Thalassaemia is characterized by ineffective erythropoiesis and anaemia, which can precipitate bone marrow expansion and extramedullary haematopoiesis. The mainstays of treatment are blood transfusion and chelation therapy, which can cause an array of complications. Only recently, however, has pain been recognized as a clinical component of thalassaemia (Trachtenberg, et al 2010). Sixty-nine per cent of 265 participants in the Thalassaemia Clinical Research Network (TCRN) Thalassaemia Longitudinal Cohort reported bodily pain (Trachtenberg, et al 2010). Additionally, 34% of transfused thalassaemia patients participating in the TCRN Low Bone Mass Observational Study reported that transfusions helped reduce or eliminate their pain (Vogiatzi, et al 2009). We hypothesized that the effects of ineffective erythropoiesis are mitigated by transfusion and thus incidence of pain will vary throughout the transfusion cycle.
The Assessment of Pain Survey study, conducted by the TCRN, was initiated to address increasing reports of pain. The primary aim of this substudy was to assess whether reports of pain vary over the transfusion cycle. Secondary aims were to assess whether the length of the transfusion cycle affects the level of pain and whether pain varies by pre-transfusion haemoglobin (Hb) level and reticulocyte count.
The TCRN of the National Heart, Lung and Blood Institute is a clinical research network, funded by the National Institutes of Health (Appendix 1). The protocol was approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all TCRN institutions. All participants signed informed consent.
Transfusion-dependent subjects (≥8 transfusions within the last 12 months) with β-thalassaemia or Hb E β-thalassaemia participating in the Assessment of Pain Survey study who reported at least mild pain in the last month as measured by Brief Pain Inventory (BPI; Cleeland 2009) were enrolled. The BPI provides information on the intensity of pain and the degree to which pain interferes with function. It has been used previously to assess pain in osteoporotic thalassaemia patients and its use has been validated in cancer patients (Cleeland 2009; Dworkin, et al 2005; Keller, et al 2004; Tan, et al 2004). Subjects were stratified into two cohorts: age 18–29 years vs. 30+ years. Stratification was chosen based on observation that patients aged 30+ years tend to have more pain than younger patients (Haines, et al 2013). Target enrolment was 25–35 subjects.
Subjects completed a daily pain assessment during three non-consecutive transfusion cycles, separated by at least three months and no more than four months apart. Subjects were transfused according to their clinical need; cycles lasted two to four weeks. Transfusion cycles were not altered for participation. Daily pain was assessed using the BPI Short Form (BPI-SF) by daily telephone call provided by Interactive Performance Technologies in association with Telecare Management of Pain (Cambridge, MA). The BPI-SF consists of 10 questions in which participants rate their worst, least and average pain in the past 24 h on a scale of 0–10 where 0 is “No pain” and 10 is “Pain as bad as you can imagine”.
Variation over the transfusion cycle was modelled with repeated measures logistic regression (for pain prevalence) or regression (for pain severity) of time in pain, controlling for age, sex and transfusion cycle. An autoregressive [AR(1)] variance structure was assumed. Time was measured as percentage of transfusion cycle completed and quadratic effects of time were utilized. Length of transfusion cycle, pre-transfusion Hb level and reticulocyte counts were added to the model. Repeated measures models, controlling for age, were used to test for differences in reported causes of pain between patients reporting frequent (≥50% of study days) vs. infrequent pain.
Thirty-two subjects enrolled, 56% were female, the mean age was 31.8 years (range, 18 – 55), and 59% were Caucasian. The average pre-transfusion Hb was 101 g/l (range, 73–128 g/l) and the average length of transfusion cycle was 23 days. Pain prevalence and severity increased with age (p<0.001; Table I). There were no significant effects of gender (p>0.4), or differences over the three transfusion cycles (p>0.5). Thirty-four per cent of subjects reported pain on at least 50% of study days, confirming that pain is an important problem.
Table I.
Pain prevalence, severity, and treatment over the thalassaemia transfusion cyclea.
| Amount of transfusion cycle completed | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0% | 25% | 50% | 75% | 100% | p-valuec | |
| Any pain other than everyday kinds | 0.14 | |||||
| Overall | 42% | 35% | 31% | 37% | 37% | |
| Age cohort | <0.001 | |||||
| 18–29 years | 23% | 18% | 15% | 20% | 18% | |
| 30+ years | 61% | 51% | 47% | 54% | 56% | |
| Length of cycle | 0.026 | |||||
| ≤ 14 days | 50% | 50% | 50% | 60% | 50% | |
| 15–21 days | 40% | 48% | 48% | 52% | 44% | |
| 22–28 days | 42% | 35% | 23% | 31% | 31% | |
| > 28 days | 41% | 14% | 14% | 18% | 32% | |
| Average (SD) amount of pain in past 24 hb | 0.08 | |||||
| Overall | 2.2 (2.9) | 1.8 (2.7) | 1.7 (2.8) | 2.1 (3.0) | 2.2 (3.1) | |
| Age cohort | <0.001 | |||||
| 18–29 years | 1.3 (2.6) | 0.9 (2.0) | 0.9 (2.1) | 1.1 (2.5) | 1.1 (2.5) | |
| 30+ years | 3.1 (3.0) | 2.7 (3.0) | 2.5 (3.0) | 3.1 (3.2) | 3.3 (3.3) | |
| Length of cycle | 0.031 | |||||
| ≤ 14 days | 3.1 (3.9) | 3.1 (3.4) | 2.3 (3.4) | 4.0 (3.9) | 3.4 (4.1) | |
| 15–21 days | 2.2 (2.9) | 2.3 (2.8) | 2.6 (2.9) | 2.7 (2.8) | 2.7 (3.2) | |
| 22–28 days | 2.3 (3.1) | 1.7 (2.7) | 1.4 (2.6) | 1.7 (2.9) | 2.0 (3.2) | |
| > 28 days | 1.6 (2.3) | 0.7 (1.8) | 0.9 (2.3) | 1.1 (2.5) | 1.4 (2.5) | |
Average over the three transfusion cycles
reported on a 0–10 scale
repeated measures of time on pain, controlling for age, sex, and transfusion cycle. Time was measured as a quadratic effect of the percent of transfusion cycle completed. The overall p-value reflects the effect of time. p-values for age and length of cycle reflect the effects of these as continuous variables, although categories are presented for descriptive purposes. There were no significant effects of gender (p>0.4).
SD, standard deviation
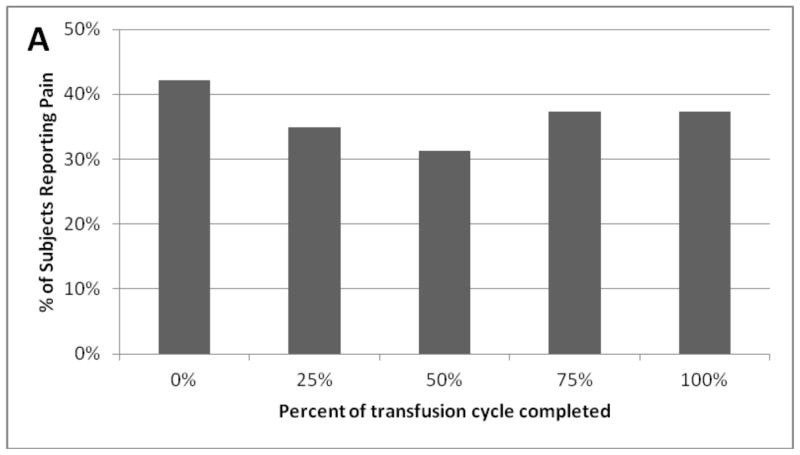
There was a non-significant trend towards lower prevalence of pain midway through the transfusion cycle compared to immediately pre-transfusion (31.3 vs. 42.2%, p=0.14; Figure 1, Table I). Pain reduction was more pronounced among subjects transfused less frequently (>4 weeks) (13.6 vs. 40.9%, p=0.026 for effect of length of transfusion cycle; Table I) whilst, there was no apparent variation among subjects transfused more frequently (≤2 week cycle); Table I). As fluctuation in Hb levels is greater with longer transfusion cycles, these effects may reflect improvement in anaemia and/or ineffective erythropoiesis.
Figure 1.
Pain prevalence over the thalassaemia transfusion cycle.
There was a trend towards lower reported prevalence of pain mid-way thorough the transfusion cycle; p = 0.14
Unexpectedly, prevalence of pain correlated with higher, not lower, pre-transfusion Hb (p=0.013) and also a lower reticulocyte count (p=0.05). Subjects transfused more frequently also reported more severe average pain compared to those with a longer transfusion cycle (3.8/10 for ≤2 week cycles vs. 0.7/10 for >4 weeks; p=0.031 for length of cycle). