Abstract
Tumor infiltrating lymphocytes (TIL) have been demonstrated to predict oncologic outcomes following resection or primary intrahepatic neoplasms and metastatic liver tumors. Despite stong immunosuppressive factors within the intrahepatic space, TIL are frequently demonstrated in liver tumors. The presence of TIL within liver tumors provides evidence of a host immune response that may be protective, but often is rendered ineffective by tumor induced immune dysfunction. In this review, we discuss techniques involved in studying TIL and subsets of TIL commonly identified. We emphasize the unique nature of the intrahepatic milieu that promotes immunosuppression, and how liver TIL and TIL ratios can be used as indicators of prognosis. Several types of primary and metastatic liver tumors are considered to highlight the similarities and important differences in TIL responses, which likely reflect how intrahepatic immunity is influenced by tumor biology. The studies we discuss indicate that tumor infiltration by suppressor cells and expression of immunoinhibitory molecules by TIL limits the anti-tumor immune function of effector T cells. Most patients fail to mount an adequate immune response to liver tumors, which provides compelling rationale for clinical study of immunotherapy for intrahepatic neoplasms.
INTRODUCTION
Tumor Infiltrating Lymphocytes (TIL) are thought to represent a specific host response to tumor antigens and may be used for therapeutic purposes following isolation, or studied for prognostic information (1, 2). A growing body of literature supports the biologic relevance of TIL as predictors of outcome for primary and metastatic tumors (3, 4). TIL have been demonstrated to predict outcomes in a wide variety of solid tumors (1, 5), with the magnitude of this effect being dependent on tumor site and disease stage (6). We previously reviewed solid tumor immunotherapy, including the therapeutic use of TIL (7). While the present review focuses on the prognostic importance of TIL in liver tumors, it is important to emphasize that TIL, along with other forms of adoptive cell therapy, will play an increasingly important role in immunotherapy.
TIL have been demonstrated to predict survival and recurrence following resection of both primary and metastatic liver tumors (3, 4, 8). TIL are frequently demonstrated in liver tumors despite strong immunosuppressive factors within the intrahepatic space. The presence of TIL within liver tumors provides evidence of a host immune response that may provide protection against disease progression, but often is rendered ineffective by tumor induced immune dysfunction. Interest in studying TIL within liver tumors is predicated upon the desire to understand the immune response to intrahepatic neoplasia, in addition to the value of TIL as biomarkers to predict outcome. In this review, we discuss techniques involved in assessing TIL, subsets of TIL commonly studied, the unique nature of the intrahepatic milieu that promotes immunosuppression, and how liver TIL can be used as indicators of prognosis. Several types of primary and metastatic liver tumors are considered to highlight the similarities and important differences in TIL responses, which likely reflect how intrahepatic immunity is influenced by tumor biology.
THE IMMUNOSUPPRESSIVE INTRAHEPATIC SPACE
At baseline, the liver has a strong tendency to promote tolerance to intrahepatic antigens (9, 10). Non-parenchymal cells (NPC) in the liver, including sinusoidal endothelial cells (11), dendritic cells (DC) (12), and natural killer (13) cells have been demonstrated to contribute to the tolerogenic intrahepatic milieu. This immunosuppressive nature of liver T cells has been described as well (11, 12, 14). The suppressive influence of liver NPC likely works in concert with tumor-induced immunosuppression to prevent eradication of intrahepatic tumors by liver TIL.
Tumors may down-regulate expression of MHC class I molecules, thereby preventing effector T cells from recognizing tumor antigens. Moreover, tumors may secrete molecules that promote the influx of suppressive immune cells in addition to augmenting suppressor cell function (15). As such, assessment of liver TIL provides important insight into the biology of the underlying neoplastic process. More importantly, a more complete understanding of the nature and functional limitations of liver TIL may reveal new therapeutic opportunities through manipulation of effector and suppressor TIL subsets.
TUMOR INFILTRATING LYMPHOCYTES SUBSETS
It is important to discriminate among different liver TIL subtypes, as they have markedly different functions within the tumor microenvironment. Since T lymphocytes are primarily responsible for antigen-specific immune responses and they typically comprise the largest proportion of TIL, much of the TIL literature is devoted to the study of T cell subsets. In a recent meta-analysis, Gooden et al. demonstrated that the extent of tumor infiltration by T cells was a significant correlate of overall survival (6). TIL other than T cells, including B cells and NK cells, have been studied as well but will not be addressed in detail in this review. Our understanding of the biologic implications of non-T cell TIL is less developed at this time.
