Abstract
Neural plasticity is widely believed to support functional recovery following brain damage. Vagus nerve stimulation paired with different forelimb movements causes long-lasting map plasticity in rat primary motor cortex that is specific to the paired movement. We tested the hypothesis that repeatedly pairing vagus nerve stimulation with upper forelimb movements would improve recovery of motor function in a rat model of stroke. Rats were separated into three groups: vagus nerve stimulation during rehab, vagus nerve stimulation after rehab, and rehab alone. Animals underwent 4 training stages: shaping (motor skill learning), pre-lesion training, post-lesion training, and therapeutic training. Rats were given a unilateral ischemic lesion within motor cortex and implanted with a left vagus nerve cuff. Animals were allowed one week of recovery before post-lesion baseline training. During the therapeutic training stage, rats received vagus nerve stimulation paired with each successful trial. All seventeen trained rats demonstrated significant contralateral forelimb impairment when performing a bradykinesia assessment task. Forelimb function was recovered completely to pre-lesion levels when vagus nerve stimulation was delivered during rehab training. Alternatively, intensive rehab training alone (without stimulation) failed to restore function to pre-lesion levels. Delivering the same amount of stimulation after rehab training did not yield improvements compared to rehab alone. These results demonstrate that vagus nerve stimulation repeatedly paired with successful forelimb movements can improve recovery after motor cortex ischemia and may be a viable option for stroke rehabilitation.
Keywords: cortical ischemia, motor cortex, plasticity, recovery, rehabilitation, stroke
Introduction
Stroke is the second most common cause of disability worldwide1. A variety of motor rehabilitation methods have been developed to improve recovery of motor function following stroke2-4. These methods are known to generate significant neural plasticity yet significant deficits typically remain after motor rehabilitation. New approaches are being developed to enhance motor rehabilitation by activating brain mechanisms to direct more effective neural plasticity5-8.
Many studies have attempted to improve stroke recovery by modulating the release of neuromodulators, such as acetylcholine and norepinephrine9,10. Stimulation of the left vagus nerve triggers a precisely timed burst of neuromodulators and enhances neural plasticity11,12. Repeatedly pairing vagus nerve stimulation (VNS) with two distinct forelimb movements resulted in movement-specific map plasticity within primary motor cortex13. VNS is well-tolerated in patients with a variety of neurological diseases11,14 and could be added to motor rehabilitation to improve recovery.
In this study, we evaluated whether the addition of VNS to motor rehabilitation can enhance recovery from cortical ischemia. After training on a motor task that requires rapid forelimb movement15, rats were injected with endothelin-1 into the rostral and caudal forelimb areas of the motor cortex. After documenting the behavioral deficit, a brief burst of VNS was delivered with each successful movement and recovery was compared with rats that received identical rehabilitation without VNS.
Methods
Subjects
Seventeen female Sprague-Dawley rats (276 ± 8 grams) were used in this experiment. The rats were housed in a 12:12 hr reversed light cycle environment to increase their daytime activity levels and were food deprived to no less than 85% of their normal body weight during training as motivation for the food pellet rewards. All handling, housing, surgical procedures, and behavioral training of the rats were approved by the University of Texas Institutional Animal Care and Use Committee.
Surgical Procedures
Unilateral Ischemic Lesion
Animals were anesthetized with ketamine hydrochloride (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and given supplemental doses as needed. Sterile saline (9% NaCl solution, 10 ml total, s.c.) solution was given to the rats before and during the surgery to prevent dehydration throughout the surgery and recovery. Bupivicaine (1 ml, s.c.) was injected into the scalp and subcutaneously in the neck at the incision sites. After placing the rat in a stereotaxic frame with a digital readout (David Kopf Instruments, Tujunga, CA), an initial incision and blunt dissection of the scalp exposed the bregma and lambda landmarks on the skull. A craniotomy was performed to expose motor cortex contralateral to the trained forelimb: anteroposterior 2.75 to −0.75mm and mediolateral 2.25 to 3.75mm in relation to bregma. The ischemic lesion was always provided in left motor cortex since all animals trained with their right forearm. Sterile saline (9% NaCl solution) was used ad libitum to prevent cranial heat accumulation during trephination. A 26-gauge tapered Hamilton syringe was fixed to a stereotaxic guidance arm. To induce ischemia, 1.0 μL of endothelin-1 (Bachem, Torrance, CA, 1 mg/mL in saline) was injected at eight different cortical locations within motor cortex: anteroposterior 2.5, 1.5, 0.5, and −0.5 mm and mediolateral 2.5 and 3.5mm from bregma. The syringe needle tip was lowered to a depth of 1.8 mm from the cortical surface and endothelin-1 was administered. The fluid was injected over a two minute period, and the syringe remained in the brain for an additional 3 minutes to allow tissue perfusion. After the final injection, KwikCast silicone polymer (World Precision Instruments, Sarasota, FL) was placed in the craniotomy and sealed with a thin layer of acrylic.
