequency of GFP+ cells in livers from the BAC transgenic mice, widespread endogenous CITED1 protein expression has been observed in fetal liver from wild-type mice at E11.5 – E14.5 (18). Thus, while the transgene includes 190kb from the 5′ region of the Cited1 gene, transgene expression is much more restricted than that of the endogenous locus.
Table 1a.
FACS sorting of embryonic liver and kidney samples for GFP expression.
| Liver (% GFP positive) | Kidney (% GFP positive) | |
|---|---|---|
|
| ||
| E 11.5 | 2.5 – 4.0% | not determined |
| E 12.5 | 0.8 – 1.0% | not determined |
| E 13.5 | 0.3 – 0.6% | not determined |
| E 14.5 | 0.3 –0.1% | 0.5% |
| E 17.5 | undetectable | 0.9% |
Expressed as percentage of total liver cells
CreER™-GFP+ cells are bipotent in cell culture
Semi-quantitative PCR analysis of E12.5 and E13.5 CreER™-GFP+ cells showed expression of albumin, α1-antitrypsin, cytokeratin 18, and vinculin, but not cytokeratin 19 (Fig. S1A, b). Because the GFP+ cells were so rare in fetal liver, we speculated that they might be progenitor cells. To test this, we isolated CreER™-GFP+ liver cells by FACS sorting, plated them at a low density (3x103/cm2), and cultured them under conditions that induce differentiation. CreER™-GFP+ cells clonally expanded in culture and gave rise to hepatocytes and cholangiocyte-like cells (Fig. S1B, a-c). Semi-quantitative PCR analysis showed that these cultures expressed markers of hepatocyte lineage (albumin, α-fetoprotein and α1-antitrypsin), cholangiocyte markers (cytokeratin19 and vinculin) and cytokeratin 18 and 8, which were expressed by both these lineages (Fig. S1B, d), indicating that CreER™-GFP+ cells were indeed bipotent.
Fate mapping of CreER™-GFP+ cells in liver
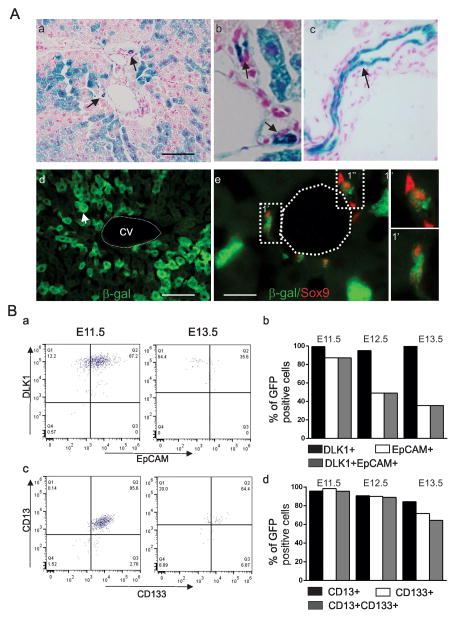
We crossed Cited1-CreER™-GFP mice with the Rosa26R-LacZ reporter mice (13). Following tamoxifen treatment (3mg/40g body weight) of embryos at E14.5, liver samples were collected at 2 months and assessed for the presence of β-gal+ cells. In the liver, hepatocytes (hexagonal large cells) and cholangiocytes of both small and large bile ducts (small cuboidal cells) were positive for β-galactosidase enzyme activity (Fig. 1A, a-c). Immunofluorescence analysis with antibody specific for β-galactosidase on liver sections showed β-gal+ cells in the liver (Fig. 1A, d). Co-staining with Sox9, a marker for adult liver progenitors (19) and β-galactosidase antibodies identified cells of bile ducts that were double positive, suggesting that the transgene-expressing cells give rise to the ductal cells (Fig. 1A, e).
Figure 1. Characterization of CreER™-GFP+ cells in fetal liver.
A. P60 liver sections showing X-gal+ liver cells, hepatocytes and bile ducts (arrows, a), higher magnification showing the small (arrows, b) and large bile ducts (c). Antibody staining of cryosections (P60) for β-galactosidase in hepatocytes (arrow, d) and in SOX9 positive (red nuclei) bile duct cells (e). 1′ and 1″ show higher magnification. Scale bar, 160 μm (a, d) and 32 μm (e). B. FACS analysis for DLK1 and EPCAM (a,b), CD13 and CD133 (c,d) in the CreER™-GFP+ cells at E11.5 – E13.5.
Expression of cell surface markers characteristic of progenitors in the CreER™-GFP+ cells
We characterized the fetal liver GFP+ cells at E11.5–E13.5 with markers characteristic of hepatic stem/progenitors by FACS analysis. Over 95% of the CreER™-GFP+ cells expressed DLK1, a fetal stem/progenitor marker which is expressed between E10.5–E16.5 and is undetectable in neonatal and adult livers (20) (Fig. 1B, a). EpCAM expression was detected in 87% of GFP+ cells in E11.5 livers (Fig. 1B, a). This decreased to 49% and 35% at E12.5 and 13.5, respectively (Fig. 1B, b). Of note, all cells positive for EpCAM were also positive for DLK1 (Fig. 1B, a). The percentage of CD13+ and CD133+ cells in the CreER™-GFP+ cells declined from 95% at E11.5, to 89% at E12.5, to 64% at E13.5 (Fig. 1B, c,d). In addition, the expression of AFP was detectable in only 3.3%, 2.5% and 13.5% of CreER™-GFP+ cells at E11.5, E12.5 and E13.5, respectively (Fig. S2A, a,b). Table 1b summarizes these findings.
Table 1b.