T cells are CD3+ are broadly categorized into CD8+ and CD4+ subsets that have distinct roles in shaping adaptive immune responses (Figure 1). CD8+ cytotoxic T lymphocytes are capable of killing tumor cells directly, and a high number of CD8+ TIL is generally associated with favorable oncologic outcomes. In contrast, CD4+ T helper (Th) lymphocytes represent a more heterogeneous population and orchestrate immune responses through production of various cytokines. Type 1 (Th1) CD4+ T cells activate cytotoxic CD8+ T cells. In contrast, type 2 (Th2) T lymphocytes promote humoral immunity through activation of B cells. In general, the CD8+ T cell response promoted by Th1 cells plays a more important role in tumor protection, as compared to Th2 responses (16). The presence of functionally diverse CD4+ TIL subtypes likely results in inconsistent associations between numbers of CD4+ TIL and outcome (4, 8). The challenge of relating CD4+ TIL to outcomes is exacerbated by the presence of CD4+ regulatory T cells (Treg) within the microenvironments of multiple tumor types.
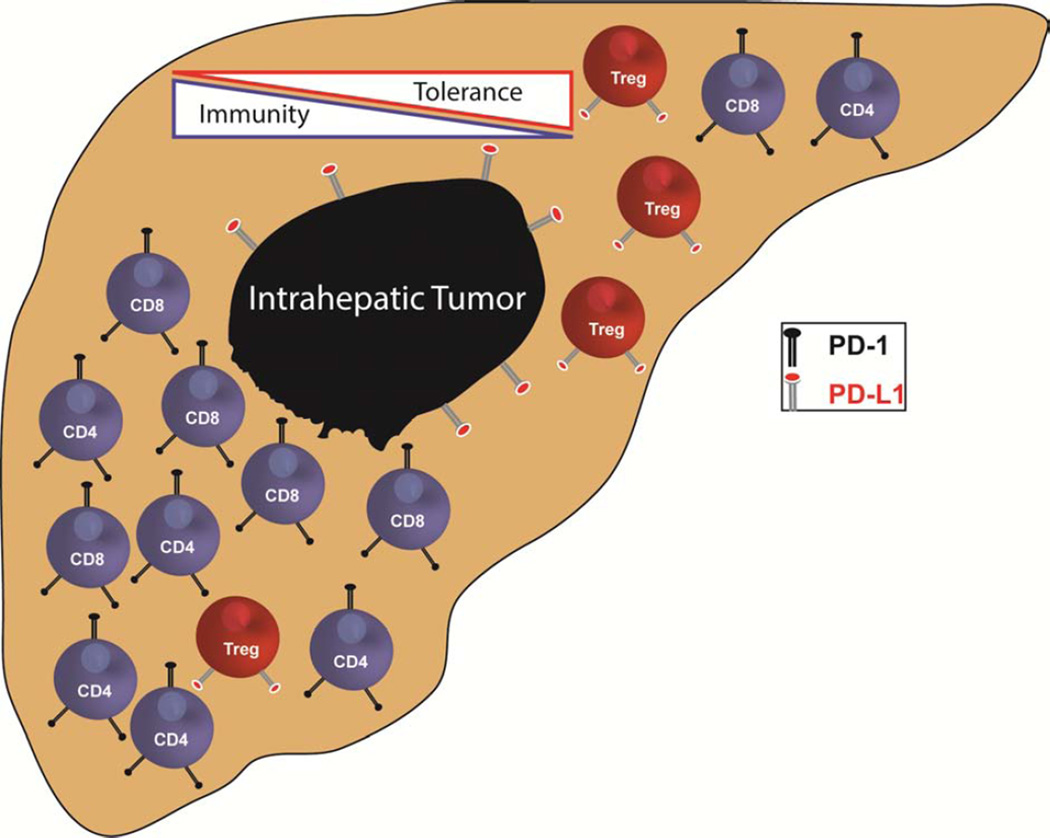
Figure 1. Liver tumor infiltrating lymphocytes.