Vagus Nerve Cuff Implantation
Following ischemic lesion, all rats received a skull-mounted two-channel connector and a bipolar stimulating nerve cuff constructed with platinum-iridium leads (5-6 kΩ impedance). Four bone screws were manually drilled into the skull at points near the lambdoid suture and over the cerebellum. The two-channel connector was attached to the cranial screws with acrylic. An incision and blunt dissection of the muscles in the neck exposed the left cervical vagus nerve. After blunt dissecting the vagus nerve from the carotid artery, the nerve was placed inside the cuff. All rats received a nerve cuff implanted around the left vagus nerve. Leads were tunneled subcutaneously and attached to the two-channel connector atop the skull. All incisions were sutured and the exposed two-channel connector was encapsulated in acrylic. A topical antibiotic cream was applied to both incision sites. As the animal returned to consciousness, a dose of ceftriaxone (20 mg total, s.c.) was administered to help revent infection. Rats were provided with amoxicillin (5 mg) and carprofen (1 mg) in tablet form for three days following the surgeries and had one week of recovery before post-lesion testing.
Behavioral Apparatus and Software
The training cage was a 20 cm × 20 cm × 20 cm wire. An automated pellet dispenser connected to a food tray on the inside of the cage. Rats reached through a 1 cm × 8 cm window slit to reach the lever outside the cage. The edge of the window was located 2 cm from the right cage wall. This arrangement restricted the rats so that they could only press the lever with their right paw. The lever was located 4.5 cm from the cage floor and at lateral distances varying from 4 cm inside to 2.5 cm outside the cage, depending on the training stage. The lever arm was affixed to a momentary switch (Med Associates, St. Albans, VT) located outside the cage. The momentary switch was connected to a digital input on the parallel port of a standard PC and trigger lines to the pellet dispenser and vagus nerve stimulator were connected to digital outputs on the same port. The state of the momentary switch was sampled every 10 ms using custom Matlab software. The software would also trigger the release of food pellet reward and trigger the VNS stimulator to deliver current to the vagus nerve cuff. Press and release times of the lever were automatically saved for offline analysis.
Bradykinesia Assessment Task
The bradykinesia assessment task allows the researcher to accurately collect hit rate performance, inter-press interval, and number of presses per trial15. Training sessions were conducted twice daily, five days a week, with daily sessions separated by at least 2 hours (Fig. 1AB). Rats pressed the lever initially located inside the training cage to receive a sugar pellet reward (45mg dustless precision pellet, BioServ, Frenchtown, NJ). In the first stage of training, rewards were delivered with a single press to facilitate lever-reward association. In the subsequent stages of training, a second press within a specified hit time window was required for reward delivery. A timer was initiated on the first press of the lever, and data was collected for 4 seconds. If the lever was depressed a second time within the hit time window, the trial was recorded as a success and a reward pellet was delivered (Fig. 1DE). If the lever was not pressed again or the second press occurred after the hit time window, the trial was recorded as a failure and no reward was given. Following the 4 second data collection period, there was a 50 millisecond pause before rats could initiate another trial. The task was made progressively more difficult as rats met the criterion for number of successful trials within a session and progressed to the next stage. As the training stages increased, the lever was gradually retracted outside the cage and the hit time window was reduced. The values for criterion, lever location and hit time window for the bradykinesia assessment task are detail in Table 1. If a rat exceeded criteria for a proceeding stage, they were automatically advanced to the stage that matched their performance. Rats were held at the pre-lesion stage until they had 10 successive sessions averaging over 85% success rate. Upon reaching this performance level, rats were given an ischemic lesion. After 7 days of recovery, rats returned to behavioral testing with the same parameters as pre-lesion to allow a direct comparison of performance. To allow for accurate measurements, all rats were tested until they had 4 sessions with greater than 10 trials each at the post-lesion stage. Rats then proceeded to the therapy stage where VNS was delivered on each successful trial (Fig. 1C). The rats continued training with these parameters twice daily in 30 minute sessions for 25 days.
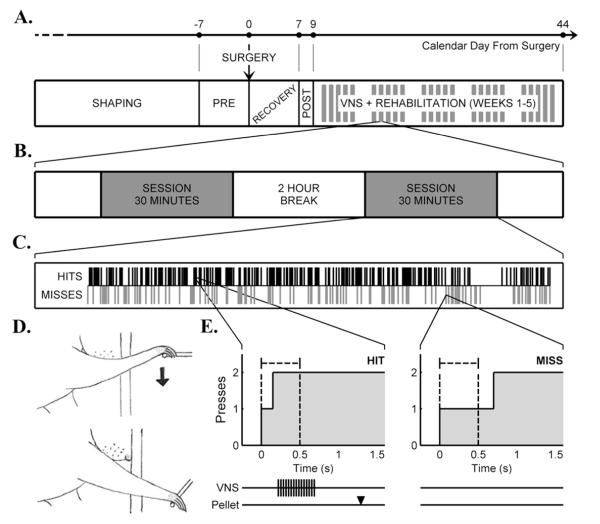
Figure 1.
A)Experimental timeline illustrating shape training, pre-lesion training, surgery, recovery, post-lesion training, and VNS+rehabilitative training. Negative value indicates training days prior to infarct, and positive values are post-infarct days. Clustered thick gray bars indicate that VNS+rehabilitative training occurred 5 days a week (weekdays only) for 25 days. B) A rehabilitative training day was composed of two 30 minute sessions per day with a two hour break between sessions. C) Shown is a representative 30 minute training session. The black bars on top specify hit trials in which VNS was delivered during rehabilitative training. The gray bars on bottom indicate miss trials were the rat failed to achieve the task requirements, no pellet or VNS delivered. D) Sketches demonstrating the movements necessary for the bradykinesia assessment task. A rat was required to press the spring-loaded lever in the downward direction twice within 0.5 s. E) Two examples of lever press data collected from a series of trials performed by a rat that received VNS during rehabilitation. The black dashed lines indicate the hit time window of 0.5 s. In the depicted hit trial, two presses occurred within the hit time window. Below the hit trial example, the repeated black bars demonstrate the VNS pulse train: 0.5 s 30 Hz pulse train at 0.8 mA 100 μs pulse width. The black arrowhead marks when the food pellet arrived in relation to stimulation. On the right, a miss trial occurred when a rat failed to press the lever twice within the dashed lines. No VNS or pellet was delivered.
Table 1. Behavioral training stage parameters.
| Training Stage |
Trial Window* (sec) |
Lever Location** (cm) |
Criterion for stage completion | Average number of sessions to stage completion |
|---|---|---|---|---|
| Stage 1 | N/A | −4.0 | 60 successful completions in 2 consecutive sessions |
10.1 ± 0.7 |
| Stage 2 | 1.0 | −2.0 | 45 successful completions in a single session |
2.4 ± 0.5 |
| Stage 3 | 0.5 | 0.5 | 80 successful completions in a single session |
1.6 ± 0.3 |
| Stage 4 | 0.5 | 1.0 | 80 successful completions in a single session |
2.9 ± 0.5 |
| Stage 5 | 0.5 | 2.0 | 80 successful completions in a single session |
6.8 ± 1.1 |
| Pre-lesion | 0.5 | 2.5 | 10 consecutive sessions averaging 85% success |
19.5 ± 4.0 |
| Post-lesion | 0.5 | 2.5 | 4 sessions of more >10 trials each | 4.0 ± 0.0 |
| Therapy | 0.5 | 2.5 | 50 sessions (25 days) of therapy training |
50.0 ± 0.0 |
Trial window refers to a specified amount of time during which a second press must occur to provide an operant food pellet reward. During Stage 1 of training, pellet rewards were delivered with a single press to facilitate lever-reward association. N/A denotes there was no trial window specified for the rat to press the lever. The remaining stages all required two presses to receive a reward.
Lever location refers to distance relative to inside cage wall. Negative values denote distance inside the cage, and positive values are outside the cage.
Application of VNS
Behavioral training was identical for all rats. Rats were assigned to the VNS during rehab group or the rehab group nine days after surgery by a standardized system of alternating group assignment. The last three rats in the rehab group received VNS two hours after the second rehab session of each day. VNS was comprised of approximately 7,500 half second stimulation events over 25 days. All rats had a rotating electrical tether connecting an external stimulator to the head mounted two-channel connector. For the rehab group, the tether wires were cut to ensure no current was delivered to the vagus cuff electrode during behavioral testing.
Rats that received VNS during rehab were given a brief burst of VNS in addition to a food reward for each successful trial. VNS was delivered within 70 ms of the second lever press (i.e. during the lever press). VNS was delivered as a 500 ms train of 15 pulses at 30 Hz. Each biphasic pulse was 0.8 mA in amplitude and 100 μs in phase duration. These parameters are identical to our earlier studies12,13. Previous studies using the same parameters employed in this study have demonstrated changes in electroencephalographic measures and neuronal spiking synchrony during VNS, indicating that the nerve is successfully stimulated12,16. Rats in the VNS after rehab group had a third session each training day during which VNS was delivered every 12 seconds for 60 minutes (300 stimulations/1hr session) totaling 7,500 stimulations over 25 days. This stimulation was given in a cage without a lever, ensuring that VNS was not delivered during lever press behavior.
Histological Processing
All rats were anesthetized with lethal dose of sodium pentobarbital (50 mg/kg, i.p.) and transcardially perfused with 250 ml of 0.02% heparin/0.1 M PB solution, followed by 450 ml of 4% paraformaldehyde/0.1 M PB solution. Brains were removed and postfixed in 4% paraformaldehyde/0.1 M PB solution, and then cryoprotected in a 30% sucrose/0.1 M PB solution. The tissue was sectioned at 40 Jm intervals and stained with cresyl violet. A rat brain atlas17 was used to help determine lesion size and location.
