Abstract
Purpose
Immunotherapy using vaccines or adoptively transferred tumor infiltrating lymphocytes (TILs) is limited by T cell functional inactivation within the solid tumor microenvironment. The purpose of this study was to determine if a similar tumor-induced inhibition occurred with genetically-modified cytotoxic T cells expressing chimeric antibody receptors (CARs) targeting tumor-associated-antigens.
Methods
Human T cells expressing CAR targeting mesothelin or fibroblast activation protein and containing CD3ζ and 4-1BB cytoplasmic domains were intravenously injected into immunodeficient mice bearing large, established human mesothelin- expressing flank tumors. CAR TILs were isolated from tumors at various time points and evaluated for effector functions and status of inhibitory pathways.
Results
CAR T cells were able to traffic into tumors with varying efficiency and proliferate. They were able to slow tumor growth, but did not cause regressions or cures. The CAR TILs underwent rapid loss of functional activity that limited their therapeutic efficacy. This hypofunction was reversible when the T cells were isolated away from the tumor. The cause of the hypofunction appeared to be multifactorial and was associated with upregulation of intrinsic T cell inhibitory enzymes (diacylglycerol kinase and SHP-1) and the expression of surface inhibitory receptors (PD-1, LAG3, TIM3, 2B4).
Conclusions
Advanced generation human CAR T cells are reversibly inactivated within the solid tumor microenvironment of some tumors by multiple mechanisms. The model described here will be an important tool for testing T cell-based strategies or systemic approaches to overcome this tumor-induced inhibition. Our results suggest that PD-1 pathway antagonism may augment human CAR T cell function.
Introduction
Adoptive T cell transfer (ACT) is a form of immunotherapy that has demonstrated increasing promise as a therapeutic option for cancer. 1–3 ACT using cytotoxic T cells that have been genetically modified to express a chimeric antibody receptor (CAR) specifically targeting a tumor-associated-antigen (TAA) or a cancer stromal antigen offers the advantages of specific, high-affinity binding of target cells in a major histocompatibility class (MHC)-independent fashion, optimization of T cell activation via incorporation of different internal co-stimulatory domains (so called “advanced generation” CARs), and relatively straightforward and efficient ex vivo preparation. 4
Recently, some dramatic tumor regressions in patients with hematologic malignancies using CARs targeting the B cell antigen CD19 have been reported.3 This has spurred a growing interest in using this approach for a variety of solid tumors.5, 6 However, if CAR T cells behave similarly to endogenous T cells (or to ex vivo expanded tumor infiltrating lymphocytes 7–10), it is likely that the efficacy of the infused T cells will be limited by a number of factors including: 1) inhibitory effects of tumor-derived cytokines, 2) metabolic challenges (i.e. lack of arginine or tryptophan), 3) a microenvironment characterized by hypoxia and low pH, 4) negative effects of intra-tumoral immune suppressor cells. 5, 6, 11–13, 5) intrinsic inhibitory pathways mediated by up regulated inhibitory receptors reacting with their cognate ligands within the tumor 14, 15 and 6) intracellular inhibitory pathways that are engaged after T cell activation which function to inhibit T cell receptor pathways and effector functions. 16 Examples of surface inhibitory receptors on TILs include CTLA4, PD-1, LAG3, 2B4, and TIM3. 17, 18. Examples of upregulated intracellular inhibitors in TILs are phosphatases (i.e. SHP-1 that dephosphorylates TCR kinases such as Lck and ZAP70 ) 19, ubiquitin-ligases (i.e. cbl-b) 20, and kinases (i.e. diacylglycerol kinase (DGK) which inactivates diacylglycerol) 21
Because advanced generation CAR T cells have intrinsic co-stimulatory activity (i.e. cytoplasmic domains from CD28 and/or 4-1BB (CD137)), it is possible that they are more resistant to these inhibitory forces. For example, there is data supporting the ability of 4-1BB co-stimulation to blunt the anergy response 22–24. However, there is no data studying the same protective ability of 4-1BB in CAR -modified T cells. Furthermore, a significant portion of this data was from research in murine T cells. 23, 25 The purpose of this study was to develop a model where suppression of T cell function using advanced generation human CAR T cells could be studied.
Materials and Methods
Generation of mesoCAR construct and lentivirus vector preparation
The single chain Fv domain of the anti-mesothelin antibody (scFv SS1), originally provided by Dr. Ira Pastan 26, was previously subcloned into the lentiviral vector pELNS bearing the EF1α promoter and incorporated the CD3ζ and 4-1BB intracellular T cell receptor (TCR) signaling domains 27. A variant of the mesoCAR construct incorporating a myc-tag between the scFv SS1 and the CD8 hinge was generated to allow for clearer detection of surface mesoCAR expression on TILs harvested from mouse flank tumors. Construction of a similar CAR, but targeting murine fibroblast activation protein (FAP), has been described previously. 28
Cell lines
For mesoCAR studies a human mesothelioma cell line derived from a patient’s tumor was used – EMP (parental). Since EMP did not have baseline expression of the tumor-associated antigen (TAA) mesothelin, it was transduced with a lentivirus to stably express human mesothelin (the transduced cell line was named EMMESO). Mesothelin expression level is shown in Supplemental Figure 1.
Mouse 3T3Balb/C cells (3T3p) were purchased from the American Type Culture Collection. Mouse FAP-expressing 3T3Balb/C cells (3T3mFAP) were created by lentiviral transduction of the parental line with murine FAP. 28
All lines were also transduced to stably express firefly luciferase (called EMPffluc, EMMESOffluc, 3T3p-ffluc, and 3T3mFAP-ffluc). The culture conditions are described in Supplemental Methods.
Isolation, bead activation, transduction, expansion of primary human T lymphocytes, and T cell effector assays
These protocols are described in the Supplemental Methods.
Animals
All animal experiment protocols were approved and conducted in accordance with the Institutional Animal Care and Use Committee. NOD/scid/IL2rγ −/− (NSG) mice were bred in the Animal Services Unit of the Wistar Institute and Children’s Hospital of Philadelphia. Female mice were used for experiments at 10 to 16 weeks of age.
In Vivo Xenograft Experiments
5 × 106 EMMESO tumor cells were injected in the flanks of NSG mice in a solution of X-Vivo media (Lonza) and Matrigel (BD Biosciences). After tumors were established (100-200mm3), the mice were randomly assigned to one of three intravenous (tail-vein) treatment groups: 1) 20 × 106 non-transduced (NT) T cells (Dynabead® activated T cells), 2) 20 × 106 mesoCAR T cells (Dynabead® activated T cells transduced with mesoCAR) 3) saline. Tumors were measured using calipers and tumor volumes were calculated using the formula . An additional experiment was performed where mice bearing EMMESO flank tumors were injected with 20 × 106 FAPCAR T cells. FAP is highly expressed on the tumor fibroblasts which make up about 6.3% of the digested EMMESO tumors.