LEFT: A favorable T cell infiltrate is depicted approaching an intrahepatic tumor. An abundance of CD4 T cells is present to support tumor cell killing by high density of cytotoxic CD8 T cells. Few regulatory T cells (Treg) are present, providing a favorable ratio of effector to suppressor T cells. RIGHT: An unfavorable T cell infiltrate is thwarting anti-tumor immunity. A high ratio of suppressive Treg to effector CD4 and CD8 T cells is promoting tolerance and will hinder tumor clearance. PDL-1 on the surface of Treg and the tumor cells will engage PD-1 on CD4 and CD8 T cells to suppress anti-tumor immune activity.
Treg are a subset of CD4+ T cells that suppress the function of effector T cells (17). Treg are classically identified by FOXP3 staining. FOXP3 is a transcription factor that serves as the master regulator of Treg differentiation and suppressive function (18). The presence of suppressors, such as Treg, tends to correlate with decreased survival or shorter recurrence-free intervals. However, it is important to note that FOXP3 is an imperfect marker for Treg in humans as it can be expressed on activated effector cells. As noted below, the use of ratios between FOXP3 and CD8 or CD4 can provide more robust information that FOXP3 alone.
THE IMPORTANCE OF TIL RATIOS
While individual TIL subsets may provide significant prognostic information, ratios of TIL subsets are generally more informative (3, 8). Host responses to solid tumors involve multiple types of immune cells (Figure 1). TIL ratios provide insight into how a particular group of cells may either promote or suppress anti-tumor immunity. For example, whether FOXP3+ Treg have a suppressive effect at the clinical level will depend on the relative numbers of effector T cells. In a recent meta-analysis, FOXP3+ TIL counts were not associated with overall survival (HR 1.19, 95% CI 0.84–1.67). However the CD8+/FOXP3+ ratio was a significant predictor of overall survival (HR 0.48, 95% CI 0.34–0.68, p<0.0001) (6). TIL ratios are more informative than individual TIL counts; ratios provide greater insight into the anti-tumor immune response as a whole (Table 1).
Table 1.
Studies of TIL in Primary and Metastatic Liver Tumors
| Author | Year | Tumor Type |
n | TIL types Studied | Correlates with Oncologic Outcomes |
|---|---|---|---|---|---|
| Wagner (27) | 2008 | CRCLM | 16 | CD3+, CD4+,
CD69+, CD25+ |
↓CD4+:CD25+ → ↓OS |
| Katz (8) | 2009 | CRCLM | 162 | CD8+, CD4+ | ↑CD8+→ ↑OS |
| Katz (4) | 2013 | CRCLM | 188 | CD3+,
CD4+,CD8+, FOXP3+ |
↑FOXP3+:CD4+→
↓OS ↑FOXP3+:CD8+→ ↓OS |
| Cai (29) | 2005 | HCC | 123 | memory T (mT) cells |
↑ mT cells → ↑RFS |
| Mathai(31) | 2012 | HCC | 131 | CD3+,CD8+,FOXP3+ | ↑FOXP3+:CD3+ →
↓OS ↑FOXP3+:CD8+ → ↓OS |
| Liao (34) | 2013 | HCC | 300 | CD4(IL17+) | ↑IL-17+→ ↓OS and ↓DFS |
| Huang (35) | 2013 | HCC | 192 | FOXP3+,
CD4+, CD8+,IL17+T cells. |
↑FOXP3+ and ↑IL17+
Tcell → ↓OS and↓DFS |
| Ye (39) | 2009 | I-CCA | 31 | PDL-1,PD-1,CD8, CD4 |
↑PDL-1 and PD-1 → ↑poor differentiation |
| Gu (36) | 2012 | I-CCA | 123 | L17+,FOXP3+, CD8+, CD66+ (neu), CD34+ |
↑IL17+→ ↓OS |
| Katz (3) | 2010 | NETLM | 39 | CD3+, CD4+,
CD8+, FOXP3+ |
↑FOXP3+ → ↓OS |
TUMOR INFILTRATING LYMPHOCYTE STUDY METHODOLOGY
In order to appropriately assess liver TIL studies, one requires a general understanding TIL study design. Immunohistochemistry (IHC) has been the primary modality used to quantify TIL. Highly specific antibodies bind to cell surface markers to identify cell types of interest (19). Anti-CD3, anti-CD4, anti-CD8, and anti-FOXP3 antibodies can be used to detect T cells and T cell subsets within tumors. Positively stained cells may then be counted manually or using automated detection software.