Expression of hepatoblast markers in CreER™ -GFP+cells.
| E11.5 | E12.5 | E13.5 | |
|---|---|---|---|
| CD13 | 95 | 91 | 85 |
| CD133 | 90 | 90 | 90 |
| CD13/CD133 | 90 | 89 | 83 |
| DLK1 | 98 | 95 | 97 |
| EpCAM | 78 | 49 | 21 |
| DLK1/EpCAM | 77 | 49 | 21 |
| AFP | 4 | 2 | 14 |
Expressed as percentage of CreER™-GFP+ cells
Hepatic colony forming units (h-cfus) from fetal liver are integrin α6 positive (low), integrin β1 positive, and negative for at least three hematopoietic stem cell markers (Ter119, CD45 and CD117 [c-kit]) (21). Like h-cfus, CreER™-GFP+ cells did not express CD117 or Ter119 (hematopoietic stem cell markers), but unlike h-cfus, a significant portion (31%) did express CD45 (Fig. S2B, a,d,e). In addition, >90% of CreER™-GFP+ cells expressed integrin β1, like h-cfus, but, unlike h-cfus, were negative for integrin α6 (Fig. S2B, a-c). About 26% of the CreER™-GFP+ cells expressed both integrin β1 and CD45 (Fig. S2B, d,e).
Q-PCR analysis of RNA isolated from the CreER™-GFP+ and CreER™-GFP− cells from E12.5 fetal liver indicated that expression of Cd13, Ecadh, Lgr5 and Dlk1 was increased in CreER™-GFP+ cells relative to CreER™-GFP− cells (Fig. S1C).
Cited1-CreER™-GFP; β-cat+/fl (Tg-β-catS) mice developed HCCs, HBs and lung metastases
To test the tumorigenic potential of CreER™-GFP+ fetal liver cells, we mosaically and somatically stabilized β-catenin (β-catS) in the CreER™-GFP+ fetal liver cells. Cited1-CreER™-GFP and Ctnnb1+/ex3(fl) littermates served as controls. Experimental animals displayed no reduced viability, but did display hepatomegaly (Table 2). Palpatable tumors were detected as early as eight weeks and, by 26 weeks, 91% (20/22) of Cited1-CreER™-GFP; Ctnnb1+/ex3(fl) mice (hereafter denoted Tg-β-catS) developed tumors (Fig. 2A). Mice were sacrificed when they became moribund and tumors were collected. Within an animal, multiple tumors of variable sizes were randomly distributed in all liver lobes (Fig. 2B, b). Of note, apparent lung metastases were also grossly observed in two of the 20 tumor-bearing mice that were sacrificed at 6 months of age (arrowheads, Fig. 2B, c). Genotypic analysis confirmed the presence of the recombined Ctnnb1 allele in the tumors (Fig. 2B, d). Interestingly, loss of the wild-type allele of Ctnnb1 (marked by *) was noted in some tumors (e.g., T2).
Table 2.
Liver weight/body weight ratio in Tg-β-cats mice at one month of age
| Genotype | Liver weight/body weight ratio | No. of mice analyzed | Fold change |
|---|---|---|---|
|
| |||
| Tg-β-cats | 0.1550 ± 0.025 | 8 | 3.03 |
| Controls | 0.0511 ± 0.002 | 9 | 1 |
Controls vs Tg-β-cats p<0.0005
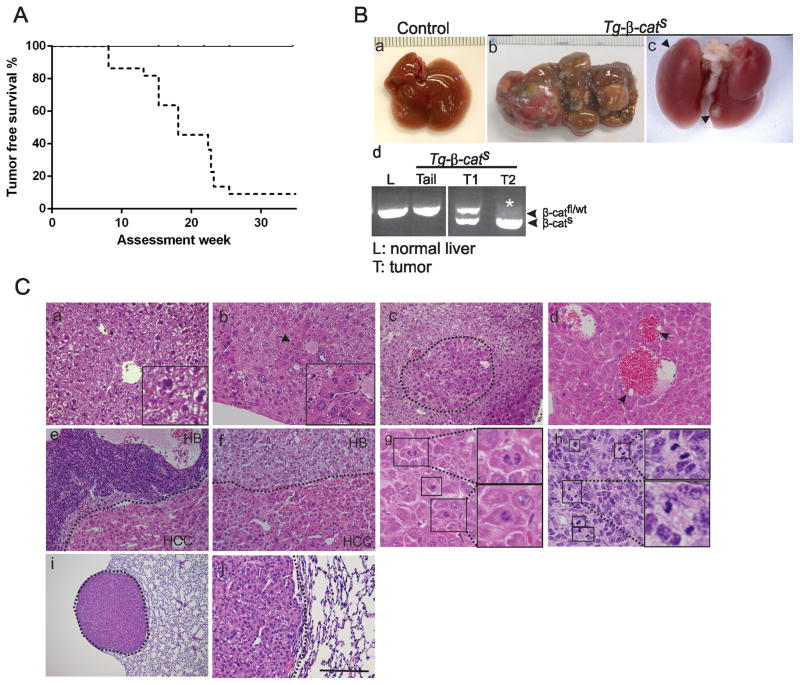
Figure 2. Development of liver tumors in Tg-β-catS mice.
A. Kaplan-Meier analysis of tumor incidence in mutant animals (dotted line) and littermate controls (solid line). B. Gross appearance of normal liver (a), liver with multifocal tumors in Tg-β-catS mice (b), and lung metastases (arrowheads, c). Representative PCR from Tg-β-catS mice showing wildtype/floxed bands in tail and liver DNA of control mice and mutant tumors (d). Asterisk (*) indicates loss of the wildtype allele in T2. C. Histological analysis of liver tumors (b–h) and associated lung metastasis (i,j). H&E staining of normal liver (a), altered hepatocytes (denoted by arrow, b) adenoma (demarcated by dotted line, c) HCC (d–f), HB embryonal (e) and HB pure fetal (f). Insets in (a) and (b) provide higher magnification (3X). Mitotic figures of HCC (g) and embryonal undifferentiated HB (h). Lung metastasis (dotted lines, i), and higher magnification (j). Scale bar, 80 μm (a,b,d,j), 500 μm (c,e,f), and 1280 μm (i).
Complete histological evaluation was carried out on liver sections from 12 tumor-bearing and control mice. Small foci of aberrant hepatocytes with increased basophilic staining was observed in most sections (Fig. 2C, b) when compared to normal liver sections (Fig. 2C, a). Nodular adenomatous lesions (Fig. 2C, c) and HCCs were most commonly present (Fig. 2C, d-f). HCCs were characterized by cytological atypia, occasional mitotic figures (Fig. 2C, g) and peliosis (arrows, Fig. 2C, d) and were very similar to human HCC.
Unexpectedly, of the 12 HCC-bearing mice, five (42%) also had histologically detectable hepatoblastomas (HBs). HBs were typically composed of undifferentiated monotypic cells, characteristic of embryonic liver cells, with scanty cytoplasm (Fig. 2C, e) and numerous mitotic figures (Fig. 2C, h). In human tumors this histology is classified as the embryonal undifferentiated subtype of HB (22). Some mice also had moderately differentiated pure fetal HB with steatosis (Table S1 & Fig. 2C, f). These observations are consistent with the fact that we targeted a progenitor population.
Histological evaluation of a lung metastasis revealed cells with increased basophilic staining, steatosis and relatively uniform nuclei (Fig. 2C, i,j). It is not clear whether the metastases arose from HCC or HB. Determining this will require further extensive evaluation.
HCCs, HBs and lung metastases showed activation of the Wnt pathway and were hyperproliferative
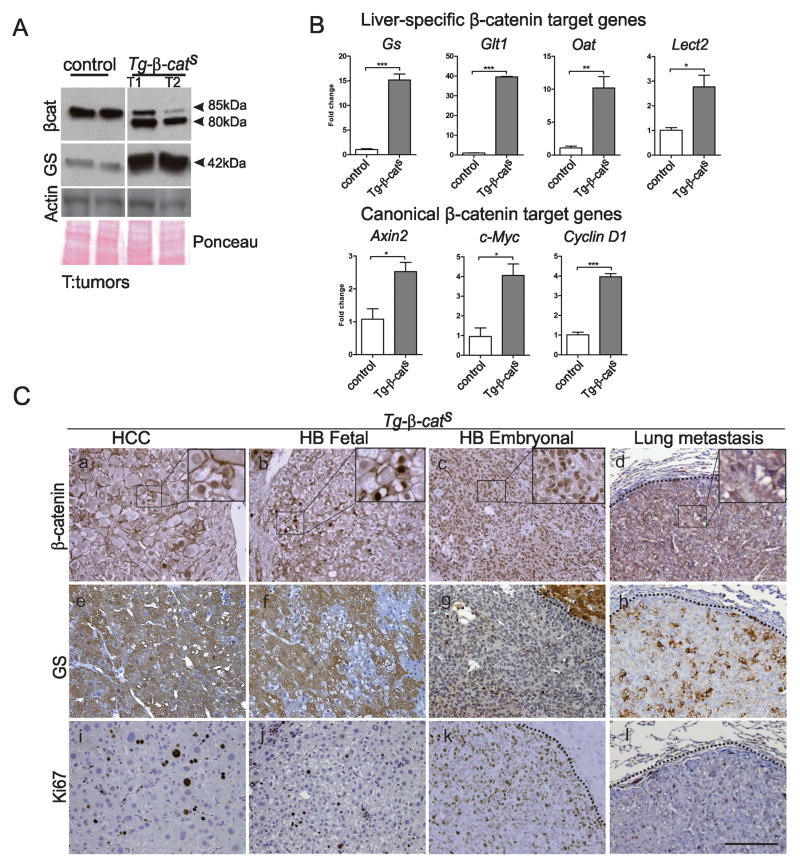
Western blot analysis confirmed the expression of the truncated β-catenin (80 kDa) encoded by the exon 3-deleted Ctnnb1 gene and also glutamine synthetase (GS) in the Tg-β-catS HCCs (Fig. 3A). Q-RT PCR analysis also revealed a significant increase in the mRNA expression of Gs and other previously reported liver-specific Wnt target genes such as glutamate receptor 1 (Glt1), ornithine amino transferase (Oat) and Leukocyte cell-derived chemotaxin 2 (Lect2) (23, 24), in Tg-β-catS tumors (Fig. 3B). A statistically significant increase in expression of Axin2, c-Myc and Cyclin D1, canonical Wnt targets, was also observed in the Tg-β-catS tumors (Fig. 3B).
Figure 3. Activation of Wnt pathway in Tg-β-catS tumors.
A. Western blot analysis of Tg-β-catS tumors and normal control livers for β-catenin and glutamine synthetase (GS). β-Actin and Ponceau stained membrane (P) were used as loading controls. B. Q-PCR analysis of liver-specific β-catenin target genes (Gs), glutamate transporter 1 (Glt1), ornithine aminotransferase (Oat) and Lect2 in Tg-β-catS tumors (n=3) and canonical target genes Axin2, c-Myc, and CyclinD, compared to littermate normal livers (n=3). C. β-catenin IHC in HCC (a), HB pure fetal (b), embryonal undifferentiated HB (c), and lung metastasis (d). GS staining in HCC, fetal HB, embryonal HB, and lung metastasis (e–h). Ki67 staining in HCC, HB, and metastasis (i–l). Scale bar, 80 μm.
β-catenin IHC demonstrated heterogeneous expression with increased cytoplasmic and nuclear localization in HCCs, pure fetal HBs, and lung metastasis from Tg-β-catS mice (Fig. 3C, a,b,d). In contrast, β-catenin staining in the embryonal undifferentiated HB was strikingly nuclear (Fig. 3C, c). In livers from littermate control mice, Wnt signaling activity, marked by the expression of GS, was restricted to a single layer of perivenular hepatocytes (Fig. S3A, b). GS immunohistochemistry revealed both an increased and an altered expression pattern in all Tg-β-catS HCCs in which it was uniformly expressed in all the hepatocytes of the tumor and was not restricted only to perivenular hepatocytes (Fig. 3C, e). In contrast, GS expression was heterogeneous in the pure fetal HB and lung metatasis (Fig. 3C, f,h). In the embryonal undifferentiated variant of HB, GS immunoreactivity was hardly detectable (Fig. 3C, g). In one month old Tg-β-catS mice that did not show any overt tumors, we detected increased cytoplasmic and nuclear localization of β-catenin, aberrant spatial expression of GS in non-perivenular areas and increased proliferation as detected by Ki67 (Fig. S3B, d-f) when compared with age-matched controls (Fig. S3B, a-c). Western blotting also confirmed these changes (Fig. S3C). Canonical Wnt targets were also upregulated in these mice (Fig. S3D).
Tumors from Tg-β-catS mice (Fig. 3C, i-k) and the lung metastasis (Fig. 3C, l) showed increased proliferation (Ki67 staining) compared to liver sections from littermate control mice (Fig. S3B, c). The embryonal undifferentiated variant of HB displayed the highest frequency of proliferating cells (Fig. 3C, k).
HCCs displayed activation of the MAPK and mTOR pathway
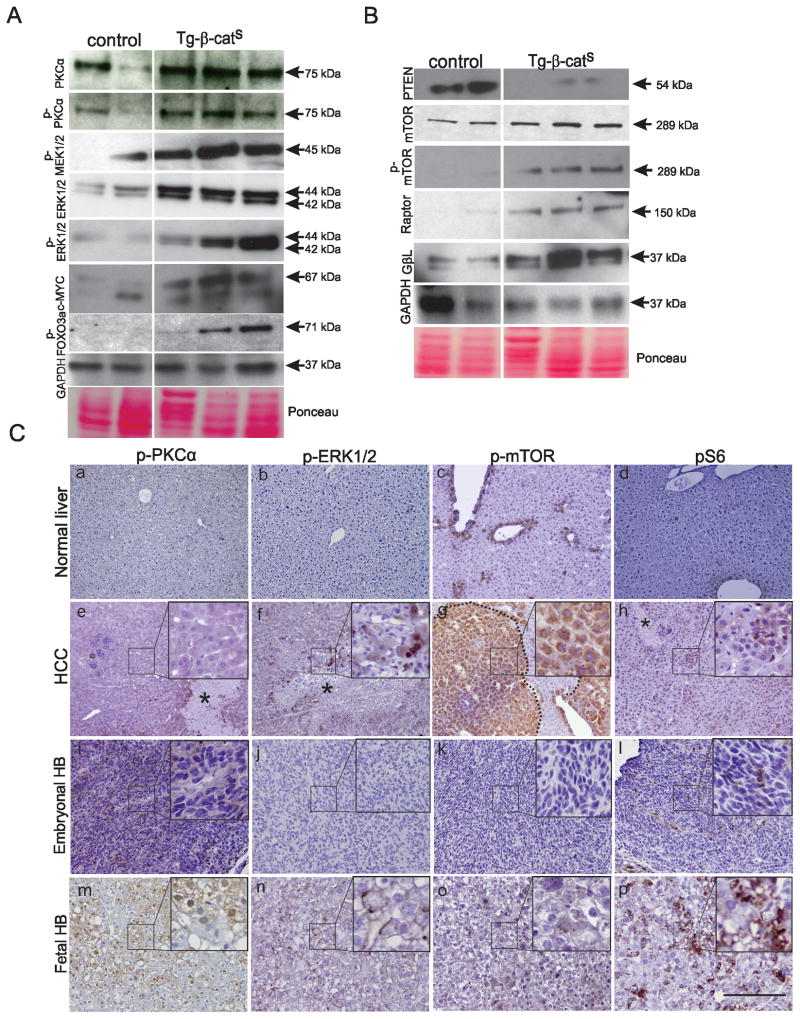
To identify key cancer signaling pathways altered in the HCCs, we performed reverse phase protein array (RPPA) analysis and compared the protein expression profile of key proteins in Tg-β-catS HCCs and normal liver of control littermates (Fig. S4). The increased expression of mTOR and MAPKpT202 detected by RPPA suggested that two key cancer signaling pathways, Ras/Raf/MEK/MAPK and PI3K/mTOR, were significantly altered in Tg-β-catS tumors. These two pathways can be activated by FGFR1 (reviewed in (25)), and FGFR1 (pT766) was also identified by RPPA as being upregulated in the Tg-β-cats tumors (Fig. S4). Western blot analysis validated the RPPA analysis data. In the Ras/Raf/MEK/MAPK pathway, we confirmed significant upregulation of MEK1/2 (phospho), PKCα (total and pT492), ERK1/2 (total and pT202/204), c-MYC (a downstream target of the pathway), and FOX03a (pS318) (a proapoptotic gene that is inhibited by phosphorylation by ERK1/2) (Fig. 4A). In the PI3K/mTOR pathway, besides upregulation of mTOR (total and pS2448) by Western analysis, we also identified upregulation of Raptor and GβL, two TORC1 complex proteins. Interestingly, PTEN, the negative upstream regulator of the mTOR pathway whose loss results in HCC (26), was significantly downregulated in Tg-β-catS tumors (Fig. 4B). Besides these two key signaling pathways, upregulation of stathmin, SRC, and CHK1, all of which have been associated with hepatocarcinogenesis (27–29), was detected by RPPA analysis (Fig. S4). Immunohistochemistry with PKCα (pT492) and ERK1/2 (pT202) antibodies confirmed their expression in HCC tissue (Fig. 4C, e,f). Similarly, upregulation of mTOR (pS2448) and S6 kinase (pS235/236) was detected in the HCC lesions (Fig. 4C, g,h). While the HB fetal variant showed upregulation of PKCα (pT492), ERK1/2 (pT202), mTOR (p2448) and S6 kinase (pS235/236) (Fig. 4C, m-p), the embryonal undifferentiated variant showed only upregulation of PKCα (pT492) and only a few phospho-S6 positive cells were detected (Fig. 4C, i, l), suggesting that alterations in signaling pathways were specific to the cell type comprising the tumor.