At different time points, tumors were harvested, microdissected, and digested in a solution of 1:2 DNAse:Collagenase with rotation at 37°C for one hour. Digested tumors were then filtered through 70um nylon mesh cell strainers, and red blood cells were lysed if needed (Pharm Lyse-BD Biosciences). After single cell suspensions were achieved,1 × 106 cells were placed in standard FACS tubes and were stained with fluorochrome conjugated anti-human CD45 or CD3 antibodies.
Groups contained 10 mice each. The in vivo experiments were repeated three times in independent fashion.
Ex vivo TIL analysis
After digestion, TILs were isolated by using an anti-human CD45 PE antibody (BD Biosciences) with the EasySEP PE Selection Kit (StemCell Technologies #18551). Once isolated, TILs were analyzed in three different ways: 1) luciferase-based killing assays, 2) intracellular cytokine expression, and/or 3) measurement of antigen-induced T cell IFNγ secretion (refer to supplemental methods for detailed protocols).
Antibodies
Refer to Supplemental Methods.
Inhibitors
The SHP-1 inhibitor sodium stibogluconate (SSG) was purchased from EMD Millipore (567565). Two different inhibitors of DGK were purchased from Sigma (DGKinh1 (nonspecific inhibitor), D5919; DGKinh2 (alpha isoform specific), D5794). Functional antibody against PD-L1 was a generous gift from Dr. Gordan Freeman. 29 Human IL2 (Proleukin, Prometheus) was acquired through the Hospital of the University of Pennsylvania pharmacy. Dose response curves were performed for both DGK inhibitors and for SSG, and the highest doses which did not induce direct tumor cell killing were used. 1uM of both DGK inhibitors and 25ug/ml of SSG were also demonstrated to be appropriate in other published investigations. 30, 31 All inhibitory studies were done twice in independent fashion with comparable results.
Immunoblotting
Lysates of T cells (40 ug) before and/or after activation with beads were run on SDS-PAGE gels, transferred and immunoblotted using standard approaches. Primary and secondary antibodies used are described in Supplemental Methods.
Statistical Analysis
All results were expressed as means +/− SEM as indicated. For studies comparing two groups, the student t test was used. For comparisons of more than two groups, we used one-way ANOVA with appropriate post hoc testing. Differences were considered significant when p <0.05.
Results
Intravenous injection of human mesoCAR T cells slows but does not eradicate human tumors
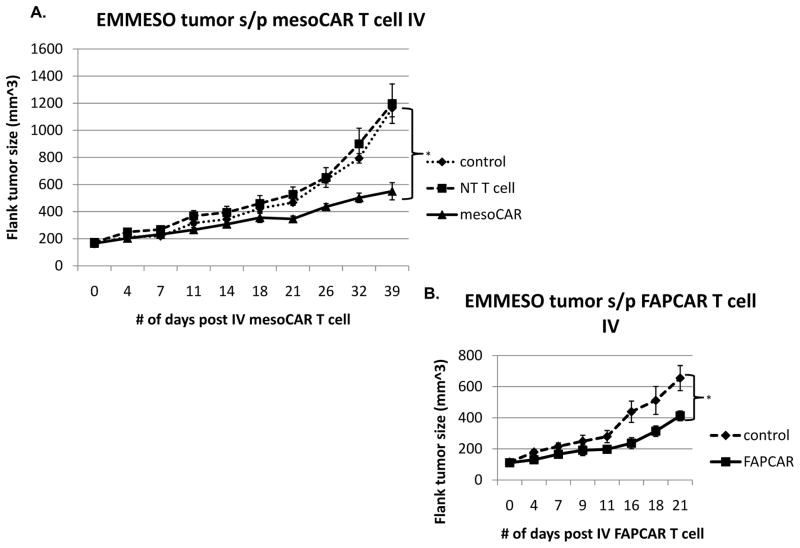
The human mesothelin-expressing mesothelioma tumor cell line EMMESO was injected into the flanks of NSG mice and allowed to grow to a size between 100 and 200 mm3. At that time, tumor- bearing mice were given one intravenous injection of 20 million T cells (mesoCAR expression was approximately 50% (data not shown)). Significant tumor growth slowing was seen after a delay 14 days (Fig 1A), however, unlike our experience with another mesothelioma cell line 27, 32, no tumor regression or cures were noted. Injection of non-transduced T cells had minimal anti-tumor effects when compared to the saline treated control (Fig 1A), indicating that the reduction in tumor growth observed in animals treated with T cells expressing mesoCAR was specifically a result of the mesoCAR.
Fig 1. CAR T cells slow tumor growth but do not cause regression.
(A) 5x106 EMMESO tumor cells were injected into the flanks of NSG mice. After they grew to ~200 mm3 in size, 20x106 mesoCAR T cells were injected via tail vein and tumors were measured for 39 days post injection. T cells were able to slow growth by day 18 but were unable to eradicate flank tumors. (B) 5x106 EMMESO tumor cells were injected into the flanks of NSG mice. After they grew to ~150 mm3 in size, 20x106 FAPCAR T cells were injected via tail vein and tumors were measured for 41 days post injection. T cells were able to slow growth by day 11 but were unable to eradicate flank tumors (* p<0.05).
Human mesoCAR T cells traffic into tumors and proliferate
To understand why we did not see tumor regression, we first evaluated whether our tumor antigen had been lost. Interestingly, as immunohistochemistry of EMMESO tumors at 40 days after T cell injection showed robust and uniform expression of mesothelin. (Supplemental Methods and Supplemental Fig 2).
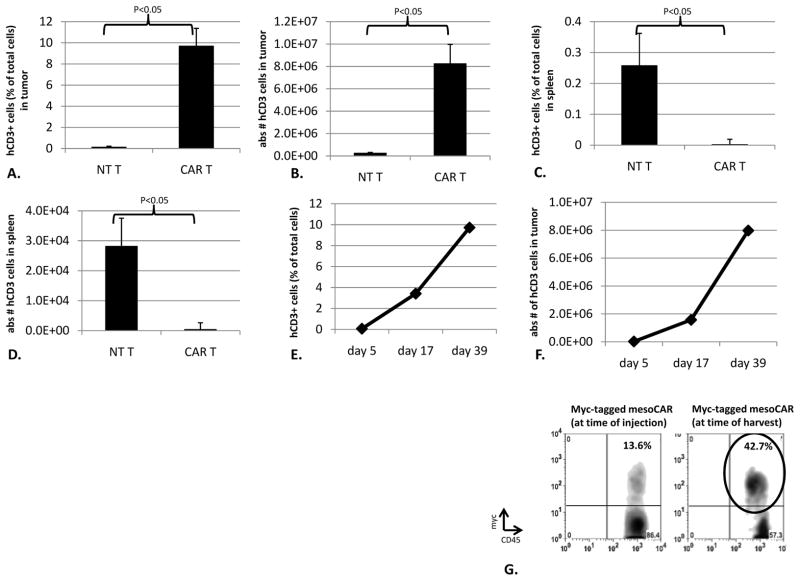
We next evaluated the number and phenotype of the human T cells approximately 40 days after CAR T cell injections by removing and digesting the tumors and spleens and identifying human CD3+ cells by flow cytometry. In EMMESO tumors from mice injected with mesoCAR T cells, we noted that approximately 10% of the digested cells (8.2 × 106) were human T cells. In contrast, in EMMESO tumors injected with non-transduced T cells, only 0.1% (2.5x105) of the digest was human T cells (Fig 2A and 2B). In contrast to our findings in the tumors, CAR T cell persistence was much lower than NT T cells in spleens. (Fig 2C and 2D). We also examined tumors at earlier time points (Fig 2E and 2F). The percentage and number of intratumoral CAR T cells increased steadily over time, starting from very small numbers detectable at Day 5.
