Abstract
Interleukin-4 (IL4), a cytokine produced mainly by immune cells, may promote the growth of epithelial tumors by mediating increased proliferation and survival. Here, we show that the type II IL4 Receptor (IL4R) is expressed and activated in human breast cancer and mouse models of breast cancer. In metastatic mouse breast cancer cells, RNAi-mediated silencing of IL4Rα, a component of the IL4 receptor, was sufficient to attenuate growth at metastatic sites. Similar results were obtained with control tumor cells in IL4-deficient mice. Decreased metastatic capacity of IL4Rα “knockdown” (KD) cells was attributed in part to reductions in proliferation and survival of breast cancer cells. Additionally, we observed an overall increase in immune infiltrates within IL4Rα KD tumors, indicating that enhanced clearance of KD tumor cells could also contribute to the reduction in KD tumor size. Pharmacological investigations suggested that IL4-induced cancer cell colonization was mediated in part by activation of Erk1/2, Akt and mTor. Reduced levels of pAkt and pErk1/2 in IL4Rα KD tumor metastases were associated with limited outgrowth, supporting roles for Akt and Erk activation in mediating the tumor-promoting effects of IL4Rα. Collectively, our results offer a preclinical proof of concept for targeting IL4/IL4Rα signaling as a therapeutic strategy to limit breast cancer metastasis.
Keywords: cytokine, proliferation, survival, mTor, Akt, Mapk
INTRODUCTION
Breast cancer is still the second leading cause of cancer-related deaths among women in the United States [1]. This is largely due to the high mortality rate in metastatic disease. Cytokines and chemokines in the tumor microenvironment are known to promote breast cancer progression and metastasis [2]. Significantly, interleukin-4 (IL4), a Th2 cytokine, is upregulated in the microenvironment of breast carcinomas [3], and the IL4 receptor (IL4R) is overexpressed by breast cancer cells themselves [4]. In the immune system, IL4 is produced predominantly by activated T cells, mast cells, basophils, and eosinophils, and is known to regulate the survival, proliferation, and differentiation of B and T lymphocytes through IL4R [5]. Therefore, it is plausible that upregulated epithelial IL4R could promote breast tumor growth.
The IL4/IL4R interaction has been shown to enhance the proliferation and survival of breast cancer cells in vitro [6,7], and the survival of other epithelial cancer cell types in vivo [4]. Still, there is no data regarding the influence of IL4R expression on the proliferation and survival of breast cancer cells in vivo, or on metastatic tumor growth in general. Furthermore, activation of signaling pathways downstream of the IL4/IL4R interaction that mediate enhanced tumor growth remain largely unresolved in breast cancer cells that overexpress a different form of the IL4R than immune cells.
Lymphoid cells express the type I IL4R composed of the common gamma chain (γc) and the IL4Rα chain [5]. Heterodimerization is necessary for receptor functionality [8]. Non-lymphoid cells including breast cancer cells, express the type II IL4R, composed of the IL4Rα and IL13 receptor alpha 1 (IL13Rα1) chains. Interleukin-13 (IL13) is the only other cytokine known to bind and activate type II IL4R’s. However, IL4 binds with higher affinity and is the prototypical ligand for the IL4R [2].
In lymphoid cells, the IL4/type I IL4R interaction can result in the activation of two primary pathways: 1) insulin receptor substrate protein (IRS)/PI3K/Akt pathway; and 2) the JAK/signal transducer and activator of transcription factor (Stat6) pathway [5]. Activated Akt [9] and Stat6 [5, 10, 11] are known regulators of proliferation and survival. However, IL4Rα signaling from the type II IL4R may differ from the type I receptor. Demonstrative of this concept, the activation of Akt in response to IL4 appears to be context and cell type dependent [5,12], and IL4 has also been shown to activate Erk in several non-hematopoietic cell types [5]. Here, we investigate whether IL4Rα, a key component of the IL4R, can promote the metastatic colonization and outgrowth of mammary tumor cells, and explore mechanisms of IL4/IL4R-induced colonization ability in vitro.
MATERIALS AND METHODS
Cell Lines and Culture
The 4T1 cell line was acquired from Dr. Albert Reynolds at Vanderbilt University. PyVT-R221a cells were isolated from a polyoma middle T oncoprotein (PyMT) tumor as previously described [13]. The R221a line was authenticated by expression of the polyoma antigen, and the morphology and growth rates of both cell lines were consistently monitored. Cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS, Atlanta Biologicals) at 37°C with 5% CO2. Mission lentiviral small hairpin RNA (shRNA) particles (Sigma) targeting two different regions of murine IL4Rα (NM_010557) along with control non-target particles were used to infect 4T1 cells. Control non-target and specific murine IL4Ra-targeted shRNA lentiviral particles (Santa Cruz Biotechnologies) were used to infect R221a cells. Post-infection, shRNA-expressing cells were selected with puromycin dihydrochloride (Sigma). The percent of IL4Rα protein knockdown was calculated by densitometry in Adobe Photoshop® following western blot analysis.
Reagents and Antibodies
Murine IL4 was purchased from BD Biosciences. The small molecule inhibitors, U1026 (EMD Millipore), Ly294002, and rapamycin (both Sigma) were dissolved in dimethylsulfoxide (DMSO, Sigma) and diluted with culture medium to the appropriate concentration for western blots and clonogenic assays. The following antibodies were used to probe western blots: murine IL4Rα, Stat6, Erk2 and β-actin from Santa Cruz Biotechnologies, anti-pStat6 [pY641] from Invitrogen, pErk1/2 [Thr202/Tyr204], Akt, pAkt [ser473], pAkt [Thr308], mTor, pmTor [ser2481], pmTor [ser2448], IRS1, and pIRS1 [ser612] from Cell Signaling Technology. An IL4Rα-Fc chimeric protein (R&D Systems) and isotype human IgG1k control antibody (SouthernBiotech) were used in clonogenic assays.
Commercial Arrays
A human tissue array containing 16 stage-IIIa/IIIb and 34 stage-IIa/IIb primary breast carcinomas from patients with lymph node metastases, cat#BR10010a, was purchased from US Biomax. The array was probed with antibodies for human IL4Rα (Santa Cruz Biotechnologies) and pStat6 [pY641] (Sigma). Samples were scored categorically as positive or negative for IL4Rα and pStat6. Conditioned media (serum free) from R221a or 4T1 combined sh-control or IL4Rα KD clones was added to an antibody array to detect sixty-two murine secreted factors (RayBiotech Inc. #AAM-CYT-3-4) per manufacturer’s instructions. Protein expression was quantified by densitometry in ImageJ® relative to positive controls.
Analysis of Murine Tumor Models
Animal procedures were conducted in accordance with Guidelines for the Care and Use of Laboratory Animals following approval by the Institutional Animal Care and Use Committee. Female age-matched BALB/c, FVB/N, and BALB/c IL4−/− mice (BALB/c-Il4tm2Nnt/J, Jackson Laboratories) were used. Two individual clones for each cell line (R221a or 4T1) were mixed to generate combined sh-control or IL4Rα KD cells pre-injection. Additional assays with 4T1 cells were performed using one KD clone, which recapitulated findings with combined clones. At necropsy, lungs were inflated with Bouin’s solution (Ricca Chemical Company) to facilitate surface tumor counts. Formalin-fixed, paraffin-embedded tissue sections from lungs and livers were stained with hematoxylin and eosin (H&E) to assess tumor burden. Tissue sections were also stained with primary antibodies for IL4Rα (Santa Cruz Biotechnologies), Ki67 (Abcam), cleaved caspase-3 (Cell Signaling Technology), Ly6B.2, CD3-ε, or F4/80 (AbD Serotec). Additionally, lung sections were probed for pStat6 [pY641] (Sigma), pErk1/2 (Santa Cruz Biotechnologies), and pAkt [ser473] (Cell Signaling). Archival paraffin-embedded PyVT and orthotopic 4T1 and R221a sh-control mammary tumors were stained for IL4Rα and pStat6 and analyzed similarly to the human tissue array. Biotinylated secondary antibodies were obtained from Vector Labs. Images were white balanced for presentation using Adobe® Photoshop.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism® software. For comparison of two conditions, data was analyzed using the parametric unpaired Student’s T test, or the nonparametric Mann-Whitney. Otherwise ANOVA or Kruskal-Wallis with post-test was used. In graphs, error bars represent the standard deviation, and P values are represented by stars where: * ≤.05, ** ≤.01, and *** ≤.001.
RESULTS
IL4Rα expression and activation in human breast cancer and murine mammary cancer models
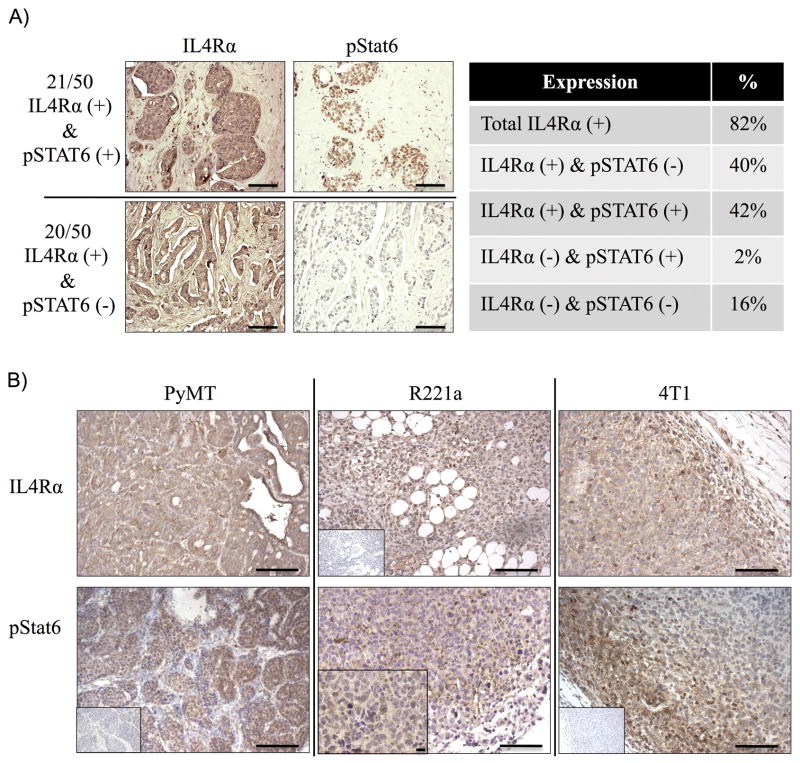
To examine the relative abundance and activation of IL4Rα in human breast cancers, we first immunostained a human tissue array consisting of 50 primary node-positive breast cancer samples for IL4Rα and downstream phosphorylation of STAT6 (pSTAT6). A total of 82% of the samples were IL4Rα positive, and 42% were positive for both IL4Rα and pSTAT6 (Fig. 1A). The majority of carcinomas were classifiable by HER2 status (38 HER2 positive and 12 HER2 negative, of which 5 were triple negative). However, sample numbers were too low to make statistically significant correlations between subtype and IL4Rα or pSTAT6 positivity. Mammary tumors from the polyoma middle T oncogene transgenic mouse, and tumors resulting from orthotopic injection of R221a and 4T1 murine mammary cancer cells, were also positive for IL4Rα and pStat6 (Fig 1B). Thus, human breast cancers and our murine models both show IL4 receptor expression and activation.
Figure 1. IL4 receptor expression and activation in human breast cancers and murine models.
A) Left: Representative images of a human tissue microarray (TMA) of breast carcinoma samples (n = 50) stained by immunohistochemistry for IL4Rα or pSTAT6. Right: Summary of categorical (positive/negative) TMA staining results. B) Representative images of IL4Rα and pStat6 immunostaining in murine mammary tumors (n=3 each). Scale bar = 100 μM. Inset: negative staining controls, and one 40X image (scale bar = 10 μM) of pStat6 in an R221a tumor.
IL4Rα promotes the growth of murine mammary tumor metastases in the lung and liver
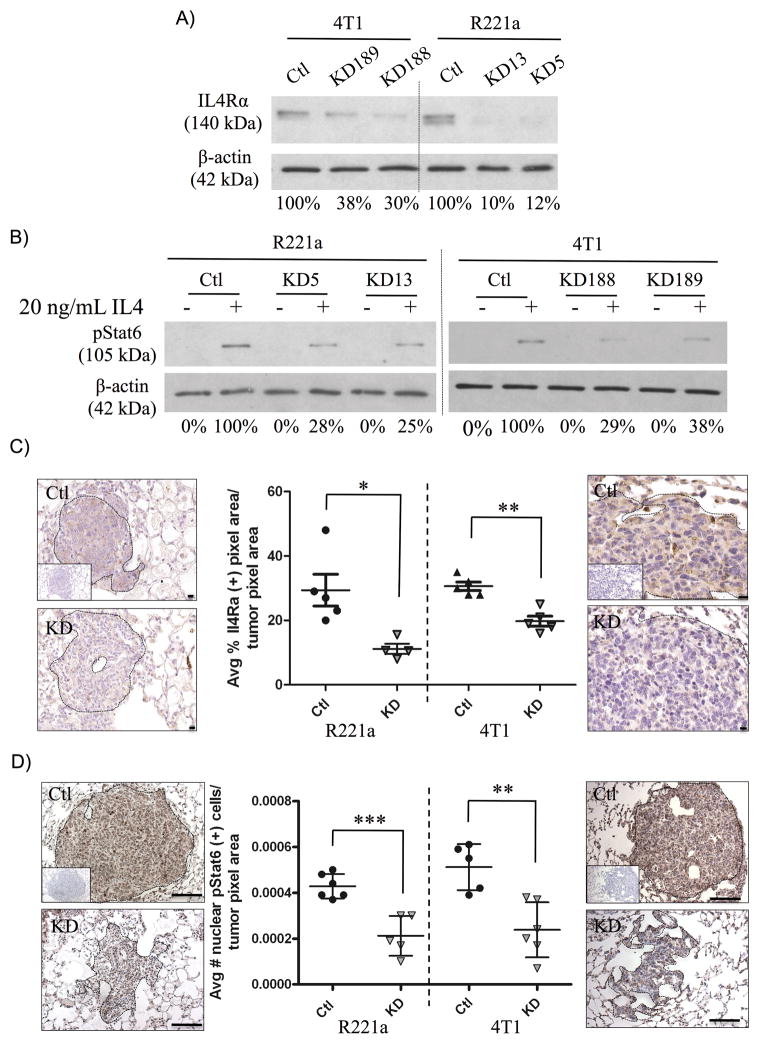
Since the IL4/IL4R interaction is species-specific, only murine mammary tumor cell lines could be used to study functional roles of IL4 receptor signaling in mouse models. The 4T1 line was derived from a spontaneous mammary cancer in a BALB/c mouse [14]. The R221A line came from a mammary tumor in an MMTV-PyVT transgenic mouse on the FVB/N background [13]. Using commercially available lentiviral particles carrying short-hairpin RNA, we generated two clones representing different knockdown sequences for the 4T1 line. A separate mix of IL4Rα-targeted shRNA sequences was used to make two different clones of R221A cells. Corresponding sh-control lines made by expression of non-targeting shRNA sequences were also generated. Western blot analysis confirmed a reduction (62–90%) in IL4Rα protein expression (Fig. 2A), and in Stat6 activation in R221a and 4T1 IL4Rα knockdown (IL4Rα KD) clones compared to sh-controls.
Figure 2. Functional knock down of IL4Rα expression in vitro and in vivo.
A) Western blot illustrating the percent knockdown of IL4Rα protein expression and B) Stat6 activation in response to 20ng/mL IL4 in individual R221a and 4T1 control (ctl) and IL4Rα KD (KD) clones. C) Representative images and quantification of immunohistochemical staining for IL4Rα (40X, scale bar = 10 μM) or D) pStat6 (20X, scale bar = 100 μM) in R221a and 4T1 sh-control and IL4Rα KD lung metastases (n = 4–6). Inset: negative staining controls.
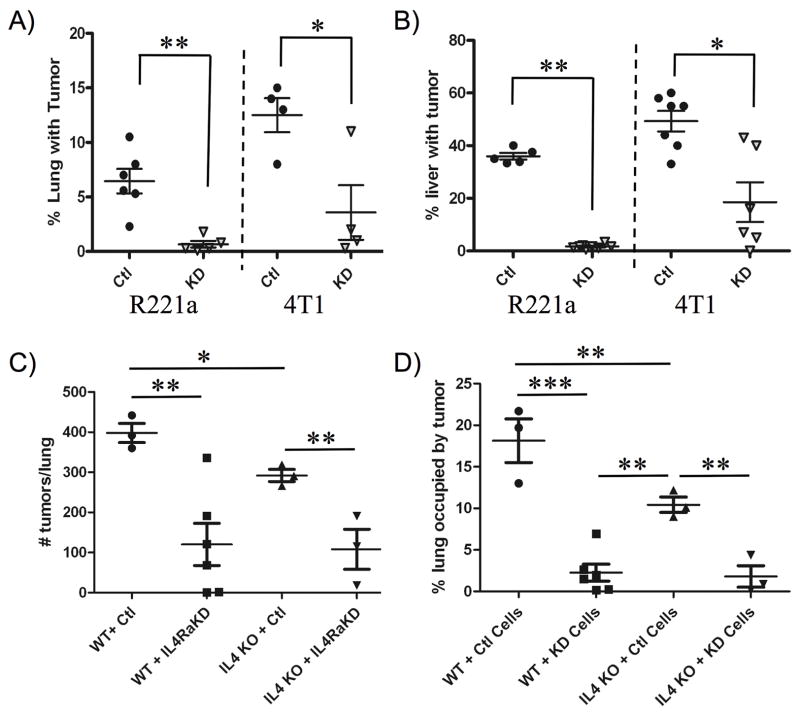
Combined sh-control or IL4Rα KD clones for each cell line (R221a or 4T1) were injected into the tail vein or spleen of age-matched female mice to examine colonization of lung and liver, respectively. At endpoint, the number of surface tumors on the lungs of tail vein injected mice was significantly reduced in tumors originating from IL4Rα KD clones for both cell lines (Fig. S1A). In addition, gross liver weights of mice receiving splenic injections of R221a IL4Rα KD clones were significantly reduced compared to sh-controls, but no significant difference in liver weight was seen with the 4T1 line (Fig. S1B). Following gross examination, lungs and livers were serially sectioned for further analysis. Immunohistochemical analysis confirmed that functional knockdown of IL4Rα was retained in vivo, as IL4Rα expression (Fig. 2C) and nuclear pStat6 levels (Fig. 2D) were significantly reduced in IL4Rα KD lung tumors compared to sh-control for both cell lines. Serial lung (Fig. S1C) and liver (Fig. S1D) sections were also stained with H&E, and used to calculate tumor burden. In both the R221a and 4T1 cell lines, reduced IL4Rα expression resulted in a significant decrease in metastatic lung and liver tumor burden (Fig. 3A, 3B).
Figure 3. The IL4/IL4Rα interaction promotes metastatic tumor growth in vivo.
Quantification of tumor burden from H&E stained A) lung and B) liver sections (3 per mouse) from mice receiving R221a or 4T1 IL4Rα KD or sh-control cells (lung: n = 4–6, liver: n = 5–8). C) Enumeration of lung surface tumors and D) quantification of lung tumor burden from H&E stained serial sections from Wild-type (WT) or IL4−/− (IL4 KO) mice receiving either 4T1 sh-control or IL4Rα KD clones by tail vein injection (n = 3–6).
We previously determined that neither R221a nor 4T1 cells produce detectable levels of IL4 by Luminex analysis (data not shown), suggesting that the cellular source of IL4 in vivo is likely the host. In order to determine the contribution of host IL4 to mammary cancer metastasis to the lung, as well as the significance of the IL4/IL4R interaction in vivo, wild type (WT) or IL4−/− (IL4 KO) BALB/c mice were injected via tail vein with either combined sh-control or IL4Rα KD 4T1 clones. Supporting the role of IL4 in promoting metastatic tumor growth, the number of lung surface tumors (Fig. 3C) and the lung tumor burden (Fig. 3D) at endpoint were significantly decreased in IL4 KO mice compared to WT mice receiving sh-control cells. These results suggest that the IL4/IL4Rα interaction is a strong promoter of lung metastatic tumor growth in vivo.
IL4Rα-associated survival and proliferation contribute to metastatic colonization and outgrowth
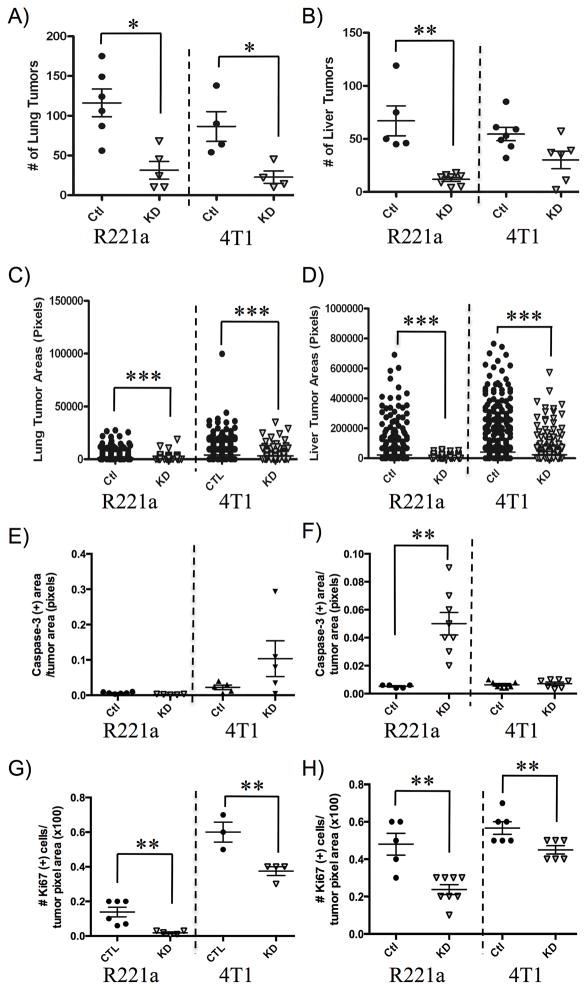
The metastatic seeding and outgrowth ability of R221a and 4T1 clones was estimated by quantifying tumor number and area, respectively, from the H&E-stained lung and liver sections (Fig. S1B, S1C). The ability of R221a IL4Rα knockdown clones to seed tumors was significantly reduced in both the lung (Fig. 4A) and liver (Fig. 4B) compared to sh-control clones. While the same was true for 4T1 IL4Rα KD clones compared to sh-controls in the lung (Fig. 4A), the decrease in seeding ability in the liver was not significant (Fig. 4B). However, in both cell lines reduced IL4Rα expression resulted in a significant decrease in lung (Fig. 4C) and liver tumor outgrowth (Fig. 4D).
Figure 4. IL4Rα-associated survival and proliferation contribute to metastatic tumor growth.
A) Enumeration of R221a or 4T1 IL4Rα KD and sh-control lung and B) liver metastatic tumors per mouse (lung: n = 4–6, liver: n = 5–8) from H&E stained sections (3 per mouse). C) Quantification of individual lung or D) liver tumor areas from the same H&E sections. E) Quantification of total cleaved caspase-3 positive area per total IL4Rα KD or sh-control lung or F) liver tumor area for each mouse (lung: n = 4–6, liver: n = 5–8). G) Quantification of the total number of Ki67 positive cells per IL4Rα KD or sh-control tumor area for each mouse lung or H) liver (lung: n = 3–6, liver: n = 5–8).
In addition to quantifying tumor number and size, lung and liver metastases were stained for cleaved caspase-3 (apoptosis marker) and Ki67 (proliferation marker) to further examine the role of IL4Rα in promoting the survival and outgrowth of metastatic tumors (Fig. S2). Differences in cleaved caspase-3 in the lungs of mice receiving either R221a or 4T1 IL4Rα KD cells by tail vein injection were not statistically significant (Fig. 4E). In R221a tumors this was likely due to the lack of assessable staining within very small KD tumors. Quantification of cleaved caspase-3 staining in liver metastases revealed a significant increase in apoptosis in IL4Rα KD tumors of R221a, but not 4T1 recipient mice compared to sh-control (Fig. 4F). Ki67 staining results were more consistent in that mice receiving either R221a or 4T1 IL4Rα KD cells exhibited a significant reduction in proliferation in both lung (Fig. 4G) and liver metastases (Fig. 4H). These data suggest that IL4Rα promotes the growth of murine mammary cancer cells at metastatic tumor sites through enhanced survival and colonization ability and increased proliferation for outgrowth.
Changes in immune infiltration contribute to IL4Rα-enhanced metastasis
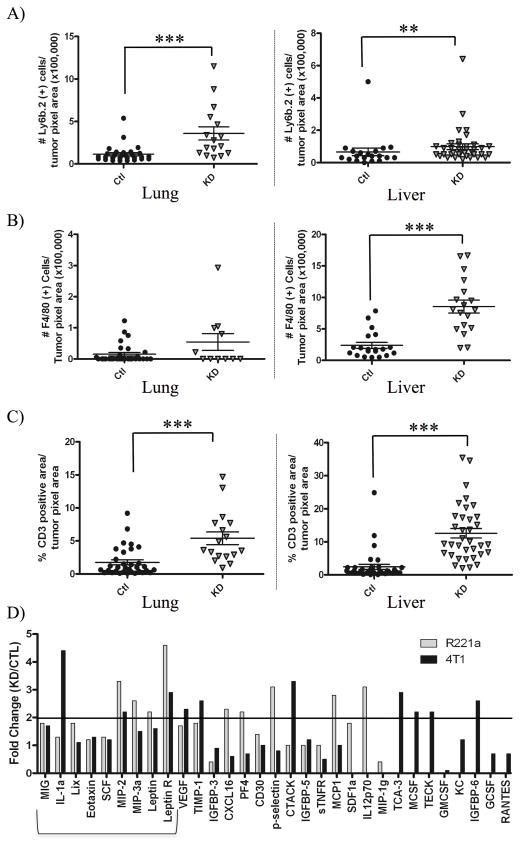
It is possible that increases in infiltrating immune cells could aid in the clearance of IL4Rα KD tumor cells with reduced survival ability, thus decreasing metastatic tumor size. We therefore quantified infiltrating immune cells in R221a and 4T1 sh-control and IL4Rα KD lung and liver metastases by immunohistochemical staining of neutrophils (Ly6B.2 positive), macrophages (F4/80 positive), and T lymphocytes (CD3 positive) (R221a: Fig. S3, 4T1: Fig. S4). There was an upward trend or significant increase in the number of each of these cell types in R221a and 4T1 lung and liver IL4Rα KD metastases compared to sh-control (R221a: Fig. 5A–C, 4T1: Fig. S5).
Figure 5. IL4Rα expression is associated with changes in immune infiltration and cytokine production.
Quantification of A) neutrophils, B) macrophages, and C) lymphocytes from immunostained lung and liver sections of mice receiving R221a or IL4Rα KD or sh-control cells (n = 4–7). D) Quantitation of the fold change in protein levels from R221a and 4T1 IL4Rα KD and sh-control conditioned media, assessed using a commercial cytokine array and densitometry. Proteins increased in IL4Rα KD clones for both cells lines that have potential to regulate immune recruitment are bracketed.
Higher levels of immune infiltrates within KD tumors could result from an increased expression of pro-inflammatory cytokines. To examine this, a murine cytokine array was used to test conditioned media from combined R221a or 4T1 sh-control or IL4Rα KD clones for the expression of sixty-two secreted factors. Five factors associated with recruitment of immune cells (MIG, IL-1a, Lix, Eotaxin, SCF, and MIP-3) were modestly upregulated by IL4Rα KD clones in comparison to sh-control clones for both cell lines (Fig. 5D). Macrophage inflammatory protein-2 (MIP-2) was the only cytokine that increased greater than 2-fold in KD clones for both cell lines. Leptin receptor has no known direct role in immune cell recruitment, however this protein was also upregulated greater than 2-fold by both R221a and 4T1 KD clones. Thus, while it is possible that increased MIP-2 production from IL4Rα KD clones may lead to enhanced leukocyte recruitment, we were unable to identify robust patterns of cytokine production that differed between sh-control and IL4Rα KD cells.
IL4-activated Akt, Erk and mTor mediate colonization ability of mammary cancer cells
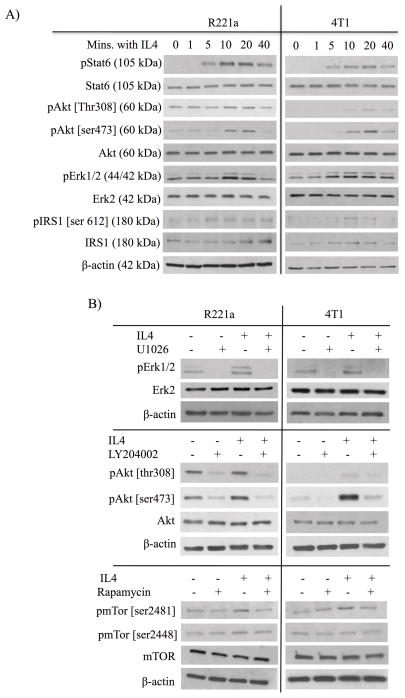
Our in vivo data establishes that the IL4 receptor mediates enhanced colonization of mammary cancer cells at metastatic sites, and that host IL4 promotes the growth of lung tumor metastases. Therefore, we used exogenous IL4 to elucidate signaling pathways that control IL4Rα-induced colonization ability in vitro. Demonstrative of functional IL4/IL4Rα signaling, treatment of R221a and 4T1 mammary cancer cells with IL4 resulted in the activation of Stat6 (Fig. 6A). Erk1/2 and Akt [ser473] were also phosphorylated in response to IL4 in both cell lines, while Akt [thr308] was only robustly phosphorylated in R221a cells (Figure 6A). Consistent with IRS1 inactivation and degradation [15,16], IRS1 [ser612] was also phosphorylated in response to IL4 in both cell lines in a temporal manner, and total IRS1 levels initially increased then rapidly decreased in the 4T1 cell line.
Figure 6. Inhibition of IL4-activated Stat6, Akt, and Erk.
Representative western blots were completed using whole cell lysates from combined R221a or 4T1 sh-control clones, and 20 ng/mL IL4. A) Timecourse analysis of effector proteins activated within 40 minutes of IL4 treatment. B) U1026 (R221a: 10 μM; 4T1: 15 μM) inhibits IL4-activated pErk1/2, LY294002 (R221a: 5 μM; 4T1: 8 μM) inhibits IL4-activated pAkt, and rapamycin (R221a: 5 nM; 4T1 3 nM) inhibits pmTor.
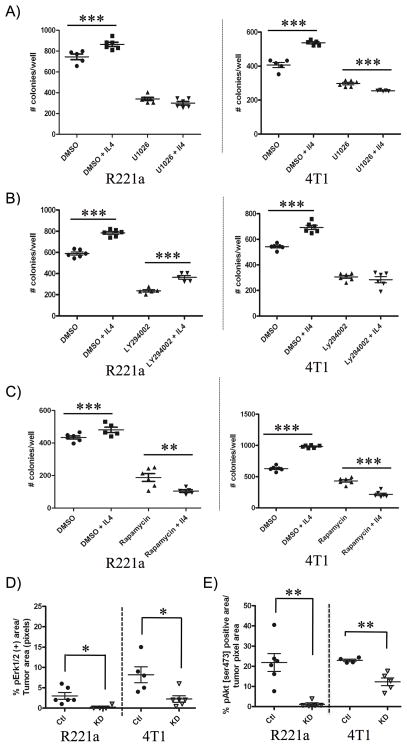
We next confirmed the specificity of the IL4/IL4Rα interaction in promoting colonization ability in vitro. R221a or 4T1 combined sh-control or IL4Rα KD cells were plated at clonal density in the presence or absence of IL4 with an IL4 blocking decoy receptor (IL4Rα-FC), or an immunoglobulin G (IgG) control. The ability of IL4Rα-FC to neutralize IL4-induced Stat6 activation at the dose used in clonogenic assays was confirmed by western blot (Fig 6A). As expected, the significant increase in colonization ability in response to IL4 was blocked by the addition of IL4Rα-FC in control clones, but neither IL4 nor IL4Rα-FC had a significant effect on the colonization ability of IL4Rα KD clones for either cell line (Fig. S6B). We then used small molecule inhibitors to test the contribution of IL4-actived Erk and Akt to IL4-induced colonization ability. U1026 was used to inhibit the activity of Erk1/2 [17], and LY294002, a known PI3K inhibitor, was used to inhibit Akt phosphorylation at both sites (thr308 and ser473) [18]. First, the effective dose of inhibitor that decreased colony formation by 50% (ED50) was determined for each cell line (data not shown). Prior to their use in clonogenic assays, selected drug doses were also confirmed to inhibit IL4-induced activation of pErk1/2 or pAkt [thr308/ser473], by western blot (Fig. 6B). Suggesting that IL4-activated Erk1/2 promotes initial colonization, U1026 treatment in both cell lines blocked IL4-induced colony formation (Fig. 7A). Treatment with LY294002 also blocked IL4-induced colony formation in the 4T1 cell line, but not the R221a line (Fig. 7B).
Figure 7. The IL4/IL4Rα interaction promotes colonization ability via Erk, Akt, and mTor activation.
R221a or 4T1 combined sh-control clones were seeded +/− IL4 (R221a: 2ng/mL, 4T1: 10 ng/mL) and +/− drug. A) U1026 (R221a: 10 μM, 4T1: 15 μM) inhibits IL4-induced colony formation. B) LY294002 inhibits colony formation in 4T1 cells (8 μM), but not R221a cells (5 μM). C) Rapamycin (R221a: 5 nM, 4T1 3 nM) inhibits IL4-induced colony formation. D) Quantification of pErk1/2 and E) pAkt [ser473] immunostaining in R221a and 4T1 IL4Rα KD and sh-control lung metastases. The percent positive area per mouse (n = 4–6) is shown.
A potential complication with LY294002 is that it also inhibits mammalian target of rapamycin (mTor) at concentrations slightly higher than the 50% inhibitory concentration (IC50) of PI3K [19]. We therefore also tested the effect of rapamycin. In both cell lines, combined IL4 and rapamycin treatment significantly reduced colony formation compared to IL4 treatment alone (Fig. 7C). We then discovered by western blot that rapamycin treatment resulted in strong inhibition of IL4-activated mTor ser2481, but not mTor ser2448, indicating that activated mTorc2 rather than mTorc1 may be important in regulating IL4-induced colony formation (Fig. 6B).
To evaluate whether the signaling pathways identified in vitro were relevant in vivo, we immunostained for pAkt [ser473], pErk1/2, and pmTor [ser2481] in R221a and 4T1 sh-control and IL4Rα KD lung metastases (Fig. S7). The anti-pmTor [ser2481] antibody selectively labeled mitotic cells with high intensity and did not provide useful information. However, we were able to confirm a significant reduction in both pErk1/2 and pAkt [ser473] in IL4Rα KD R221a and 4T1 lung metastases compared to sh-controls (Fig. 7D, E).
DISCUSSION
The biological effects of the IL4/IL4Rα interaction have recently been extended from immune cells to epithelial cancer cells expressing the Type II IL4R (IL4Rα and IL13Rα1 chains). Previous studies have established the importance of this interaction in promoting pro-tumorigenic phenotypes including proliferation and survival ability in several types of epithelial cancers [20]. However, the majority of these studies utilized in vitro assays and subcutaneous xenograft models in nude mice. Here, we have established the relevance of IL4Rα expression in human breast cancer and in two syngeneic murine models of breast cancer (Fig. 1). Using murine models we have defined a novel role of epithelial IL4Rα in promoting metastatic mammary tumor growth.
Using R221a and 4T1 IL4Rα knockdown (KD) clones and wild-type mice in experimental metastasis assays we demonstrated that reduced IL4Rα expression results in significantly attenuated metastatic tumor burden in both the lung and the liver (Fig. 3). This reduced burden was also seen in the lungs of IL4−/− mice injected with control cells, indicating that reducing either the ligand or receptor can attenuate metastatic growth (Fig 3C, D). Notably, IL4−/− mice injected with IL4Rα KD cells showed a greater reduction in tumor burden than either IL4−/− or IL4Rα KD alone. This could be partially explained by incomplete KD of IL4Rα, but may also reflect a role for the other type II IL4R ligand, IL13 in vivo. Decreased metastatic ability of IL4Rα knockdown clones was attributed to 1) reduced tumor cell survival as determined by seeding ability (number of tumors) and an increase in apoptosis by cleaved caspase-3 positivity, and 2) a reduction in proliferation determined by tumor outgrowth ability (tumor size) and a decrease in Ki67 positivity (Fig. 4). In some cases, differences in these parameters were not significant at endpoint, which may be attributable to changes that occurred earlier in tumor development. Additionally, there are large differences in the growth rates of the cell lines. The aggressive and highly metastatic 4T1 line is typically used to establish models representative of clinical stage four breast cancer whereas R221a cells rarely metastasize spontaneously, and may represent less aggressive breast cancers. This growth disparity is most evident in the H&E stained images of the 4T1 livers (Fig. S1D), where large tumors were difficult to delineate from each other.
In addition to proliferation and survival, we examined whether immune infiltrates contributed to the reduction in KD tumor size. We saw increases in almost all leukocyte populations examined in both R221a and 4T1 KD metastases (R221a: Fig. 4, 4T1 Fig. S5). Thus our results overall suggest that more efficient clearance of KD tumor cells could contribute to the reduction in tumor size visualized at endpoint compared to sh-control tumors.
It is feasible that enhanced production of pro-inflammatory cytokines from IL4Rα KD cells could recruit immune cells to these tumors. However, out of sixty-two secreted pro-inflammatory factors examined by cytokine array, only MIP-2 was increased greater than 2-fold by IL4Rα KD cells in comparison to sh-controls for both cell lines (Fig. 5D). MIP-2 secreted from epithelial cells enhances neutrophil and lymphocyte recruitment [21]. Leptin/leptin receptor signaling reportedly induces MIP-2 production in pre-neoplastic colon cells [22]. Therefore, the increased expression of leptin (> 1.5-fold) and its receptor (> 2-fold) within IL4Rα KD tumors could also facilitate neutrophil and lymphocyte recruitment. Still, strong patterns of cytokine production from IL4Rα KD cells were lacking, indicating that other mechanisms may contribute to IL4Rα-induced survival and proliferation ability at metastatic sites.
The Jak/Stat6 pathway is activated by the IL4/IL4Rα interaction in lymphoid cells [5], and in several epithelial cancer cell types including breast cancer (Fig. 1, [7]). We were able to confirm activation of IL4R in vitro via phosphorylation of downstream Stat6 in response to IL4 (Fig. 6A). Stat6 has been shown to regulate pro-metastatic processes including migration and invasion [23] and enhanced survival and proliferation in vitro [4, 7, 21], although one study showed that Stat6 activation decreased between ductal carcinomas in situ and invasive breast ductal carcinomas [24]. That study did not examine IL4Rα expression, or other IL4/IL4Rα-activated pathways including PI3K/Akt and MAPK/Erk that may be important for the promotion of metastatic phenotypes in vivo.
Using the Mek inhibitor, U1026, we determined that IL4-activated Erk1/2 promotes the colonization ability of mammary cancer cells in vitro (Fig. 7A). The PI3K/Akt pathway inhibitor, LY294002, blocked IL4-activated Akt in both cell lines, but only abrogated colony formation in the R221a line (Fig. 7B). The R221a cells express the middle T oncoprotein, a strong driver of constitutive PI3K/Akt and Src kinase activity, which would be unaffected by LY294002 treatment [25]. These results confirm previous reports that activation of Akt in response to IL4 is cell type/context dependent [5, 12].
We next examined whether IL4-activated mTor could promote IL4-induced colony formation for two reasons: 1) Akt was phosphorylated at ser473 in both cell lines in response to IL4 (Fig. 6A); and 2) LY294002 is known to fit in the active catalytic domain of mTor and inhibit its activity at slightly higher concentrations than the IC50 for PI3K inhibition [19]. Rapamycin, a potent mTorc1 inhibitor, has also been shown to inhibit ser2481 phosphorylation and mTorc2 [26]. Use of this compound in vitro demonstrated that IL4-activated mTorc2 [ser2481], but not mTorc1 [ser2448], is a potent promoter of colonization ability in both the R221a and 4T1 cell lines (Fig. 6B, Fig. 7C). Our findings are consistent with a previous report demonstrating that mTorc2 but not mTORC1 mediates the survival of breast cancer cells [27].
Insulin receptor proteins (IRS) proteins mediate the activation of several signaling pathways in response to IL4 [27,28]. IRS1 expression in breast cancers is often decreased in high-grade invasive tumors compared to well-differentiated tumors [24,31,32]. Also, IRS1 in murine lung metastases is inactivated by serine phosphorylation, and loss of IRS1 expression in murine mammary cancer cells led to increased lung metastases [32]. Serine phosphorylation of IRS1 for inactivation and/or proteasomal degradation is known to be mediated by several kinases including mTor, Akt, and Mapk [33], three effector proteins activated in response to IL4 (Fig. 6). In R221a and 4T1 cells, ser612 is phosphorylated in a time dependent manner after IL4 (Fig. 6A). This may explain the eventual decrease of total IRS1 protein levels in 4T1 cells (Fig. 6A). These results indicate that IL4 signaling may induce the inactivation and proteasomal degradation of IRS1, thus removing a potential metastasis-suppressor.
In conclusion, we have demonstrated for the first time that the IL4/IL4Rα interaction promotes mammary metastatic tumor growth, and that loss of IL4Rα in mammary cancer cells results in reduced survival and proliferation and decreased colonization and outgrowth at metastatic sites. It is known that the Mapk/Erk, and PI3K/Akt/mTor signaling axes are potent drivers of breast cancer growth [26,27]. We have shown that Erk, Akt, and mTor are downstream effectors of the IL4Rα-associated pro-metastatic colonization phenotype. Additionally, suppression of IRS1 signaling may be another mechanism by which IL4/IL4Rα promotes mammary cancer metastatic tumor growth. Overall, systemic inhibition of IL4Rα may have promise as an anti-cancer therapy, provided that any adverse effects on immune cells that signal through the type I IL4 receptor could be minimized. Significantly, modified IL4 ligands termed “superkines,” are capable of specifically targeting the type II IL4 receptor, and are being tested for improving cytokine therapy selectivity [36]. We raise the possibility of using such agents to target the type II IL4 receptor on breast cancer cells as a novel means to thwart metastatic tumor growth.
Supplementary Material
Acknowledgments
Financial support: This work was supported by DOD award W81XWH-09-1-0446, and by R01 CA157781 to BF. KTV was supported by the Cellular, Biochemical and Molecular Sciences training program (5T32GM008554). Use of the Translational Pathology Shared Resource was supported by Cancer Center Support Grant # P30 CA068485. Additionally, support was obtained from the National Center for Research Resources, Grant UL1 RR024975-01, now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06.
We gratefully acknowledge Drs. Sung Hoon Cho and Mark Boothby for Luminex measurements, Dr. Joe Roland and the Epithelial Biology Core for access to the Gelcount instrument and Leica SCN400 slide scanner, and Dr. Christine Lovly for consultation and the IRS1 antibody.
Footnotes
No conflicts of interest to declare.
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5:31–6. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camp BJ, Dyhrman ST, Memoli VA, Mott LA, Barth RJ. In situ cytokine production by breast cancer tumor-infiltrating lymphocytes. Ann Surg Oncol. 1996;3:76–84. doi: 10.1007/BF02305798. [DOI] [PubMed] [Google Scholar]
- 4.Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15:762–72. doi: 10.1038/sj.cdd.4402305. [DOI] [PubMed] [Google Scholar]
- 5.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Ann Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 6.Roca H, Craig MJ, Ying C, Varsos ZS, Czarnieski P, Alva AS, et al. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin upregulation. J Cell Biochem. 2011;113:1569–80. doi: 10.1002/jcb.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang WJ, Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42:39–47. doi: 10.1016/j.cyto.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–3. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 9.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–8. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–8. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 13.Martin MD, Carter KJ, Jean-Philippe SJ, Chang M, Mobashery S, Thiolloy S, et al. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008;68:6251–9. doi: 10.1158/0008-5472.CAN-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dexter DL, Kowalski HM, Blazar BA, Fligiel Z, Vogel R, Heppner GH. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978;38:3174–81. [PubMed] [Google Scholar]
- 15.Pederson TM, Kramer DL, Rondinone CM. Serine/Threonine phosphorylation of IRS-1 triggers its degradation: Possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Sarikas A, Dias-Santagata DC, Dolios G, Lafontant PJ, Tsai S-C, et al. The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitin-dependent degradation. Mol Cell. 2008;30:403–14. doi: 10.1016/j.molcel.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goueli S, Hsiao K, Lu T, Simpson D. U0126: A Novel, Selective and Potent Inhibitor of MAP Kinase Kinase (MEK) Promega Notes. 1998;69:6. [Google Scholar]
- 18.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 19.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–67. [PMC free article] [PubMed] [Google Scholar]
- 20.Hallett MA, Venmar KT, Fingleton B. Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res. 2012;72:6338–43. doi: 10.1158/0008-5472.CAN-12-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsuka Y. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenton JI, Hursting SD, Perkins SN, Hord NG. Leptin induces an Apc genotype-associated colon epithelial cell chemokine production pattern associated with macrophage chemotaxis and activation. Carcinogenesis. 2007;28:455–64. doi: 10.1093/carcin/bgl137. [DOI] [PubMed] [Google Scholar]
- 23.Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, Yuan J, et al. IL-4/Stat6 activities correlate with apoptosis and metastasis in colon cancer cells. Biochem Biophys Res Commun. 2008;369:554–60. doi: 10.1016/j.bbrc.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 24.Porter HA, Perry A, Kingsley C, Tran NL, Keegan AD. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett. 2013;338:239–48. doi: 10.1016/j.canlet.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Jia S, Zhu Y, Utermark T, Signoretti S, Loda M, et al. Transgenic expression of polyomavirus middle T antigen in the mouse prostate gives rise to carcinoma. J Virol. 2011;85:5581–92. doi: 10.1128/JVI.02609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Lin J, Wang X, Yao G, Wang L, Zheng H, et al. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Cancer Res Treat. 2012;134:1057–66. doi: 10.1007/s10549-012-2036-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang LM, Myers MG, Sun XJ, Aaronson SA, White M, Pierce JH. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993;261:1591–4. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- 29.Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG, Glasheen E, et al. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–7. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 30.Schnarr B, Strunz K, Ohsam J, Benner A, Wacker J, Mayer D. Down-regulation of insulin-like growth factor-I receptor and insulin receptor substrate-1 expression in advanced human breast cancer. Int J Cancer. 2000;89:506–13. doi: 10.1002/1097-0215(20001120)89:6<506::aid-ijc7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Sisci D, Morelli C, Garofalo C, Romeo F, Morabito L, Casaburi F, et al. Expression of nuclear insulin receptor substrate 1 in breast cancer. J Clin Pathol. 2007;60:633–41. doi: 10.1136/jcp.2006.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol Cell Biol. 2006;26:9338–51. doi: 10.1128/MCB.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gual P, Le Marchand-Brustel Y, Tanti J-F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Cidado J, Park BH. Targeting the PI3K/Akt/mTOR pathway for breast cancer therapy. J Mammary Gland Biol Neoplasia. 2012;17:205–16. doi: 10.1007/s10911-012-9264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzivion G, McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EWT, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta - Mol Cell Res. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Junttila IS, Creusot RJ, Moraga I, Bates DL, Wong MT, Alonso MN, et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat Chem Biol. 2012;8:990–8. doi: 10.1038/nchembio.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.