Figure 4. Activation of the MAPK and mTOR pathways in Tg-β-catS tumors.
A, B. Western blot analysis of MAPK and mTOR pathway components in HCC from Tg-β-catS HCCs compared to control livers. GAPDH and Ponceau stained membranes were used as loading controls. C. IHC analysis of phospho-PKCα (a,e,I,m), phospho-ERK1/2 (b,f,j,n), phospho-mTOR (c,g,k,o), and pS6 (d,h,l,p) in the normal liver (a–d), HCCs (eh), embryonal HB (i–l) and fetal HB (m–p) of Tg-β-catS mice. Insets show higher magnification (e–p). Necrotic areas denoted by *. Scale bar, 80μm.
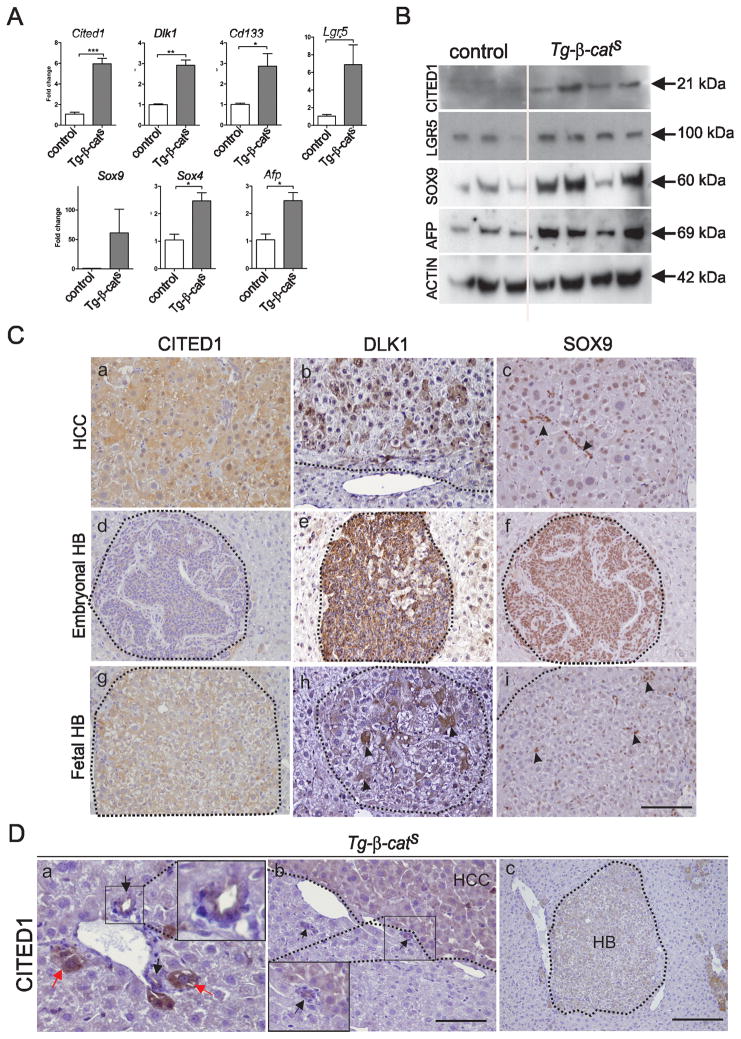
Tumors express stem/progenitor markers
To test if the HCCs in our model expressed stem/progenitor markers characteristic of the cells from which they arose, we performed Q-RT PCR. A statistically significant increase in the expression of Cited1, Dlk1, CD133, and Lgr5 was observed (Fig. 5A). In addition, the expression of Afp, a marker for premature hepatocytes and also a Wnt target gene in liver, and Sox9 and Sox4, markers for adult progenitors in liver, were also significantly increased in tumors (Fig. 5A). Increased Cited1, Lgr5, Sox9 and Afp was also confirmed at the protein level by Western blotting (Fig. 5B). By IHC, CITED1 expression was detected uniformly in all hepatocytes of the HCCs (Fig. 5C, a), whereas DLK1 expression was more heterogeneous (Fig. 5C, b) and SOX9 expression was nuclear and confined to small cells in tumors (arrows, Fig. 5C, c). In the embryonal undifferentiated variant of HB, CITED1 expression was weak, whereas DLK1 and SOX9 expression was robust with almost all cells expressing these markers (Fig. 5C, d,e,f). In the pure fetal HB, CITED1 expression was uniform and robust (Fig. 5C, g), high DLK1 expression was restricted to a few hepatocyte cells, (Fig. 5C, h), and SOX9 expression was nuclear and restricted to a very few cells as in the HCCs (arrows, Fig. 5C, i). CITED1 expression was seen in bile ducts (black arrows, Fig. 5D, a) and hepatocytes emerging from them (red arrows, Fig. 5D, a) and in tumors (HCCs and HBs) around bile ducts (Fig. 5D, b,c).
Figure 5. Expression of hepatic stem/progenitor markers in Tg-β-catS tumors.
A. RT-PCR analysis of HCCs (n=3) and control livers (n=3) for expression of stem/progenitor markers. B. Western blot analysis of stem/progenitor markers in Tg-β-catS HCCs. C. IHC showing expression of CITED1 (a,d,g), DLK1 (b,e,h), and SOX9 (arrowheads, c, i and f) in HCC (a–c) embryonal HB (d–f) and fetal HB (g–i). Scale bar, 70 μm. D. IHC showing CITED1 expression in bile duct epithelium (black arrows, a) and in hepatocytes emerging around the bile ducts (red arrows, a). Tumors (HCC, b and HB, c) originating around the bile ducts. Insets in (a) and (b) show CITED1 expression in bile duct epithelium. Scale bar; a,b, 70 μm; c, 160 μm.
Discussion
We report here the identification of a novel population of fetal liver progenitor cells and the development of endogenous and metastatic liver tumors when β-catenin stabilization/Wnt pathway activation -- alone -- is targeted to these cells. These tumors also showed upregulation of the Ras/Raf/MEK/MAPK and PI3K/mTOR pathways.
Wnt pathway activation by CTNNB1 mutation is observed in 20–40% of human HCCs and 50–90% of HB (30–32). However, in mice activation of the Wnt pathway by over-expression of wild type or stable mutants of β-catenin in hepatocytes failed to result in tumor development (6, 7, 33). For tumors to develop in these mice, Wnt pathway activation had to be accompanied by additional alterations such as activation of either Ha-Ras or Akt (9, 10) or treatment with hepatotoxins (34). In this study, we hypothesized that β-catenin stabilization in an early fetal progenitor would be sufficient for tumor development. Therefore, using the Cited1-CreER™-GFP mouse strain (11) we targeted Wnt pathway activation to CreER™-GFP+ cells, a unique, and previously uncharacterized, early fetal liver progenitor.
Both in vitro differentiation studies and in vivo lineage tracing studies demonstrated that CreER™-GFP+ cells are progenitors of hepatocytes and cholangiocytes in the adult liver. Thus they are functionally like fetal hepatic stem/progenitors which have been characterized, in part, by expression of cell surface markers such as DLK1, EpCAM, E-cadherin, CD13, and CD133 and which are considered to be bipotent progenitors for both hepatocytes and cholangiocytes (35). CreER™-GFP+ cells also express DLK1, CD13, EpCAM, CD133, and E-cadherin, each of which has been reported to be a marker for fetal stem/progenitor cells. However, unlike previously characterized hepatic colony forming units (h-cfus) (16), the CreER™-GFP+ cells do not express integrin α6 and a fraction did express CD45 (a hematopoietic stem cell marker). Notably, the expression of AFP was detected in only a small percentage of cells. The expression of CD45 is unique to the CreER™-GFP+ cells. Recent studies in humans suggest that hepatic progenitor-like cells also express hematopoietic markers such as CD45 and CD109 (36). Additionally, co-immunofluorescence studies demonstrated that CreER™-GFP+ cells also gave rise to SOX9-expressing periductal cells in adult liver, which are considered to be adult liver progenitors. Thus the CreER™-GFP+ cells are a unique cell population which represents ~4% of the liver in E11.5 mice, a time point at which there is an increase in hepatic parenchyma and hepatoblast expansion. These cells were almost undetectable by E17.5, by which time liver development is near to completion.
In animals in which β-catenin was stabilized in the CreER™-GFP+ cells (Tg-β-catS mice), over 90% developed HCC by 26 weeks. This is in striking contrast to the absence of tumors when β-catenin is stabilized in adult hepatocytes using a Cre-expressing adenovirus or using a adolaseB promoter-driven transgene expressing a mutant stable β-catenin protein (7). Our conditional genetic model using the Tg-β-catS mice demonstrates that the CreER™-GFP+ liver stem/progenitor cells can be transformed by just β-catenin activation. Only a small fraction of CreER™-GFP+ cells express AFP. Future studies will be required to determine whether the tumors in the Tg-β-catS mice arise from the AFP+ and/or the AFP− fraction of the CreER™-GFP+ cells. Of note, activation of Wnt signaling pathway in fetal hepatoblasts using an Afp-driven Cre transgene is embryonic lethal, making it impossible to evaluate potential tumorigenesis of the large population of Afp+ fetal liver progenitors (37).
Like human HCCs, the Tg-β-catS tumors histologically showed nuclear atypia, mitotic figures, and peliosis, and robustly and heterogeneously expressed GS and nuclear β-catenin. Approximately ~40% of mice that developed HCCs also developed HBs. In the Tg-β-catS mice, β-catenin was stabilized at ~E14.5, at which time hepatoblast lineage bifurcation begins. Because of this, it is possible that β-catenin stabilization occurred in a heterogeneous population of fetal progenitors, some of which were more differentiated than others. This is fully consistent with the occurrence of both HCC and HB in these mice. Like human HBs that are classified into distinct classes based on their histology (22), we observed two distinct histologies: a pure fetal moderately differentiated HB and an embryonal undifferentiated HB. Some mutant mice showed both histologies of HB. Like human HBs, both types of murine HBs displayed increased nuclear β-catenin expression, homogeneously in the embryonal HBs and heterogeneously in the fetal histology HBs. GS expression in the fetal moderately differentiated HBs was similar to that in the HCCs, although more heterogeneous. GS expression was lower in the embryonal undifferentiated HBs, consistent with the expression of GS in more differentiated cells of the liver.
Lung metastases were grossly observable in some Tg-β-catS mice, an unusual occurrence in mouse models. 46% and 44% of human HCC and HB patients, respectively, have metastatic disease, primarily in the lung (38, 39). As was the case for the primary Tg-β-catS tumors, we observed robust β-catenin expression in a lung metastasis.
The observation of HCCs and sometimes, additionally, HBs, raises a question about the cell type(s) from which these tumors arose. HBs are thought to arise from maturation arrest of infant liver stem cells and HCCs to develop from more differentiated cells that are nevertheless from the same lineage (40, 41). For human HCC, a fetal cell origin has been suggested for a subset of tumors based on their fetal liver gene expression profile, and the development of HCCs following ex vivo genetic manipulation of murine hepatoblasts is consistent with this (42, 43). Alternatively, HCCs may arise following dedifferentiation of adult hepatocytes and re-expression of fetal markers during the course of malignant transformation (2). The recent observation of CITED1 expression in regenerating hepatocytes following partial hepatectomy or DDC treatment (18) suggests that fetal markers can be re-expressed in adult hepatocytes during regeneration.
Another possibility is that the HCCs arose from the fetal CreER™-GFP+ cell-derived SOX9+ adult progenitors that we identified in our mice. Examination of the livers of Tg-β-catS mice revealed that small HCCs were almost invariably located around bile ducts, similar to the localization of the SOX9+ stem/progenitors, consistent with the notion that tumors may have arisen from adult progenitors.
Activating mutations of the Wnt signaling pathway are very common in both mouse and human HCC (30), and in human tumors Wnt target genes, both canonical and liver-specific targets, are upregulated (23, 24, 44, 45). We observed increased cytoplasmic and nuclear localization of β-catenin in the liver of young (one month old) Tg-β-catS mice and also in hepatocytes in the Tg-β-catS HCCs. Notably, both liver and HCCs also showed an increase in both canonical (c-Myc, Cyclin D1 and Axin2) and non-canonical Wnt targets (Gs, Glt1, Oat and Lect2). Such induction of canonical Wnt targets was not observed in a model in which stabilized β-catenin was expressed in hepatocytes, nor did these mice develop tumors (7). These differences between the two models suggest that the ability of Wnt pathway activation to result in HCCs critically depends on the activation of canonical Wnt targets.
Tg-β-catS tumors also expressed FGFR1, commonly observed in human HCCs, and displayed activation of the Ras/Raf/MAPK and the PI3K/AKT/mTOR pathways, both of which can be activated by FGFR1 and are present in human HCCs characterized by aggressive behavior (46). Specifically, PKCα, which has been reported to contribute to HCC proliferation, migration, and invasion via activation of MAPK (47, 48), was up-regulated in Tg-β-catS tumors as was pMEK1/2 and pERK1/2. Phospho-FOXO3A, a proapoptotic protein which is negatively regulated via phosphorylation by pERK1/2, was increased. PTEN was downregulated in tumors, whereas components of the TORC1 complex, p-mTOR, RAPTOR, and GβL, were upregulated, consistent with the known role of PTEN in negatively regulating the mTOR pathway. As noted previously, tumors displayed an increase in both liver-specific and canonical β-catenin targets. One, c-MYC, is also an effector of the MEK/ERK pathway, and another, GS, has been reported to enhance tumor growth by activation of the mTOR pathway (49, 50). Thus the activation of the Wnt pathway engineered into the CreER™-GFP+ fetal liver progenitors may have not only initiated tumorigenesis, but may have also enhanced the effect of mTOR and MEK/ERK pathway activation. We also observed up-regulation of Stathmin, SRC, and CHK1, which have also been implicated in hepatocarcinogenesis (27–29).
By RPPA analysis, Tg-β-catS HCCs uniformly displayed activation of the mTOR and MEK/ERK pathways and this was subsequently verified by IHC. In contrast to HCCs, we observed distinct patterns of protein/phospho-protein expression in the two histologic types of HBs. The fetal HB corresponded more closely to HCC with upregulation of PKCα, ERK1/2, mTOR and S6 proteins, whereas the embryonal undifferentiated HB showed significant upregulation of only PKCα. The expression of β-catenin was also distinct in these two histologies of HB, with the embryonal undifferentiated HB displaying strikingly nuclear localization and the pure fetal HB resembling the HCCs. These results suggest that the specific signaling pathways altered in the tumors are dependent upon the cell type within the tumor.
In summary, we have identified a unique population of progenitor cells in fetal liver which are marked by the expression of the Cited1-CreER™-GFP transgene. Somatic β-catenin stabilization in these cells results in the frequent development of both HCCs and HBs with spontaneous lung metastases. Our model provides valuable evidence for a common origin of HCC and HB from the Tg-β-catS stem/progenitor cell. This model will be extremely valuable for understanding the pathobiology of human HCCs, in particular those that display an especially poor prognosis and are thought to be of stem/progenitor origin.
Supplementary Material
Acknowledgments
Financial Support
Support from NIH grants CA34936, DK069599, NCI CCSG grant CA16672, CPRIT RP100329, and CPRIT RP110324. SM is a recipient of the Dodie P. Hawn Fellowship in Genetics at MD Anderson Cancer Center.
Footnotes
Conflict of interest
No conflicts to disclose.
References
- 1.El-Serag HB. CURRENT CONCEPTS Hepatocellular Carcinoma. New England Journal of Medicine. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Thorgeirsson SS, Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature Medicine. 2006;12:410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 4.Guichard C, Imbeaud S, Amaddeo G, Ben Maad I, Letouze E, Pelletier L, et al. Landscape of Somatic Mutation in Hepatocellular Carcinoma. Journal of Hepatology. 2012;56:S8-S. [Google Scholar]
- 5.Monga SPS. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. International Journal of Biochemistry & Cell Biology. 2011;43:1021–9. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada N, Miyoshi H, Murai N, Oshima H, Tamai Y, Oshima M, et al. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res. 2002;62:1971–7. [PubMed] [Google Scholar]
- 7.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–9. [PubMed] [Google Scholar]
- 8.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Dar MJ, Khillan J, et al. Accelerated Liver Regeneration and Hepatocarcinogenesis in Mice Overexpressing Serine-45 Mutant beta-Catenin. Hepatology. 2010;51:1603–13. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 10.Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, et al. Coactivation of AKT and beta-Catenin in Mice Rapidly Induces Formation of Lipogenic Liver Tumors. Cancer Research. 2011;71:2718–27. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, et al. Fate mapping using Cited1-CreER(T2) mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Developmental Biology. 2008;313:234–45. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo Journal. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 14.Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Molecular and cellular biology. 2005;25:6846–56. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibes R, Qiu YH, Hennessy B, Andreeff M, Miiis GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Molecular Cancer Therapeutics. 2006;5:2512–21. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki A, Taniguchi H, Zheng YW, Takada Y, Fukunaga K, Seino K, et al. Clonal colony formation of hepatic stem/progenitor cells enhanced by embryonic fibroblast conditioning medium. Transplantation Proceedings. 2000;32:2328–30. doi: 10.1016/s0041-1345(00)01685-7. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Zheng YW, Kondo R, Kusakabe M, Takada Y, Fukao K, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–9. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AJ, de Caestecker C, Pierce J, Boyle SC, Ayers GD, Zhao Z, et al. CITED1 expression in liver development and hepatoblastoma. Neoplasia (New York, N Y ) 2012;14:1153–63. doi: 10.1593/neo.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature genetics. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 20.Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. Journal of cell science. 2003;116:1775–86. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, et al. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. Journal of Cell Biology. 2002;156:173–84. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann A. The emerging family of hepatoblastoma tumours: from ontogenesis to oncogenesis. European journal of cancer (Oxford, England : 1990) 2005;41:1503–14. doi: 10.1016/j.ejca.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 24.Ovejero C, Cavard C, Perianin A, Hakvoort T, Vermeulen J, Godard C, et al. Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology. 2004;40:167–76. doi: 10.1002/hep.20286. [DOI] [PubMed] [Google Scholar]
- 25.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature reviews Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 26.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. The Journal of clinical investigation. 2004;113:1774–83. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan L, Guo K, Li Y, Kang X, Sun L, Shu H, et al. Up-regulated expression of stathmin may be associated with hepatocarcinogenesis. Oncology reports. 2010;23:1037–43. doi: 10.3892/or_00000730. [DOI] [PubMed] [Google Scholar]
- 28.Hong J, Hu K, Yuan Y, Sang Y, Bu Q, Chen G, et al. CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. The Journal of clinical investigation. 2012;122:2165–75. doi: 10.1172/JCI61380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masaki T, Okada M, Shiratori Y, Rengifo W, Matsumoto K, Maeda S, et al. pp60c-src activation in hepatocellular carcinoma of humans and LEC rats. Hepatology (Baltimore, Md ) 1998;27:1257–64. doi: 10.1002/hep.510270511. [DOI] [PubMed] [Google Scholar]
- 30.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–51. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer research. 1999;59:269–73. [PubMed] [Google Scholar]
- 32.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–45. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287–95. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- 35.Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, et al. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol. 2012;9:231–40. doi: 10.1038/nrgastro.2012.23. [DOI] [PubMed] [Google Scholar]
- 36.Jun LJ, Xin, Liyuan Zhang, Jian Wu, Longyan Jiang, Qian Zhou, Jun Li, Jing Guo, Hongcui Cao, Lanjuan L, Li Human hepatic progenitor cells express hematopoietic cell markers CD45 and CD109. International Journal of Medicinal Sciences. 2014;11:65–79. doi: 10.7150/ijms.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47:247–58. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 38.Yang T, Lu J-H, Lin C, Shi S, Chen T-H, Zhao R-H, et al. Concomitant lung metastasis in patients with advanced hepatocellular carcinoma. World journal of gastroenterology : WJG. 2012;18:2533–9. doi: 10.3748/wjg.v18.i20.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchiyama M, Iwafuchi M, Naito M, Yagi M, Iinuma Y, Kanada S, et al. A study of therapy for pediatric hepatoblastoma: prevention and treatment of pulmonary metastasis. Eur J Pediatr Surg. 1999;9:142–5. doi: 10.1055/s-2008-1072230. [DOI] [PubMed] [Google Scholar]
- 40.Fiegel HC, Gluer S, Roth B, Rischewski J, von Schweinitz D, Ure B, et al. Stem-like cells in human hepatoblastoma. Journal of Histochemistry & Cytochemistry. 2004;52:1495–501. doi: 10.1369/jhc.4A6297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sell S, Leffert HL. Liver cancer stem cells. Journal of Clinical Oncology. 2008;26:2800–5. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J-S, Heo J, Libbrecht L, Chu I-S, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature medicine. 2006;12:410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 43.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida N, Fukuda Y, Komeda T, Kita R, Sando T, Furukawa M, et al. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer research. 1994;54:3107–10. [PubMed] [Google Scholar]
- 45.Yuen MF, Wu PC, Lai VC, Lau JY, Lai CL. Expression of c-Myc, c-Fos, and c-jun in hepatocellular carcinoma. Cancer. 2001;91:106–12. doi: 10.1002/1097-0142(20010101)91:1<106::aid-cncr14>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Thorgeirsson SS, Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Wu T-T, Hsieh Y-H, Wu C-C, Hsieh Y-S, Huang C-Y, Liu J-Y. Overexpression of protein kinase C alpha mRNA in human hepatocellular carcinoma: a potential marker of disease prognosis. Clin Chim Acta. 2007;382:54–8. doi: 10.1016/j.cca.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Wu T-T, Hsieh Y-H, Hsieh Y-S, Liu J-Y. Reduction of PKC alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. Journal of cellular biochemistry. 2008;103:9–20. doi: 10.1002/jcb.21378. [DOI] [PubMed] [Google Scholar]
- 49.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haussinger D, Schliess F. Glutamine metabolism and signaling in the liver. Frontiers in bioscience : a journal and virtual library. 2007;12:371–91. doi: 10.2741/2070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.