Fig 2. CAR T cells infiltrate, survive, proliferate, and retain transgene expression in tumors.
39 days after tail vein T cell injection, flank tumors were harvested and digested and the quantity of human CD3+ cells were assessed. The percentage (A) and absolute number (B) of intra-tumoral CAR T cells was much higher than non-transduced T cells. (9.7% (8.2x106) CAR T cells vs. 0.16% (2.5x105) NT T cells (p<0.05)) The percentage (C) and absolute number (D) of intra-splenic CAR T cells was much lower than non-transduced T cells (<0.05% (3.89x102) CAR T cells vs. 0.25% (2.82x104) NT T cells (p<0.05)). The percentage (E) and absolute number (F) of intra-tumoral CAR T cells was also assessed at earlier time points and demonstrated a marked increase over time. (0.07% (2.16x104) in the first week after T cell injection to 9.7% (7.98x106) in the 6th week after T cell injection. (G) When myc-tagged mesoCAR TILs were isolated from flank tumors 40 days after injection, they retained surface expression of myc-tagged mesoCAR after infiltration into tumors. Percentage of Myc-tag expressing T cells after infiltration was actually higher than at the time of injection (42.7% vs. 13.6%)
Tumor infiltrating human mesoCAR T cells continue to express CAR receptors on their surface
To evaluate loss or downregulation of the surface expression of the CARs, we injected mice bearing EMMESO tumors with T cells expressing a CAR that had been engineered to express a myc-tag in the extracellular domain. CAR expression (using an anti-myc antibody) on human T cells from tumors 40 days after injection were compared to the expression on CAR T cells that had been originally injected (Fig 2G). In this experiment, the percentage of TILs expressing CAR on their surface increased to more than 42% compared to 13% at the time of injection.
Tumor-infiltrating human CAR T cells become hypofunctional
The data above suggested that although the human T cells were present in large numbers they had become hypofunctional. Given that the level of CAR expression on the CAR TILs was equal to or greater than that of the cells prior to injection (Fig 2G), we compared their functional activity. We isolated and analyzed the mesoCAR TILs from EMMESO tumors 40 days after injection (all studies were started immediately after isolation) and compared them to the same batch of mesoCAR T cells that had been used for the original injection and frozen away (“cryo mesoCAR”). These cells were studied after thawing and incubating in 37°C/5% CO2 for 18hrs to mirror handling prior to injection.
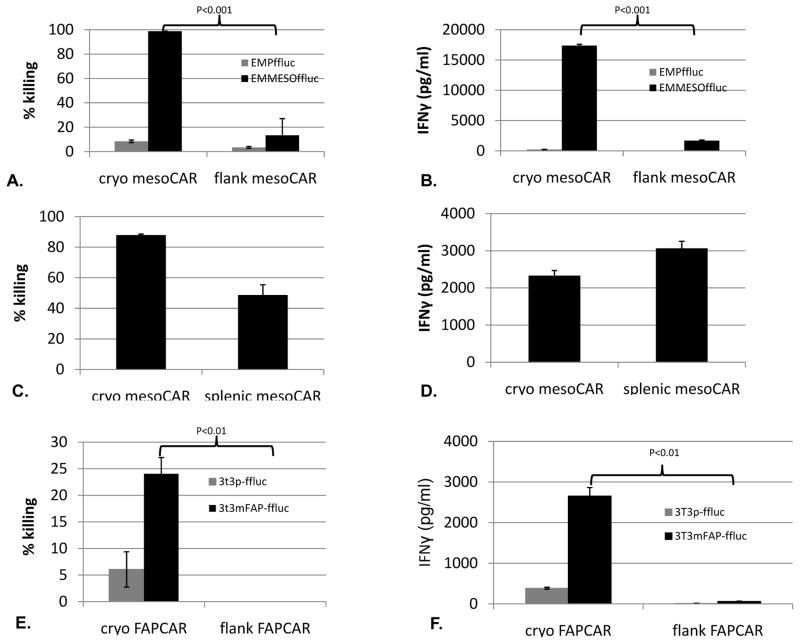
When we added cryo mesoCAR T cells and flank mesoCAR TILs to cultured EMMESO cells expressing firefly luciferase (EMMESOffluc) at a 20:1 ratio for 18 hours, the cryo mesoCAR T cells were highly efficient in killing tumor cells (>95%), while the mesoCAR TILs killed only about 10% (Fig 3A, p<0.001). Similarly, whereas cryo mesoCAR T cells released large amounts of IFNγ into the supernatant, the mesoCAR-TIL secreted very little (Fig 3B, p<0.001). Neither CAR TILs nor cryo mesoCAR T cells had significant anti-tumor activity against EMPffluc demonstrating that the response was specific to mesothelin TAA. The ex vivo killing ability of mesoCAR T cells isolated from the spleens of the same tumor-bearing mice demonstrated much less loss of function than the T cells isolated from the tumor suggesting these effects were specific to the tumor microenvironment. (Fig 3C and 3D).
Fig 3. CAR T cells undergo tumor-induced hypofunction of cytolytic and cytokine secretion ability.
(A) Cytotoxicity: Flank mesoCAR TILs from Day 39 demonstrated hypofunctional killing of EMMESOffluc compared to cryo mesoCAR T cell controls (98.7% vs. 12.3%, p<0.001). TILs/T cells were cocultured with firefly luciferase expressing EMMESO tumors at 20:1 E:T ratio for 18 hours. Measurement of luciferase activity of remaining tumor cells was used to calculate % killing. B. IFNγ secretion: Flank mesoCAR TILs demonstrate hypofunctional secretion of IFNγ in response to EMMESOffluc tumor compared to cryo mesoCAR T cell controls (17,387pg/ml vs. 1689pg/ml, p<0.001). Coculture was performed at a ratio of 20:1 (effector to target) for 18 hours in 37°C, 5%CO2. EMPffluc targets were also tested to confirm antigen-specific tumor killing ability of both the cryo mesoCAR T cells and the mesoCAR TILs. (C) Isolated spleen infiltrated T cells demonstrated much less loss of tumor killing function as compared to TILs (A) and (D) much less loss of tumor induced IFNγ secretion as compared to TILs (B). (E) Flank FAPCAR TILs from Day 28 demonstrated hypofunctional killing of 3T3mFAP-ffluc compared to cryo FAPCAR T cell controls (24% vs. 0%, p<0.01). TILs/T cells were cocultured with firefly luciferase expressing 3T3p and 3T3mFAP tumors at 10:1 E:T ratio for 18 hours. Measurement of luciferase activity of remaining tumor cells was used to calculate % killing. (F) IFNγ secretion: Flank FAPCAR TILs demonstrate hypofunction secretion of IFNγ in response to 3T3mFAP-ffluc tumor compared to cryo FAPCAR T cell controls (2653.7pg/ml vs. 60.85pg/ml, p<0.01).
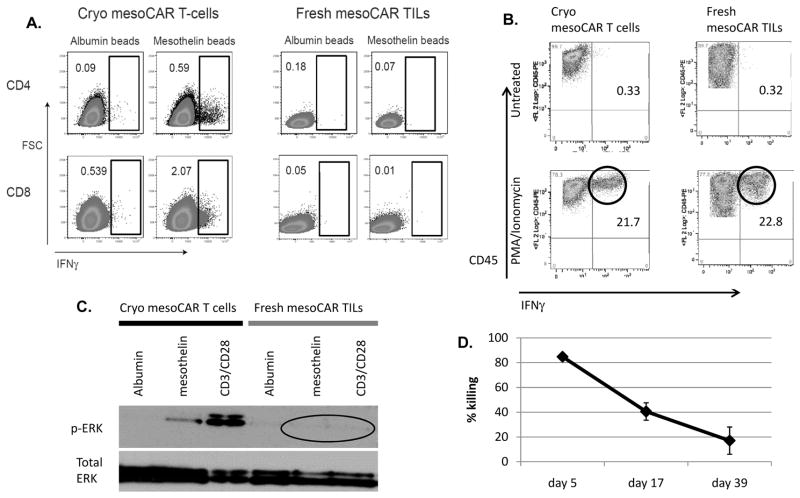
We next examined the ability of the CAR T cells and CAR TILs to produce cytokines (using intracellular cytokine staining by flow cytometry) (Fig 4A). When exposed to albumin-coated beads (control), neither the cryo mesoCAR T cells nor fresh mesoCAR TILs (CD4 and CD8 cells) produced IFNγ. After exposure to mesothelin-coated beads, a clear subpopulation of CD4 (0.59%) and CD8 (2.07%) cryo mesoCAR T cells made IFNγ (Fig 4A, left panels). In contrast, the mesoCAR TILs did not produce IFNγ after exposure to their surrogate antigen (Fig 4A, right panels) despite having relatively high expression of mesoCAR on the surface (Fig 2G). We saw very similar responses with IL-2 production (Supplemental Fig 3). We next exposed the same cells to PMA/Ionomycin (Fig 4B). Approximately 20% of the freshly thawed mesoCAR T cells made IFNγ (Fig 4B, lower left panel). In contrast to the results with the beads, a similar percentage of the mesoCAR TILs (22%) produced IFNγ after PMA/ionomycin (Fig 4B, lower right panel).
Fig 4. CAR TIL hypofunction progresses rapidly after injection and may be due to defects in proximal signaling.
(A) Upon exposure to either albumin-labeled beads (negative control) or mesothelin-labeled beads, the mesoCAR TILs were found to be hypofunctional in their ability to produce IFNγ in response to the antigen-coated beads. (B) Upon exposure to 0.1ug/ml PMA and 2ug/ml ionomycin, the mesoCAR TILs demonstrated preserved ability to produce IFNγ as measured by intracellular cytokine staining by flow cytometry. (C) Upon exposure to either albumin labeled beads (negative control), mesothelin labeled beads, or CD3/CD28 beads (positive control) for 15 minutes, mesoCAR TILs were found to be hypofunctional in their ability to produce signal via phosphorylation of ERK in response to antigen or CD3/28 stimuli. (D) When flank tumors were harvested at early (day 5), mid (day 17), and late (day 39) timepoints, isolated EMMESO TILs were cocultured with firefly luciferase expressing EMMESO tumor at 20:1 E:T ratio. After 18 hours, % killing was calculated after measuring luciferase activity of the remaining tumor cells. We demonstrated that EMMESO TILs undergo rapidly increasing tumor-induced hypofunction after IV injection .
We also assessed the ability of the T cells to signal by assessing phospho-ERK expression using immunoblotting 20 minutes after exposure to beads (Fig 4C). Phospho-ERK expression was minimal in both types of CAR T cells after exposure to control beads. Cryo mesoCAR T cells exhibited ERK activation via phosphorylation after exposure to both mesothelin and anti-CD3/CD28-coated beads (Fig 4C - left panel). In contrast, no phospho-ERK was detected in the mesoCAR TILs after exposure to mesothelin or anti-CD3/CD28-coated beads (Fig 4C - right panel).
It was also of interest to determine the kinetics of inducing T cell hypofunction. MesoCAR TILs were thus isolated from tumors at 5 days, 17 days, and 39 days after injection and their ability to kill tumor cells ex vivo was determined (Fig 4D). MesoCAR TILs isolated at Day 5 were still highly active, killing 85% of EMMESO tumor cells as assessed by our in vitro killing assay. In contrast, mesoCAR TILs isolated on days 17 and 39 had progressive hypofunction.
The hypofunction seen in human mesoCAR TILs is reversible
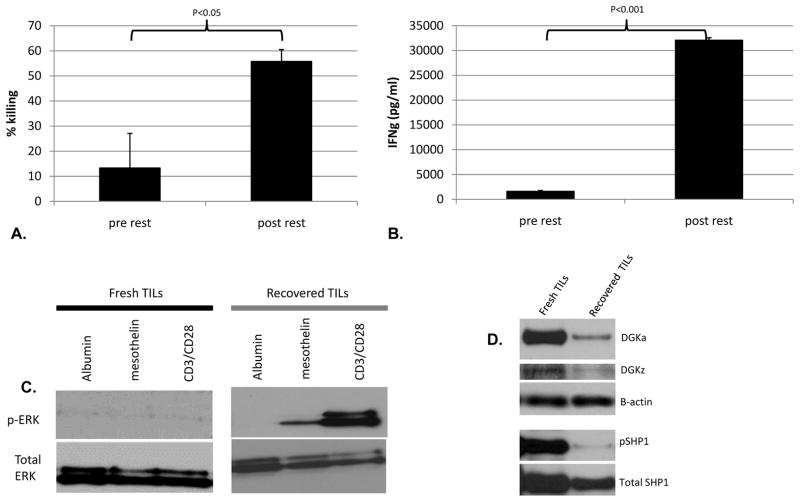
To determine if the hypofunction was reversible, mesoCAR TILs isolated from Day 39 tumors were “rested” for 24 hours away from the tumor in either media alone or media plus low dose IL-2 and then had their effector functions assessed. As shown in Figures 5A and 5B, substantial recovery of killing ability and IFNγ release was seen after 24 hours away from the tumor. The presence of IL-2 in the resting media accelerated recovery, but was not required (Supplemental Fig 4). We saw a similar recovery in the ability to produce cytokines (at the single cell level using flow cytometry) for IFNγ and TNFα in response to EMMESO tumor (Supplemental Fig 5).
Fig 5. CAR TIL cytolytic and cytokine secreting functions recover after rest away from tumor. DGK and pSHP1 expression is increased in hypofunctional CAR TILs.
Human TILs isolated from flank EMMESO tumors at Day 39 and “rested” away from tumor for 24 hours demonstrated significant recovery in their (A) EMMESOffluc tumor killing ability and (B) their ability to secrete IFNγ in response to EMMESOffluc tumor. (C) Compared to freshly harvested EMMESO TILs, resting away from tumor led to recovery of antigen-specific signaling function of EMMESO TILs as measured by detection of phosphorylated ERK. (D) DGKa and DGKz and pSHP1 significantly elevated in TILs freshly isolated from EMMESO. Expression of both isoforms of DGK as well as pSHP1 decreased dramatically after over night rest of TILs.
The ability to phosphorylate ERK after surrogate antigen stimulation or ligation of the endogenous TCR was also restored after 24 hours of “rest” (Fig 5C). Thus a profound but reversible functional impairment exists in human CAR TILs in mice with progressive tumor growth.
Human mesoCAR TILs express increased levels of inhibitory receptors
We next evaluated the expression of four inhibitory receptors (IR), that have been previously described in hypofunctional TILs isolated from humans, using flow cytometry on: i) the cryo mesoCAR T cells that were used for injection, ii) freshly isolated mesoCAR TILs from EMMESO tumor at Day 39, and iii) “recovered” mesoCAR TILs that had been removed from EMMESO tumor and “rested” 24 hours (Supplemental Table 1). CAR TILs expressed high levels of inhibitory receptors. These levels were generally much lower after 24 hours of recovery away from the tumor microenvironment. For the CD4 CAR TILs, PD-1 went from 73% to 53%, LAG-3 went from 63% to 3%, and TIM3 went from 24% to 1%. 2B4 expression was high and remained elevated after rest (67% to 88%). For the CD8 CAR TILs, PD-1 went from 26% to 21%, LAG-3 went from 48% to 13%, and TIM3 went from 56% to 1%. 2B4 expression was high and remained elevated after rest (96% to 98%).
We also evaluated three of these IR’s on the human T cells that could be isolated from the spleens of the EMMESO mice. Interestingly, the expression levels of PD-1, TIM3, and LAG3 were all lower on the splenic T cells compared with the TILs (Supplemental Table 2), supporting the hypothesis that the tumor microenvironment induces the upregulation of IR’s.
Human mesoCAR TILs express increased levels of intracellular inhibitory enzymes
We also explored the expression levels of two intrinsic inhibitors of T cell function that have been implicated in TIL dysfunction, SHP-1 and DGK, using immunoblotting (Fig 5D). The levels of both isoforms of DGK (alpha and zeta), as well as the phosphorylated form of SHP1 (pSHP1), were significantly elevated in mesoCAR TILs that were freshly isolated from EMMESO flank tumor compared to rested TILs. This was also confirmed for DGKα using flow cytometry where 23% of fresh EMMESO TILs expressed DGKα . Expression was undetectable after overnight rest (data not shown).
Blockade of inhibitors in human mesoCAR T cells enhances their ex vivo killing function
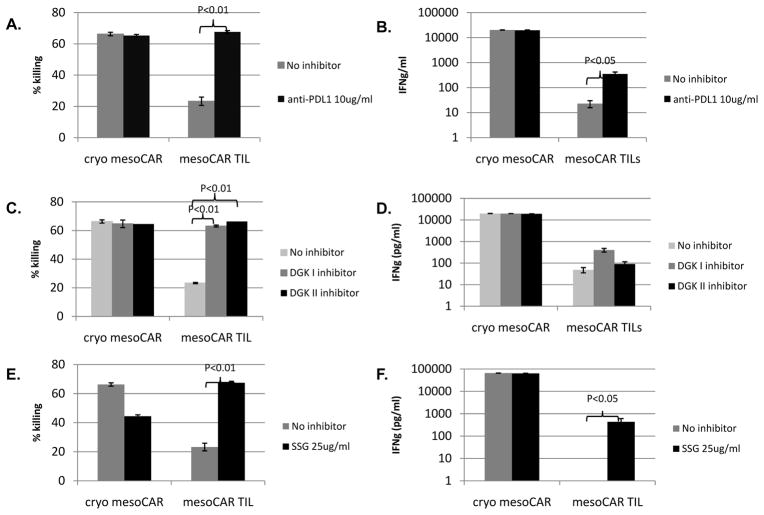
Given these expression data, we studied the potential functional importance of specific inhibitory pathways in mesoCAR TILs by introducing available blocking agents during the ex vivo killing and cytokine release assays. Addition of an anti-PDL1 antibody significantly restored the killing activity and ability to secrete IFNγ by the mesoCAR TILs (Fig 6A, 6B). The relatively high dose of 10μg/ml anti-PDL1 antibody was based on previously published investigations in cancer immunotherapy. 33 Addition of either a type I or type II DGK inhibitor also significantly increased the killing ability (Fig 6C), but without significantly increasing tumor-induced IFNγ secretion (Fig 6D). Addition of the SHP1 inhibitor, SSG, slightly inhibited the killing ability of cryo mesoCAR T cells, but significantly increased that of the mesoCAR TILs (Fig 6E), as well as significantly increasing tumor-induced IFNγ secretion (Fig 6F).
Fig 6. PDL1 blockade and DGK inhibition are able to restore CAR TIL function.
10ug/ml of anti-PDL1 antibody was added to the co-culture killing assay and was able to restore (A) mesoCAR TIL killing of EMMESOffluc (B) and tumor-induced mesoCAR TIL secretion of IFNg after fresh isolation from flank tumor. (C) 1uM of type I and II DGK inhibitor was added to the co-culture killing assay and was able to restore mesoCAR TIL killing of EMMESOffluc with (D) minimal increase in tumor-induced mesoCAR TIL secretion of IFNg after fresh isolation from flank tumor. 25ug/ml of SSG was added to the co-culture killing assay and was able to restore (E) mesoCAR TIL killing of EMMESOffluc (F) and tumor-induced mesoCAR TIL secretion of IFNg after fresh isolation from flank tumor.