TIL IHC may be studied by staining whole mount slides or tissue microarrays (TMA). While examination of whole slides permits TIL analysis from large portions of one specimen at a time, TMAs afford the advantage of placing a large number of tissue cores on a single slide. A TMA slide therefore enables simultaneous staining of many specimens followed by high throughput analysis. The TMA approach increases efficiency by reducing data acquisition time, reagent use, and storage space requirements. TMA studies have shown excellent concordance with the traditional whole slide approaches (20). An important limitation of TMAs is the potential for sampling error as cores are selected from tissue blocks. Utilization of multiple cores per sample and use of tissue blocks with viable tumor decreases the impact of sampling error.
Two issues contribute to heterogeneity among TIL studies. First, cutoff points used to define levels of TIL as “high” or “low” vary considerably among different studies. Various studies use percentiles, tertiles, median, or statistically optimal values (3, 21). Other studies merely rely on presence or absence of TIL (22). In several studies cutoff points were not described (6). Second, TIL location within tumors has been examined in various ways. TIL within tumor stroma and tumor epithelium have been analyzed separately by some authors, while others have focused on TIL as a whole regardless of intratumoral location (23, 24). At this time, it is unclear if there is an optimal approach for cutoff point methodology or if intratumoral lymphocyte location significantly impacts the correlation between TIL counts and oncologic outcome.
COLORECTAL LIVER METASTASES
Surgical resection of colorectal liver metastases (CRLM) can achieve 5-year survival rates of up to 58%, and 17 to 25% of patients are free of disease at 10 years (25, 26). TIL are independent predictors of oncologic outcome following resection of CRLM. CRLM TIL provide valuable information that can predict the likelihood of recurrence and death following resection. Both CD4+ and CD8+ T cells are activated within CRLM, and activated CD4+ T helper cells may promote tumor selective activity of cytotoxic CD8+ T lymphocytes (27). Following hepatic resection, patients with high numbers of CD8+ intratumoral T cells were more likely to survive 10 years or more (8). Among patients who survived for 10 years or more, 31% had high levels of CD8 T cells. In contrast, only 8% of those who survived less than 2 years had a high level of CD8+ TIL. To exclude dependence on particular cutoff points, correlations between cell type and survival time were confirmed by using TIL numbers as continuous variables in regression models (8).
As noted earlier, TIL ratios provide important insight into the immune response against solid tumors. Among patients with CRLM who survived for at least 10 years following resection, 19% had a favorable or high CD8+:CD4+ TIL ratio compared to only 2% among patients who died of their disease within 2 years (8). Unfavorable or low CD8+:CD4+ TIL ratios were seen in 63% of patients who died within 2 years in comparison to only 10% of patients who survived more than 10 years after resection. A high CD8+:CD4+ TIL ratio was an independent predictor of long-term survival following CRLM resection after adjusting for multiple variables including clinical risk score and extrahepatic disease (OR 15.6, p<0.01). TIL ratios proved to be stronger correlates of survival than individual TIL subset counts.
In a more recent study, overall survival at 5 years for patients with high level of CD4+ TIL was 55% compared to 35% in patients with low levels of CD4+ TILs (p=0.004) (4). Consistent with the earlier report discussed above, the 5 year overall survival was 59% in those with high number of CD8+ and 35% in those with low levels of CD8+ TILs (p=0.04). In addition, a high CD4+:CD3+ TIL ratio was a significant correlate of improved overall survival (p=0.01) and recurrence-free survival (p=0.05). A high CD8+:CD3+ TIL ratio was a significant predictor of overall survival (p=0.05) as well. On average, 174 CD3+, 26 CD4+, 95 CD8+, and 44 FoxP3+ TILs were seen in TMA cores.
TIL ratios including FOXP3+ Treg are also useful in predicting outcome following resection of CRLM. As noted above, Treg are a subset of CD4+ T cells identified by detection of FOXP3 transcription factor expression (18). High ratios of FOXP3+ to either CD4+ or CD8+ TIL were independent predictors of shorter overall survival in patients with CRLM. Overall survival at 5 years for patients with a high FOXP3+:CD4+ TIL ratio was 34% compared to 51% for patients with a low ratio (OR=1.6, p=0.03). Similarly, 5 year survival was 35% in those with high FOXP3+:CD8+ ratio compared to 46% in those with a low FOXP3+:CD8+ TIL ratio (HR=1.5, p=0.05) (4). Separate multivariate analyses were constructed for each cell marker and ratio, controlling for factors including clinical risk score and extrahepatic disease. In contrast, FOXP3+ counts alone were not significant predictors of outcome.