Statistics
All data are reported as the mean ± SEM. All comparisons were planned in the experimental design a priori, and significant differences were determined using student t-tests and/or analysis of variance. Statistical tests for each comparison are noted in the text. Histological analysis used One-Way ANOVA’s to compare lesion size across all groups. Behavioral data was also analyzed with ANOVA. Student t-tests were used for post-hoc analysis when appropriate for group comparisons. Significant differences are noted in the figures as * P < 0.05, ** = P < 0.01, *** = P < 0.001. Error bars indicate mean ± SEM.
Results
Pre-Lesion Performance
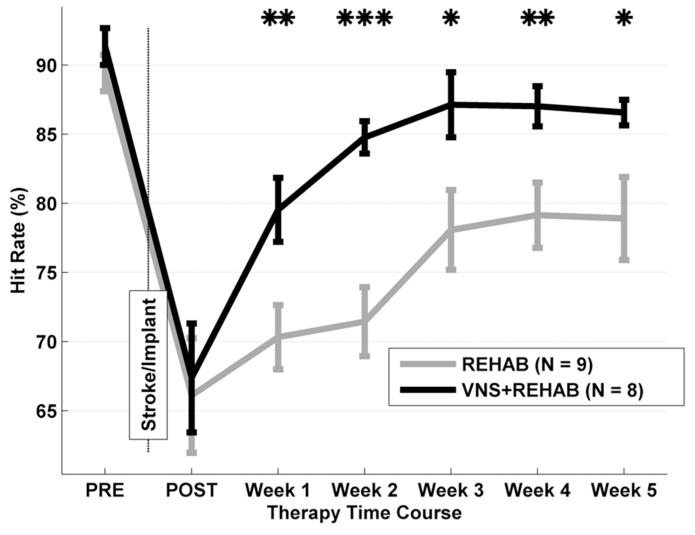
Prior to ET-1 ischemia, all seventeen rats were able to successfully press the lever twice within 500 ms on 90.3 ± 1.0% of trials (Fig. 2). There was no significant difference in hit rate performance among groups (ANOVA F[2,1] = 3.56, P = 0.06). The inter-press interval data also showed no significance among groups (F[2,1] = 2.61, P = 0.13). The rats completed the second press with an average inter-press interval of 268 ± 17 ms, well within the 500 ms hit window. Rats were proficient on all measures of the task prior to lesion. Performance during earlier shaping stages have been described previously (Hays et al., 2013).
Figure 2.
Lever press performance on the bradykinesia assessment task. Rats were trained to press a lever located outside the cage twice within 500 msec to receive a food reward. Prior to the lesion (PRE) both groups were equally proficient at the task. Ischemic lesion of the motor cortex reduced performance equally in both groups (POST). During all 5 weeks of therapy the VNS during rehab group (black) performed significantly better compared to the rehab group (gray). Data are means ± S.E.M. (* p <0.05).
Histology
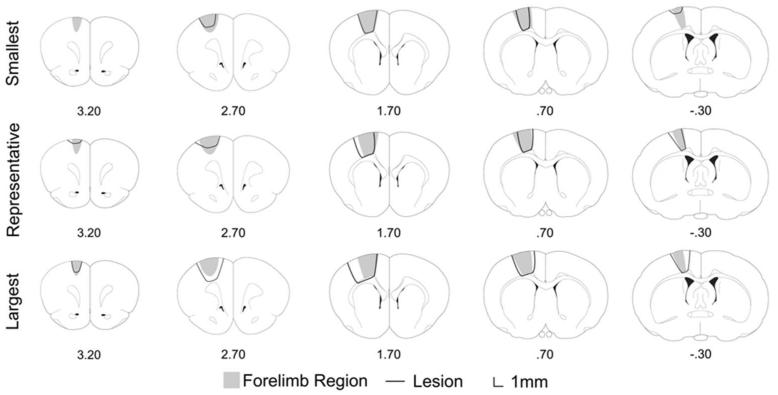
Unilateral ET-1 injections consistently caused an ischemic lesion spanning rostral and caudal forelimb areas within motor cortex18. Coronal sections were collected throughout the lesion area for thirteen animals. Cortical layers I-VI were destroyed by the infarct. In ten brains, the underlying white matter (external capsule) showed no damage, the remaining 3 brains showed superficial white matter damage. Errors in histological procedures prevented analysis of the remaining four brains. The lesion was reconstructed using ImageJ software (National Institutes of Health, Bethesda, MD). Lesions typically ranged from anteroposterior 3.2 mm to −0.7 mm (Fig. 3). The mean ± SD infarct volume across all animals was 9.38 ± 3.74 mm3, and one-way ANOVA showed no significant difference across all groups (F[2,10] = 0.43, P = 0.66). The lesion size was consistent across groups and comparable to previous studies using similar lesion methods19,20.
Figure 3.
Schematic representations of the smallest, representative, and largest lesion following intracortical ET-1 infarct. Grey region represents the location of motor cortex, and the black line trace represents the area of infarct. Coordinates are relative to bregma.
Post-Lesion Performance
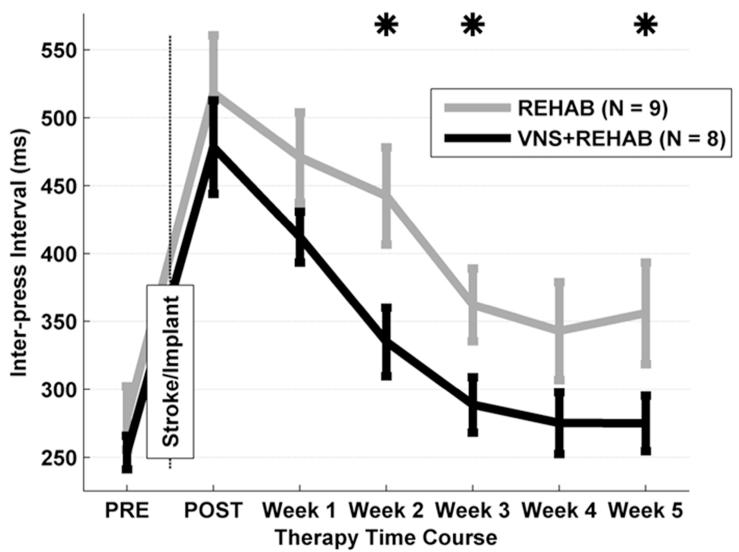
Unilateral ET-1 lesions worsened the hit rate performance for all seventeen rats to 66.7 ± 2.8% (Fig. 2). There was no significant difference for both hit rate performance (ANOVA, F[2,1]= 1.34, P = 0.49) and inter-press interval (F[2,1]= 1.34, P = 0.31) across groups. As expected, the lesion caused a significant decrease in hit rate of 23.6 ± 2.5% compared to pre-lesion performance (F[2,1]= 56.13, P < 0.001). This level of impairment is consistent with other reports of rats performing a pellet grasp task with a similar ischemic lesion (Fang et al 2010; Gilmour et al 2003). Rats demonstrated approximately a two-fold reduction in forearm movement speed as measured by an average inter-press interval of 502 ± 33 ms (P < 0.001 compared to PRE; Fig. 4). The results establish that all groups were similarly impaired in performance after ischemic lesion.
Figure 4.
Inter-press interval on the bradykinesia assessment task. Using the bradykinesia assessment task, forelimb movement speed is measured by the latency between the first and second lever press. Prior to lesion (PRE), both groups rapidly press the lever. After lesion (POST), the latency between lever presses is increased similarly in both groups, suggestive of a slowing of forelimb speed. During weeks 2, 3, and 5 the VNS during rehab group (black) performed significantly better compared to the rehab group (gray). Data are means ± S.E.M. (* p <0.05).
Motor impairment persists even after five weeks of daily rehab
The hit rate performance of the rehab group modestly improved during the course of therapy (Fig. 2). Rats showed an improvement during weeks 3-5 compared to post-lesion (P < 0.01 for weeks 3-5), but remained significantly impaired compared to pre-lesion levels throughout the entire therapy (P > 0.05 for all weeks). The data presented here demonstrates that motor rehabilitation only partially restores function, but is insufficient to fully recover forelimb function.
Vagus nerve stimulation with each correct movement eliminates motor impairment
A repeated measures ANOVA on hit rate performance across the post-lesion interval revealed a significant effect of therapy (F[2,84]= 18.99, P < 0.001) and weeks (F[5,84]= 9.52, P < 0.001) without a significant interaction (F[10,84]= 0.92, P = 0.52). Rats that received VNS during rehab significantly improved hit rate within the first week of therapy compared to post-lesion (+12.1 ± 2.2%, P < 0.001; Fig. 2). Additionally, hit rate performance improved significantly after the second week of therapy compared to the first week (+5.2 ± 2.3%, P < 0.05). By the third week of VNS during rehab therapy, hit rate performance was no longer significantly impaired compared to pre-lesion levels (VNS during rehab, PRE: 91.4 ± 1.3%, Week 3: 87.1 ± 2.4%, P = 0.18). In contrast, rats that received rehab alone did not significantly improve after a week of therapy compared to post-lesion (+4.2 ± 3.8%, P = 0.27) or after the second week of rehab compared to the first week (+1.1 ± 2.5%, P = 0.64). Performance of the rehab group was significantly reduced compared to pre-lesion levels at every time point. VNS delivered during motor rehabilitation induced a rapid improvement and return to pre-lesion performance, highlighting the beneficial effects of VNS combined with motor rehabilitation compared to rehabilitation alone.
Vagus nerve stimulation did not improve motor function when delivered after rehabilitation
VNS delivered after rehab did not to yield improved recovery compared to rehab alone. The VNS after rehab group was significantly impaired on the task during weeks 1, 2, 4, and 5 (hit rate impairment compared to baseline at Week 1: −19.4 ± 5.2%, P < 0.05; Week 2: −14.4 ± 1.5%, P < 0.01; Week 3: −8.9 ± 4.8%, P = 0.15; Week 4: −12.8 ± 3.4%, P < 0.05; Week 5: −13.9 ± 3.8%, P < 0.05). Because VNS after rehab offered no benefit compared to rehab only, data from rats in the VNS after rehab group were combined with data from rats receiving rehab only, and will be referred to as the rehab group. These results may suggest that VNS alone is not sufficient to confer therapeutic benefits.
Group comparison confirms the benefit of VNS during rehabilitation
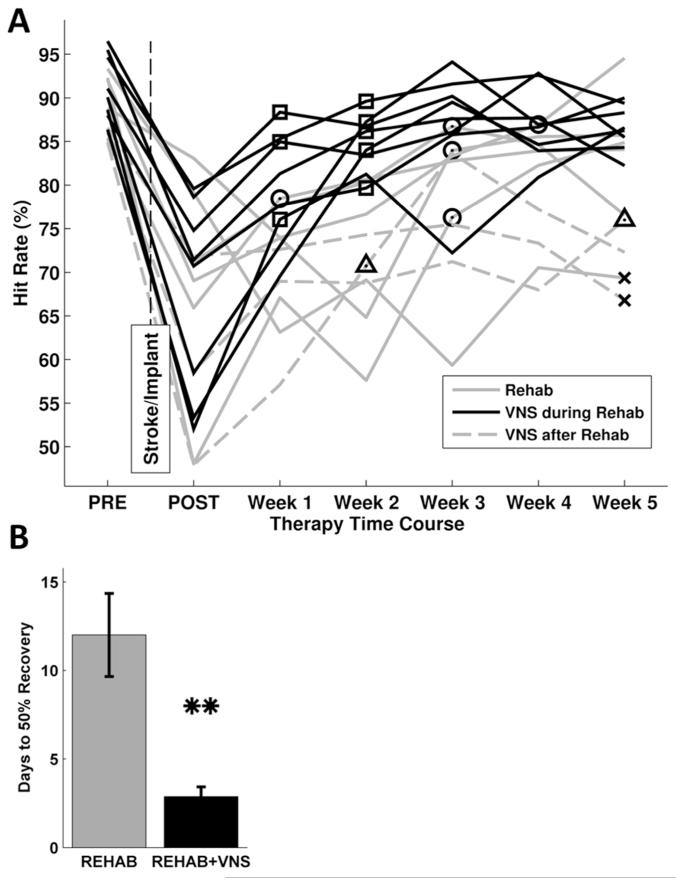
VNS during rehab resulted in significantly better successful hit rate performance than the rehab group during every week of therapy (P < 0.05 for all weeks, Fig. 2). On average, rats that received VNS during rehab rapidly recovered to 50% of their pre-lesion performance within 2.3 ± 0.4 days, while the rehab group took 10 ± 2.5 days (P < 0.01, Fig. 5b). Six out of nine rats in the rehab group continued to exhibit a significant motor impairment on the last week of therapy. Only two of the rats in the VNS during rehab group exhibited an impairment compared to pre-lesion performance. All rats in the VNS during rehab group returned to 50% of their pre-lesion performance by week 2 (Fig. 5a). These data demonstrate that VNS during rehab provides a substantial benefit in recovery of forelimb function compared to rehab alone.
Figure 5.
A) Individual rat performance on the bradykinesia assessment task. Symbols indicate when an animal regained 50% of pre-lesion forelimb function. X indicates that an animal that did not regain 50% of forelimb function at any point during therapy. All rats that received VNS during rehab regained 50% of forelimb function by week 2 (squares). One animal in both the rehab alone (circles) and VNS after rehab group (triangles) did not regain 50% of forelimb function. B) Number of days for each group to regain 50% of forelimb function based on lever press success rate performance. The VNS during rehab group demonstrated 50% recovery of pre-lesion performance within the first week of therapy, while rats that received rehab alone reached 50% recovery during the third week of therapy on average. Data are means ± S.E.M. (* p <0.05).
Vagus nerve stimulation improves inter-press interval
ANOVA on inter-press interval revealed a significant effect of therapy (F[1,4]= 14.24, P < 0.001) and weeks (F[1,4]= 5.77, P < 0.001) without a significant interaction (F[1,4]= 0.15, P = 0.96). Delivery of VNS during rehab resulted in an improvement in forelimb speed compared to the rehab group (Fig. 4). Inter-press interval returned to pre-lesion levels by Week 3 of therapy for rats that received VNS during rehab (VNS during rehab; Week 3: 288 ± 21 ms, P = 0.22). In contrast, rats that received rehab returned to pre-lesion levels by week 4 (Rehab; Week 4: 343 ± 36 ms, P = 0.17). The reduction in the time between first and second lever presses demonstrates that VNS delivered during motor rehabilitation is effective at reducing bradykinesia following ischemic lesion.
Vagus nerve stimulation does not increase the number of trials
Because stimulation of the vagus nerve has a wide variety of effects, we sought to rule out several possible off-target effects. VNS during rehab did not result in a significant difference in the number of trials during the five weeks of therapy. Rehab rats attempted slightly more total trials compared to VNS during rehab rats (Rehab: 4736 ± 237 trials, VNS during rehab: 4403 ± 388 trials), therefore increased usage of the forelimb cannot account for the improvements in performance. The body weight of each group was also not different across groups (Two way ANOVA, F[1,4]= 2.66, p = 0.11), suggesting that overall health was unaffected by the therapy. We never observed movement triggered by VNS. VNS also did not appear to distract rats during task performance. These observations suggest that differences in motivation or gross physiological effects are unlikely to account for the functional improvement in rats receiving VNS after each correct trial.
Discussion
This study evaluated the efficacy of pairing stimulation of the vagus nerve with forelimb movement to restore motor function after ischemic damage to motor cortex. Forelimb function, as measured by the bradykinesia assessment task, recovered completely when a brief burst of VNS was delivered with each successful trial. The same degree of intensive daily motor rehabilitation without VNS failed to restore normal function. These results demonstrate that VNS paired with motor training can improve recovery of forelimb function following ischemic lesion of rat motor cortex even compared to intensive daily rehabilitation. VNS thus provides a potential new method to improve stroke rehabilitation in patients21,22.
Neuroplasticity is thought to be the key mechanism for functional recovery after brain damage23. As such, many current therapies for motor rehabilitation incorporate methods to enhance plasticity with motor training. Pharmacological strategies, such as amphetamine and fluoxetine, are thought to enhance neuroplasticity by modulating neurotransmitter effects and thereby promote functional recovery24,25. Stimulation of the vagus nerve causes the release of neurotransmitters that drive plasticity, including acetylcholine, norepinephrine, and serotonin26. Modulation of these neurotransmitters can improve functional recovery, while their ablation can occlude recovery9,27,28. Because VNS can stimulate the release of plasticity-enhancing neurotransmitters, it provides a novel means to enhance neuroplasticity.
VNS offers a much higher degree of temporal precision than drugs, allowing precisely controlled release of neuromodulators and therefore more precisely targeted plasticity. Previous studies have documented the importance of precise timing of VNS in the induction of plasticity12,13. VNS delivered milliseconds after forelimb movement increases the representation of the coincident movement in the motor cortex, while other movements occurring within seconds of VNS delivery did not display map plasticity13. VNS directed plasticity is also spatially precise, as VNS paired with specific movements enhances only the representation of the paired movement in motor cortex. Corroborating the need for high temporal precision, VNS paired precisely with the presentation of a tone increases the representation of the paired tone in the auditory cortex and not another tone frequency that is interleaved but separated from VNS by many seconds12. The high temporal and spatial precision of VNS makes it possible to regulate plasticity in a moment by moment manner, such that only successful trials are paired with VNS.
The observation that our therapy improves recovery compared to a control group that received thousands of repetitions of a task that forced them to use their impaired limb (constraint induced movement therapy) suggests VNS can make effective rehabilitation therapies better and possibly restore normal function. It remains to be seen whether this therapy can benefit rats with more severe lesions29,30. Lesions that prevent VNS from triggering release of acetylcholine or norepinephrine would be expected to block the beneficial effects of VNS9,27, but this has not been shown. It is possible that careful optimization of the VNS parameters (current, pulse width, duration, etc.) would be needed in such conditions. Insufficient generalization of performance gains from trained tasks to other skills often limits the impact of rehabilitation in clinical settings31. It would be valuable to determine whether pairing VNS with successful completion of one task could generalize to performance on another (e.g. pellet retrieval). Pairing VNS with unsuccessful trials would also be important for understanding how pairing may be influencing plasticity, and whether the effects would be beneficial, detrimental, or irrelevant towards recovery.
VNS is well tolerated by patients with a wide range of neurological conditions. VNS causes no significant changes heart rate, blood oxygenation level, or resting behavior in animals, and no changes in cardiac function from basal physiological levels in patients11,12,32. Over 60,000 patients have received vagus nerve stimulation for the treatment of refractory epilepsy and depression. VNS therapy for these conditions is delivered continuously for years and only causes minor side effects11,14. Pairing brief bursts of VNS with rehabilitation requires only 1% of the VNS used to treat epilepsy33. The minimal amount of VNS required suggests that VNS could be safely paired with rehabilitation of stroke and possibly other neurological disorders.
Conclusion/Implications
This study provides a demonstration that pairing VNS with motor rehabilitation can improve recovery following stroke. VNS delivered during motor rehabilitation restored rapid improvement and return to pre-lesion performance. Rehab training alone was insufficient to restore normal performance. These results suggest that targeted plasticity therapy is a potentially viable new therapy for recovering motor function after stroke.
Acknowledgements
We would like to thank Duc Cao, Nabila Alam, Fizza Naqvi, Hector Henriquez, and Sarosh Shah for help with behavioral training. We would like to thank Dhirender Ratra, Monica Javidnia, and Tommy Vu for help with histology. We would also like to thank Scott Barbay and Delaina Walker-Batson for their comments on earlier versions of the manuscript.
Source of Funding
This research was supported by MicroTransponder, Incorporated.
Footnotes
Disclosure Statement
Michael P. Kilgard is a consultant and shareholder of MicroTransponder, Incorporated.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- (1).Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovascular Diseases. 2003;16:280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- (2).Johansson BB. Brain plasticity and stroke rehabilitation The Willis lecture. Stroke. 2000;31:223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- (3).Liepert J, Bauder H, Miltner WHR, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- (4).Volpe BT, Lynch D, Rykman-Berland A, Ferraro M, Galgano M, Hogan N, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:305–310. doi: 10.1177/1545968307311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- (6).Plautz E, Barbay S, Frost S, Friel K, Dancause N, Zoubina E, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- (7).Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- (8).Yamamoto T, Katayama Y, Watanabe M, Sumi K, Obuchi T, Kobayashi K, et al. Changes in Motor Function Induced by Chronic Motor Cortex Stimulation in Post-Stroke Pain Patients. Stereotact Funct Neurosurg. 2011;89:381–389. doi: 10.1159/000332060. [DOI] [PubMed] [Google Scholar]
- (9).Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- (10).Feeney DM, Westerberg VS. Norepinephrine and brain damage: Alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Canadian Journal of Psychology/Revue canadienne de psychologie. 1990;44:233. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- (11).George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- (12).Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- (14).Sjogren MJC, Hellstrom PTO, Jonsson MAG, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psychiatry. 2002;63:972–980. doi: 10.4088/jcp.v63n1103. [DOI] [PubMed] [Google Scholar]
- (15).Hays SA, Khodaparast N, Sloan AM, Fayyaz T, Hulsey DR, Ruiz AD, et al. The bradykinesia assessment task: an automated method to measure forelimb speed in rodents. Journal of Neuroscience Methods. 2013 doi: 10.1016/j.jneumeth.2012.12.022. [DOI] [PubMed] [Google Scholar]
- (16).Nichols J, Nichols A, Smirnakis S, Engineer N, Kilgard M, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- (17).Paxinos G, Watson C. Academic press; 2007. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- (18).Neafsey E, Bold E, Haas G, Hurley-Gius K, Quirk G, Sievert C, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res Rev. 1986;11:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- (19).Fang P, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1-40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–549. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Gilmour G, Iversen SD, O’Neill MF, Bannerman DM. The effects of intracortical endothelin-1 injections on skilled forelimb use: implications for modelling recovery of function after stroke. Behav Brain Res. 2004;150:171–183. doi: 10.1016/j.bbr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- (21).Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lozano AM. Harnessing plasticity to reset dysfunctional neurons. N Engl J Med. 2011;364:1367–1368. doi: 10.1056/NEJMcibr1100496. [DOI] [PubMed] [Google Scholar]
- (23).Hosp JA, Luft AR. Cortical plasticity during motor learning and recovery after ischemic stroke. Neural Plast. 2011:2011. doi: 10.1155/2011/871296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Martinsson L, Eksborg S. Drugs for stroke recovery: the example of amphetamines. Drugs Aging. 2004;21:67–79. doi: 10.2165/00002512-200421020-00001. [DOI] [PubMed] [Google Scholar]
- (25).Pleger B, Schwenkreis P, Grünberg C, Malin JP, Tegenthoff M. Fluoxetine facilitates use-dependent excitability of human primary motor cortex. Clinical neurophysiology. 2004;115:2157–2163. doi: 10.1016/j.clinph.2004.04.015. [DOI] [PubMed] [Google Scholar]
- (26).Detari L, Juhasz G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiol Hung. 1983;61:147–154. [PubMed] [Google Scholar]
- (27).Goldstein LB, Coviello A, Miller GD, Davis JN. Norepinephrine depletion impairs motor recovery following sensorimotor cortex injury in the rat. Restorative Neurol Neurosci. 1991;3:41–47. doi: 10.3233/RNN-1991-3105. [DOI] [PubMed] [Google Scholar]
- (28).Pappius HM, Dadoun R, McHugh M. The effect of p-chlorophenylalanine on cerebral metabolism and biogenic amine content of traumatized brain. Journal of Cerebral Blood Flow & Metabolism. 1988;8:324–334. doi: 10.1038/jcbfm.1988.67. [DOI] [PubMed] [Google Scholar]
- (29).Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma. 2010;27:2221–2232. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).MacLellan CL, Gyawali S, Colbourne F. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behav Brain Res. 2006;175:82–89. doi: 10.1016/j.bbr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- (31).Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- (32).Handforth A, DeGiorgio C, Schachter S, Uthman B, Naritoku D, Tecoma E, et al. Vagus nerve stimulation therapy for partial-onset seizures A randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- (33).Morris GL, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology. 1999;53:1731–1731. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]