Tumor-infiltrating human T cells against other tumors and other tumor-associated antigens also become hypofunctional
To investigate the generalizability our findings, we studied mesoCAR T cells in an additional human mesothelioma model (M30 cells) and saw virtually identical induction of hypofunction as we did with EMMESO (data not shown). To demonstrate that these effects were independent of the specific TAA, we evaluated T cells modified with a CAR directed against murine fibroblast activating protein (FAP), which is expressed on the mouse fibroblasts in the stroma formed in the EMMESO flank tumors. Similar to our findings using murine T cells and mouse tumors 28, injection of 107 FAPCAR T cells significantly slowed the growth of EMMESO tumors, but did not eradicate them by Day 41 after injection (Fig 1B). FAPCAR TILs isolated from flank tumors at Day 28 post injection (by methods described above) also demonstrated profound hypofunction in cytolytic activity and IFNγ secretion similar to that seen in the mesoCAR TILs. (Fig 3E, 3F)
Discussion
Adoptive T cell transfer (ACT) using chimeric antigen receptor-transduced T cells has demonstrated increasing promise as a therapeutic option for cancer, especially in hematopoietic tumors. 3, 34 Given that T cell inactivation has been reported in solid tumors 5, 6, 11, the purpose of this study was to develop a model where this process could be assessed using advanced generation human CAR T cells. We found that a single intravenous injection of human mesoCAR T cells or FAPCAR T cells into immunodeficient mice induced a significant decrease in the tumor growth rate compared to that of controls, but did not result in tumor regressions or cures. By harvesting and analyzing the tumors at multiple time points, we were able to show that this failure to cure the tumors was not due to loss of tumor antigen, loss of the chimeric antigen receptor on the surface of the T cells, nor a failure of the CAR T cells to accumulate within the tumor. Instead, our data shows that loss of anti-tumor efficacy was due to a progressive loss of CAR T cell effector function primarily caused by the tumor microenvironment. This T cell hypofunction is very similar to that described previously in mouse and human TILs 7–9, 18, 35 and in non-genetically modified adoptively transferred T cells in mouse models. 10, 36, 37.
Interestingly, although the CAR TILs isolated from tumors had a profound but reversible functional defect, they accumulated to high numbers in the tumor microenvironment. This suggests that the dysfunctional CAR TILs survive and retain proliferative capacity, and is consistent with the hypothesis that the 4-1BB cytoplasmic domain in the CAR supports T cell survival, but does not protect T cells from hypofunction. Our animal data, thus, predicts that even though human CAR T cells may accumulate within solid tumors in patients, their efficacy may be limited by a progressive inactivation within certain solid tumors. The relative kinetics of CAR T cell accumulation versus their rate of inactivation within tumors will ultimately determine the overall anti- tumor efficacy, and this balance will likely be tumor-specific.
Given these observations, we evaluated the nature of this tumor-induced CAR T cell hypofunction. One feature was that the hypofunction was rapidly and almost fully reversible when the CAR TILs were removed from the tumor microenvironment. Although supplemental IL-2 accelerated the recovery, it was not necessary. We believe this reversibility was due to a general recovery of T cell function due to the removal of inhibitory factors that reside in the tumor microenvironment. However, it is formally possible that our observations were due to a selection and expansion of the most functional TILs, with a concomitant loss of the hypofunctional TILs during the rest period. This possibility is difficult to exclude and may require advanced molecular strategies like “bar-coding” the T cells to track them during the rest period. However, we find the explanation of recovery of anti-tumor function more likely, based on published work from other groups’ investigations 38-40. If one assumes that the CAR TILs are not irreversibly inactivated, it may be best to describe their phenotype as “hypofunctional” rather than “exhausted” or “anergic” which infer a permanent state of dysfunction. 41, 42
A second feature of our hypofunctional CAR TILs was that although TIL hypofunction was evident by 17 days post-injection, tumor volume did not progress dramatically at that point but rather “plateaued.” This was likely due to a balance between progressively decreasing TIL function (Fig. 4D) and increasing number of TILs in the tumor (Fig. 2E) as a result of the proliferative 41BB signaling incorporated into the CAR construct. Potentially, combining strategies that increase the rate or duration of TIL proliferation and decrease TIL hypofunction would widen the “therapeutic sweet spot”, leading to tumor regression.
A third feature of our hypofunctional CAR TILs was that the block appeared to be “proximal” in the T cell signaling pathway. After stimulation by antigen or by CD3/CD28 crosslinking, CAR TILs failed to secrete cytokines or to phosphorylate ERK. CAR TILs also had defects in the phosphorylation of Lck, ZAP-70, and SLP-76 (data not shown). However, when the CAR TILs were exposed to PMA/Ionomycin, bypassing the early TCR activation steps, the cells were fully capable of making cytokines. Although these features of reversibility and defects in proximal signaling have been well documented previously in mouse 17, 43 and to some extent, human TILs 44, 45, the exact mechanisms responsible for this phenotype remain elusive. Both cytoplasmic and cell surface candidates have been implicated. For instance, Rappl et al. elucidated one possible mechanism of terminally-differentiated, late stage T cell hypofunction as being due to impaired TCR synapse formation from immobility of TCR membrane surface components, which can be bypassed by introducing a 1st generation CAR construct that confers tumor reactivity by signaling through normal T cell signaling components. 46 The mechanism of the hypofunction described here seems to be different as demonstrated by dysfunctional proximal T cell signaling. The described in vivo model is inadequate to assess TCR function as it lacks human antigen-presenting cells.
With regard to the mechanisms of hypofunction, we evaluated two intrinsic inhibitory enzymes previously identified in hypofunctional TILs. The first candidate was the phosphatase SHP-1, which can inactivate a number of the kinases in the early TCR signaling cascade. 19, 47, 48 Investigators have shown in mice that TILs have elevated SHP-1 activity and that inhibition of SHP-1 in non-lytic TILs in vitro restored their tumor-cytolytic ability. 43. Conversely, mice deficient in SHP-1 showed increased effector T cell activity. 48 The second candidate was diacylglycerol kinase (DGK), a key enzyme that inactivates diacylglycerol (DAG), a downstream messenger necessary for translating the TCR signal into T cell stimulation. 21, 44, 49 Loss of DGK leads to resistance to T cell anergy and increased CD8 T cell function. 21, 44, 49 Our group has recently shown that loss of the alpha or zeta isoform of DGK augments murine and human CAR T cell effector function. 31, 50 Both SHP-1 and DGK have been shown to be upregulated in hypofunctional TILs. 21, 44, 49, 51.
Our data support the importance of both SHP-1 and DGK in the induction of hypofunction of the human CAR TILs. First, CAR TILs had high expression of both SHP-1 and DGK, which rapidly declined 24 hours after the TILs were removed from the tumors. Second, when we blocked DGK or SHP-1 activity using chemical inhibitors in our ex vivo killing assay, we were able to reduce the defects in tumor killing, with lesser effects on IFNγ secretion. Consistent with these data, Prinz et. al. demonstrated increased CD8 activity in human TILs from renal cell cancers after blocking DGK. 44 The factors within the tumor microenvironment that upregulate these inhibitory enzymes, as well as approaches to inhibit these enzymes in vivo, are active areas of research in our lab. In addition to cytoplasmic inhibitory pathways, it has become increasingly recognized that expression of some cell surface inhibitory receptors on T cells can induce dysfunction. 18 Overall, we observed a similar phenotype as that described by previous studies in human TILs, with increased expression of PD-1, TIM3, Lag3, and 2B4. 52-55 Interestingly, our data suggest that the tumor microenvironment appeared to play an important role in “shaping” the expression pattern of specific IRs on the T cells. First, the expression of IRs was much higher on TILs, rather than on human T cells isolated from spleens of the same animals. Second, after overnight “rest” away from the tumor microenvironment, expression levels of most of these receptors decreased dramatically. Finally, we were able to test the functional significance of the PD1/PD-L1 interaction in the EMMESO model. Addition of a blocking PD-L1 antibody to our ex vivo CAR TIL killing assay was able to restore the defect in tumor cell killing.
We believe the model that we have described here has many potential advantages and possible uses. First, it allows study of human (not mouse) CAR T cells. This is important as we have found the behavior of human CAR T cells to be very different that of murine CAR T cells with regard to key parameters such as persistence, sensitivity to activation-induced cell death, and the activation state at the time of injection. Furthermore, it allows the study of CAR T cells that are prepared identically to those being injected into patients. This should enhance the generalizability of the findings to clinical trials. Second, the use of a uniform preparation of CAR T cells prepared from one donor allows one to focus on microenvironmental differences rather than intrinsic T cell variability. Third, the use of CAR T cells targeted to specific targets allows the study of antigen-specific T cell interactions rather than just general TCR activation induced by CD3/CD28 beads or PMA/Ionomycin stimulation, as has been used in most studies of human TILs. Fourth, the model uses well-characterized human solid tumor cells that can be genetically modified, if needed. This allows the systematic study of different tumor microenvironments. Although we have used flank tumors for convenience, orthotopic tumor cell placement is possible (and probably desirable). Most importantly, the CAR T cells studied in the model seem to very closely resemble naturally occurring human TILs in all of our assays. Finally, the CAR T cell hypofunction in this model seems to be generalizable across different tumors and CARs recognizing different targets. The in vivo experiment detailed above was repeated using other MPM cell lines in the NSG xenograft model. After a single intravenous dose of mesoCAR T cells in NSG mice bearing flank tumors from another human MPM cell line (M30), we observed slowing of tumor growth (with no cures), and the mesoCAR TILs also demonstrated tumor-induced hypofunction that improved with overnight rest away from tumor. The hypofunction was also demonstrated in T cells redirected against a non-mesothelin antigen (mFAP) after infiltration in our NSG xenograft model. Whether the immunosuppression occurred due to EMMESO tumor or the murine fibroblasts within the tumor stroma will be investigated. Other groups have demonstrated tumor associated fibroblasts also play a significant immunosuppressive role. 56
Limitations of the pre-clinical model should also be acknowledged. The NSG mice have a hybrid immune system, thus limiting the ability to study the effects of important tumor immune cells such at T-regulatory cells, NK cells, dendritic cells, B cells, and myeloid cells. The innate immune cells present in NSG mice are not isologous with the human T cells and tumor cells. The use of mice with more fully “humanized” immune systems may be advantageous. Finally, there may be some species incompatibility issues (i.e. some ligands in murine microenvironment might not react with human T cell receptors). Despite these potential problems, the CAR TILs isolated from the tumors do seem to strongly resemble human TILs suggesting that the key interactions that induce T cell hypofunction are present.
In addition to studying mechanisms of tumor-induced T cell hypofunction, models such as ours will allow testing of a wide variety of therapeutic approaches in an in vivo setting where anti-human reagents can be used. One strategy would be systemic administration of agents that might affect the tumor microenvironment. These could include agents like: 1) inhibitors of soluble inhibitory mediators such as TGF-β or PGE2, 2) activating cytokines such as IL-2, IL12, IL-15, or type 1 interferons, 3) small molecular inhibitors of intrinsic inhibitors like DGK and SHP-1, 4) agents that might alter tumor pH or oxidative status, and 5) anti-T cell inhibitory receptor antibodies like anti-PD-1, anti-PDL-1, anti-TIM3, etc. Another promising strategy will be to introduce genetic changes into the human T cells along with the CAR to prevent or modulate hypofunction. This approach would allow highly specific alterations and avoid any systemic toxicity. There are a wide variety of possibilities that include introduction of new gene genes such as cytokines (i.e. IL-12 57 or stimulatory proteins, such as constitutively active AKT 58. It should also be possible to express shRNA, dominant negative constructs, or intracellular antibodies to reduce or block the expression or activities of inhibitory surface receptors (like the TGF-β receptor 59, PD-1 and Tim 3) or intrinsic inhibitors (like DGK and SHP-1). Based on our present findings, we plan to tests anti-PD-1 antagonists with CAR T cells in future clinical trials.
Supplementary Material
Statement of Translational Relevance.
Adoptive cellular immunotherapy (ACT) using T cells genetically modified to express chimeric antigen receptors (CARs) against tumor-associated antigens (TAAs) has shown great promise in the treatment of blood-borne malignant disease, but may be limited by the strong immunosuppressive environment within solid tumors. We present a novel model demonstrating that reversible tumor-induced hypofunction of CAR T cells does occur in solid tumors. This model will be important in understanding the mechanisms of this effect and developing strategies to reduce or eliminate this hypofunction, paving the way for future clinical trials.
Acknowledgments
This work was supported by the NCI (National Cancer Institute) grants (P01 CA 66726-07 / R01 CA 1477-95 /RO1 CA172/ RO1 CA141144/K08 CA 163941-01), a grant from the Cancer Research Institute, Novartis Pharmaceuticals Corporation, and the Lung Cancer Translational Center of Excellence at UPenn
We would like to acknowledge the Human Immunology Core at the University of Pennsylvania for supplying the healthy donor T cells used for the studies in this paper and Albert Lo, Kazim Panjwani, Michael Allegreza and Sanjana Kirloskar for their assistance with some of the experiments.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann Oncol. 2012 Sep 23;(Suppl 8):viii35–40. doi: 10.1093/annonc/mds261. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011 Jul 1;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011 Aug 25;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadelain M, Brentjens R, Riviere I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013 Apr 4; doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med Jul. 2012;18(7):377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG. Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer Immunol Immunother Jul. 2012;61(7):953–962. doi: 10.1007/s00262-012-1254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 8.Mescher MF, Popescu FE, Gerner M, Hammerbeck CD, Curtsinger JM. Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors. Semin Cancer Biol Aug. 2007;17(4):299–308. doi: 10.1016/j.semcancer.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell JD. The induction and maintenance of T cell anergy. Clin Immunol Sep. 2006;120(3):239–246. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Russ AJ, Wentworth L, Xu K, Rakhmilevich A, Seroogy CM, Sondel PM, et al. Suppression of T-cell expansion by melanoma is exerted on resting cells. Ann Surg Oncol Dec. 2011;18(13):3848–3857. doi: 10.1245/s10434-011-1667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol Jul. 2012;33(7):364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol Apr. 2013;25(2):268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Mayer A, Vaupel P. Hypoxia, lactate accumulation, and acidosis: siblings or accomplices driving tumor progression and resistance to therapy? Adv Exp Med Biol. 2013;789:203–209. doi: 10.1007/978-1-4614-7411-1_28. [DOI] [PubMed] [Google Scholar]
- 14.Abate-Daga D, Hanada KI, Davis JL, Yang JC, Rosenberg SA, Morgan RA. Expression profiling of TCR-engineered T cells demonstrates over-expression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013 Jul 16;122(8):1399–1410. doi: 10.1182/blood-2013-04-495531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HJ, Kusnadi A, Lee EJ, Kim WW, Cho BC, Lee IJ, et al. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012 Jul-Aug;278(1–2):76–83. doi: 10.1016/j.cellimm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep Jan. 2008;9(1):50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010 Dec 15;185(12):7133–7140. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013 Jan 5;25(2):214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paling NR, Welham MJ. Role of the protein tyrosine phosphatase SHP-1 (Src homology phosphatase-1) in the regulation of interleukin-3-induced survival, proliferation and signalling. Biochem J. 2002 Dec 15;368(Pt 3):885–894. doi: 10.1042/BJ20021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopewell EL, Zhao W, Fulp WJ, Bronk CC, Lopez AS, Massengill M, et al. Lung tumor NF-kappaB signaling promotes T cell-mediated immune surveillance. J Clin Invest. 2013 Jun 3;123(6):2509–2522. doi: 10.1172/JCI67250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong XP, Olenchock BA, Koretzky GA. The role of diacylglycerol kinases in T cell anergy. Ernst Schering Found Symp Proc. 2007;(3):139–149. [PubMed] [Google Scholar]
- 22.Habib-Agahi M, Phan TT, Searle PF. Co-stimulation with 4-1BB ligand allows extended T-cell proliferation, synergizes with CD80/CD86 and can reactivate anergic T cells. Int Immunol Dec. 2007;19(12):1383–1394. doi: 10.1093/intimm/dxm106. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox RA, Tamada K, Flies DB, Zhu G, Chapoval AI, Blazar BR, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004 Jan 1;103(1):177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Merchant MS, Chua KS, Khanna C, Helman LJ, Telford B, et al. Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther Sep-Oct. 2003;2(5):579–586. doi: 10.4161/cbt.2.5.545. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest Mar. 2002;109(5):651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high-affinity human monoclonal antibody to mesothelin. Int J Cancer. 2011 May 1;128(9):2020–2030. doi: 10.1002/ijc.25557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LS, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting Fibroblast Activation Protein in Tumor Stroma with Chimeric Antigen Receptor T Cells Can Inhibit Tumor Growth and Augment Host Immunity without Severe Toxicity. Cancer Immunol Research. 2013;2(2):154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003 Feb 1;170(3):1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 30.Yi T, Pathak MK, Lindner DJ, Ketterer ME, Farver C, Borden EC. Anticancer activity of sodium stibogluconate in synergy with IFNs. J Immunol. 2002 Nov 15;169(10):5978–5985. doi: 10.4049/jimmunol.169.10.5978. [DOI] [PubMed] [Google Scholar]
- 31.Riese MJ, Wang LC, Moon EK, Joshi RP, Ranganathan A, June CH, et al. Enhanced Effector Responses in Activated CD8+ T Cells Deficient in Diacylglycerol Kinases. Cancer Res. 2013 Jun 15;73(12):3566–3577. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010 Nov 15;70(22):9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010 Apr 1;184(7):3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013 Mar 20;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Mol Med Jun. 2000;6(6):465–479. [PMC free article] [PubMed] [Google Scholar]
- 36.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004 Apr 15;64(8):2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev Oct. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 38.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008 Mar 1;371(9614):771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 39.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010 Sep 27;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB. CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001 Nov 1;167(9):5042–5051. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 41.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol Oct. 2010;22(5):552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ. T cell exhaustion. Nat Immunol Jun. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 43.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007 Dec 1;67(23):11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol. 2012 Jun 15;188(12):5990–6000. doi: 10.4049/jimmunol.1103028. [DOI] [PubMed] [Google Scholar]
- 45.Whiteside TL. Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol Immunother Oct. 1999;48(7):346–352. doi: 10.1007/s002620050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rappl G, Riet T, Awerkiew S, Schmidt A, Hombach AA, Pfister H, et al. The CD3-zeta chimeric antigen receptor overcomes TCR Hypo-responsiveness of human terminal late-stage T cells. PLoS One. 2012;7(1):e30713. doi: 10.1371/journal.pone.0030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004 Jul 15;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 48.Fowler CC, Pao LI, Blattman JN, Greenberg PD. SHP-1 in T cells limits the production of CD8 effector cells without impacting the formation of long-lived central memory cells. J Immunol. 2010 Sep 15;185(6):3256–3267. doi: 10.4049/jimmunol.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanjuan MA, Jones DR, Izquierdo M, Merida I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J Cell Biol. 2001 Apr 2;153(1):207–220. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riese MJ, Grewal J, Das J, Zou T, Patil V, Chakraborty AK, et al. Decreased diacylglycerol metabolism enhances ERK activation and augments CD8+ T cell functional responses. J Biol Chem. 2010 Feb 18;286(7):5254–5265. doi: 10.1074/jbc.M110.171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koneru M, Schaer D, Monu N, Ayala A, Frey AB. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005 Feb 15;174(4):1830–1840. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009 Aug 20;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011 May 15;71(10):3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008 Jun 15;180(12):8159–8167. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 56.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007 May 1;178(9):5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 57.Chmielewski M, Abken H. CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol Immunother Aug. 2012;61(8):1269–1277. doi: 10.1007/s00262-012-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther Nov. 2010;18(11):2006–2017. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iyer S, Wang ZG, Akhtari M, Zhao W, Seth P. Targeting TGFbeta signaling for cancer therapy. Cancer Biol Ther Mar. 2005;4(3):261–266. doi: 10.4161/cbt.4.3.1566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.