It is important to consider why Treg counts alone were less predictive of outcome following CRLM resection than were ratios of Treg to other TIL subsets. The ability of Treg to suppress an immune response depends on how effectively they can inhibit the function of effector CD4+ and CD8+ TIL. Even highly suppressive Treg will have little inhibitory effect on an anti-tumor immune response if effector T cells greatly outnumber the suppressors. In contrast, a small number of suppressor cells may thwart anti-tumor immunity if the overall intratumoral TIL density is low. As such, ratios of Treg to other TIL subtypes may be more informative than Treg counts alone. The immunosuppressive potential of Treg is more apparent when considered in the context of the overall immune response using TIL ratios.
An important lesson from studies of CRLM is that while favorable TIL profiles are associated with better outcomes, the majority of patients do not demonstrate an effective intratumoral immune response. With respect to liver tumors, we speculate that immunosuppressive environmental cues within the liver as a whole, as well as within liver tumors, limit the function of effector T cells. This presents a therapeutic opportunity to deliver effective adoptive cellular immunotherapy in conjunction with suppressive pathway inhibition to overcome the factors curtailing endogenous anti-tumor immunity, as previously reviewed (7).
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. Even after major resection or transplantation, 5-year recurrence rates range from 75 to 100% (28). The immune response to HCC, as measured by TIL density, is useful for HCC patient risk stratification. TIL responses to HCC are likely to be driven by more complex biology than CRCLM given the underlying inflammatory processes that drive HCC carcinogenesis and progression. High densities of DC, memory T cells, and tumor-associated macrophages have been reported to be favorable prognostic factors in HCC (29, 30). Cai et al. stratified their analyses by the ratio of memory T cells to DC, and demonstrated that both intratumoral memory T cells and DC were significant predictors of tumor free survival (29).
HCC TIL subsets have been compared to T cell populations in adjacent normal liver tissue as well. This is of particular interest in HCC as opposed to CRLM, given that many HCC patients suffer from whole-liver inflammation. Relative to normal liver, T cells within HCC tumors reflect a more immunosuppressed state. Mathai et al. demonstrated that HCC TIL populations have a higher number of Treg compared to normal surrounding liver. Increased ratios of FOXP3 to CD3 (p=0.0016) and FOXP3 to CD8 (p=0.0044) both were independently associated with poorer differentiation. The FOXP3:CD8 ratio was also associated with higher nuclear grade (p=0.0179) and recurrence (p=0.0183) in HCC patients. The overall (HR=1.153; 95% CI, 1.019–1.304; p=0.0235) and disease-free (HR=1.138; 95% CI, 1.016–1.273; p=0.0249) survivals time were decreased in patients with high FOXP3:CD8 ratios, seen on both univariate and multivariate analysis (31). The authors including well established prognostic factors in their statistical models, including tumor differentiation and vascular invasion.
In addition to a high level Treg infiltration, TIL within HCC specimens demonstrated increased programmed death-1 (PD-1, CD279) expression (32). PD-1 is a cell surface receptor that negatively regulates T cell function (Figure 1). As such, the presence of Treg and PD-1+ TIL is reflective of an immunosuppressive milieu and explains the correlation with poor outcome. Expression of PD-L1 on tumor tissue was also a predictor of shorter overall survival (32). PD-1 is of particular interest given recent clinical trials showing that PD-1/PD-L1 axis blockade is an effective immunotherapeutic strategy for solid tumors (33).
Like Treg, Th17 cells are a subset of CD4+ TIL and they are characterized by IL-17 production. Th17 cells promote chronic inflammation in a variety of settings, the nature of which may hinder effective anti-tumor immunity (34). The density of IL-17+ TIL cells correlated with HCC development and affect prognosis as well (35). Huang et al. studied the balance between IL-17+ and FOXP3+ TIL in 136 patients with chronic hepatitis B infection, atypical hyperplasia, and HCC. FOXP3+ and IL-17+ TIL absolute numbers and densities were compared. FOXP3+ Treg increased progressively in tissue from subjects with chronic hepatitis B, atypical hyperplasia, and HCC. Patients with the combination of high FOXP3+ and IL-17+ TIL within HCC tumors had worse survival and recurrence times compared to other patients in the study (35). Treg and Th17 cells may cooperate to thwart effective anti-tumor immunity. Treg directly suppress effector T cell function while Th17 cells promote chronic, non-productive inflammation.
INTRAHEPATIC CHOLANGIOCARCINOMA
Cholangiocarcinoma (CCA) is the second most common primary intrahepatic malignancy, and like HCC is often associated with inciting inflammatory events. The majority of the patients presenting with this disease are not candidates for curative surgical intervention. Improving our understanding of the immunologic response to CCA may offer insights into novel therapeutic options and improve disease biology assessments. A total of 123 patients with intrahepatic CCA who underwent curative surgical resection were evaluated by Gu et al. The authors used TMA to analyze the distribution of IL-17+, FOXP3+ and CD8+ TIL, as well as microvessel density within CCA specimens. Mean values of cells per core were utilized for analysis. The mean numbers of IL-17+ and FOXP3+ TIL were 306 and 34, respectively. IL-17+ and FOXP3+ TIL were enriched predominantly in intratumoral (IT) locations, whereas CD8+ lymphocytes were most abundant at the tumor invasive front. In their multivariate analysis, IL-17+ TIL and intratumoral neutrophils were independent predictors of decreased patient survival after controlling for standard risk factors, including vascular invasion, AJCC TNM stage, and node metastases (36). Th17 cells play a role in neutrophil recruitment and both cells types have the capacity to promote tumor growth by stimulating glycolysis and production of growth factors (37, 38).
As noted above, the immunoinhibitory molecule PD-1 can suppress effector T cell function, and its expression among TIL may be an indicator of ineffective anti-tumor immunity. Ye and colleagues studied the expression of PD-1 and its receptor, programmed death ligand-1 (PD-L1) in intrahepatic CCA (39). All specimens were stained and scored for the intensity of PD-L1, with subsequent development of a scoring system from 0 to 3. Tumor expression od PD-L1 was correlated with tumor stage and degree of differentiation on univariate analysis. This likely reflects the importance of tumor PD-L1 interacting with the PD-1 on TILs to inhibit their function, ultimately resulting in tumor progression (39). Should further studies confirm the importance of the PD-1/PD-L1 axis in CCA progression, trials using anti-PD-1 antibodies would be worthy of consideration for CCA patients.
In a recently published study by Goeppert et al., T lymphocytes were shown to be the most prevalent immune cells in biliary tract cancers using TMA. Immune cell densities were assessed by TMA, and the cell counts were stratified according to the median value. Interestingly, the authors observed that the densities of adaptive immune cells decreased from dysplasia to primary carcinoma to metastases. The density of intraepithelial tumor infiltrating CD4+ (p=0.002) and CD8+ (p=0.015) TIL correlated with longer overall survival. In contrast to studies described above, intraepithelial FOXP3+ TIL correlated with improved OS in their multivariate analysis, highlighting the difficulty with using FOXP3 as a single marker for suppressive Treg (40). Takagi et al. demonstrated that the number of CD8+ (p=0.0113) or CD4+ (p=0.0028) cells correlated with DC density. Mature DC likely enhance CD8+ and CD4+ TIL cell infiltration and thereby improve prognosis in patients with CCA (41). As with studying TIL subset ratios, consideration of intratumoral DC density in concert with T cell infiltration provides a more complete picture of the immune network within tumor specimens.
NEUROENDOCRINE TUMOR LIVER METASTASES
Neuroendocrine tumor liver metastases (NETLM) most commonly arise from primary tumors in the pancreas or small bowel. They account for 1–2% of all malignancies and are typically relatively slow growing neoplasms (42). However, NETLM are a frequent cause of death in patients with pancreatic or gastrointestinal neuroendocrine tumors (43). In a report of 39 patients with NETLM, 97% had some degree of T cell infiltration. TIL were counted and scored based on their densities in the tumor per 10 HPF. Potentially immunosuppressive Treg were seen in 55% of intermediate/high grade tumors, whereas only 16% of low grade NETLM demonstrated intratumoral Treg (p=0.02). Following resection of NETLM, higher Treg densities in these tumors were associated with an overall survival of 47 months, in comparison to 108 months in patients with lower levels of FOXP3+ TIL (p=0.029, univariate analysis) (3). The prognostic importance of Treg in NETLM patients suggests that NETLM may be responsive to immunotherapy directed at reversing suppression within the intrahepatic space.
CONCLUSIONS
TIL are indicators of the host immune response to intrahepatic primary and metastatic tumors. As valuable prognostic markers, TIL provide useful information about tumor biology in both primary and metastatic liver tumors. TIL ratios are particularly informative, offering greater insight into how immunosuppression within the liver impairs anti-tumor immunity. A robust intratumoral T cell response in some patients indicates the potential for effective immunity to liver tumors. However, the immunosuppressive intrahepatic space represents an important barrier to effective anti-tumor immunity, as reflected by the small proportion of patients with liver tumors who demonstrate favorable TIL profiles. Data reviewed herein suggests that tumor infiltration by suppressor cells and expression of PD-1 by TIL limits the anti-tumor immune function of CD4+ and CD8+ effector T cells. As such, most patients fail to mount an adequate immune response to intrahepatic neoplasms, which provides compelling rationale for immunotherapy strategies designed to target liver tumors and reverse tumor-induced immunosuppression.
HIGHLIGHTS.
Tumor infiltrating lymphocytes are important predictors of oncologic outcome for patients with primary and metastatic liver cancer
Ratios of tumor infiltrating lymphocyte subsets provide important prognostic information
The intrahepatic space is immunosuppressive and most patients fail to mount effective anti-tumor immune responses to primary or metastatic liver tumors
Acknowledgments
Financial Support: Support for this work was provided by the National Institute of Health (1K08CA160662-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Hadi Khan: conception, writing and revision
Venu G. Pillarisetty: design and revision
Steven C. Katz: conception, design, writing and critical revision
REFERENCES
- 1.Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138:989–995. [PubMed] [Google Scholar]
- 2.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 3.Katz SC, Donkor C, Glasgow K, Pillarisetty VG, Gönen M, Espat NJ, Klimstra DS, D’Angelica MI, Allen PJ, Jarnagin W. T cell infiltrate and outcome following resection of intermediate □ grade primary neuroendocrine tumours and liver metastases. HPB. 2010;12:674–683. doi: 10.1111/j.1477-2574.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC, Hedvat CV, Jarnagin WR, Fong Y, D’Angelica MI. Regulatory T Cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Annals of surgical oncology. 2013;20:946–955. doi: 10.1245/s10434-012-2668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belldegrun A, Muul LM, Rosenberg SA. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer research. 1988;48:206–214. [PubMed] [Google Scholar]
- 6.Gooden MJ, Bock GHde, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saied A, Pillarisetty VG, Katz SC. Immunotherapy for solid tumors--a review for surgeons. J Surg Res. 2014;187:525–535. doi: 10.1016/j.jss.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Jarnagin W, Fong Y, Blumgart L, D’Angelica M, DeMatteo RP. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Annals of surgical oncology. 2009;16:2524–2530. doi: 10.1245/s10434-009-0585-3. [DOI] [PubMed] [Google Scholar]
- 9.Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215:744–745. doi: 10.1038/215744a0. [DOI] [PubMed] [Google Scholar]
- 10.Vierling JM. Liver Immunology. Philadelphia, PA: Hanley & Belfus, Inc; 2003. Animal models of autoimmune diseases of the liver; pp. 263–290. [Google Scholar]
- 11.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. The Journal of Immunology. 2004;173:230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 12.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. The Journal of Immunology. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 13.Emoto M, Kaufmann SHE. Liver NKT cells: an account of heterogeneity. TRENDS in Immunology. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 14.Katz SC, Pillarisetty VG, Bleier JI, Kingham TP, Chaudhry UI, Shah AB, DeMatteo RP. Conventional liver CD4 T cells are functionally distinct and suppressed by environmental factors. Hepatology. 2005;42:293–300. doi: 10.1002/hep.20795. [DOI] [PubMed] [Google Scholar]
- 15.Bubenik J. MHC class I down-regulation: tumour escape from immune surveillance? (review) Int J Oncol. 2004;25:487–491. [PubMed] [Google Scholar]
- 16.Yu P, Fu Y-X. Tumor-infiltrating T lymphocytes: friends or foes? Laboratory investigation. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 19.Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42:405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 20.Packeisen J, Buerger H, Krech R, Boecker W. Tissue microarrays: a new approach for quality control in immunohistochemistry. J Clin Pathol. 2002;55:613–615. doi: 10.1136/jcp.55.8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherwood AM, Emerson RO, Scherer D, Habermann N, Buck K, Staffa J, Desmarais C, Halama N, Jaeger D, Schirmacher P. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunology, Immunotherapy. 2013:1–9. doi: 10.1007/s00262-013-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cintolo JA, Gimotty P, Blair A, Guerry D, Elder DE, Hammond R, Elenitsas R, Xu X, Fraker D, Schuchter LM, Czerniecki BJ, Karakousis G. Local immune response predicts survival in patients with thick (t4) melanomas. Ann Surg Oncol. 2013;20:3610–3617. doi: 10.1245/s10434-013-3086-3. [DOI] [PubMed] [Google Scholar]
- 23.Feichtenbeiner A, Haas M, Buttner M, Grabenbauer GG, Fietkau R, Distel LV. Critical role of spatial interaction between CD8(+) and Foxp3 (+) cells in human gastric cancer: the distance matters. Cancer Immunol Immunother. 2014;63:111–119. doi: 10.1007/s00262-013-1491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin JZ, Upadhyay V, Prabhakar B, Maker AV. Shedding LIGHT (TNFSF14) on the tumor microenvironment of colorectal cancer liver metastases. J Transl Med. 2013;11:70. doi: 10.1186/1479-5876-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of surgery. 1999;230:309. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of Clinical Oncology. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 27.Wagner P, Koch M, Palm S, Galindo L, Autenrieth D, Schmitz-Winnenthal FH, Büchler MW, Beckhove P, Weitz J. Detection and functional analysis of tumor infiltrating T-lymphocytes (TIL) in liver metastases from colorectal cancer. Annals of surgical oncology. 2008;15:2310–2317. doi: 10.1245/s10434-008-9971-5. [DOI] [PubMed] [Google Scholar]
- 28.Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802–805. doi: 10.1002/hep.1840140510. [DOI] [PubMed] [Google Scholar]
- 29.Cai XY, Qiu SJ, Wu ZQ, Ye SL, Fan J, Zhou J, Tang ZY. [Relationship between dendritic cells and memory T lymphocytes in tumor site and prognosis of hepatocellular carcinoma] Zhonghua Yi Xue Za Zhi. 2005;85:671–675. [PubMed] [Google Scholar]
- 30.Li Y-W, Qiu S-J, Fan J, Gao Q, Zhou J, Xiao Y-S, Xu Y, Wang X-Y, Sun J, Huang X-W. Tumor-infiltrating macrophages can predict favorable prognosis in hepatocellular carcinoma after resection. Journal of cancer research and clinical oncology. 2009;135:439–449. doi: 10.1007/s00432-008-0469-0. [DOI] [PubMed] [Google Scholar]
- 31.Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. The American Journal of Surgical Pathology. 2012;36:980–986. doi: 10.1097/PAS.0b013e31824e9b7c. [DOI] [PubMed] [Google Scholar]
- 32.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer research. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New England Journal of Medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Wang F, Wang Y, Zhu Z, Gao Y, Ma Z, Xu R, Du Z. Intrahepatic interleukin-17(+) T cells and FoxP3(+) regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:851–859. doi: 10.1111/jgh.12418. [DOI] [PubMed] [Google Scholar]
- 36.Gu F-M, Gao Q, Shi G-M, Zhang X, Wang J, Jiang J-H, Wang X-Y, Shi Y-H, Ding Z-B, Fan J. Intratumoral IL-17+ Cells and Neutrophils show Strong Prognostic Significance in Intrahepatic Cholangiocarcinoma. Annals of surgical oncology. 2012;19:2506–2514. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Lai Y, Chen H, Guo H, Su I, Chen HHW. Interleukin □17□ producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013 doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 38.Straus DS. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Molecular cancer. 2013 doi: 10.1186/1476-4598-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Y, Zhou L, Xie X, Jiang G, Xie H, Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–504. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- 40.Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, Joehrens K, Warth A, Renner M, Mehrabi A, Hafezi M, Thelen A, Schirmacher P, Weichert W. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109:2665–2674. doi: 10.1038/bjc.2013.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takagi S, Miyagawa S-I, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, Iijima S, Noike T, Kobayashi A, Kawasaki S. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Human pathology. 2004;35:881–886. doi: 10.1016/j.humpath.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 42.DEMÝRKAN BHM, Eriksson B. Systemic treatment of neuroendocrine tumors with hepatic metastases. Turk J Gastroenterol. 2012;23:427–437. doi: 10.4318/tjg.2012.0552. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? Journal of the American College of Surgeons. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]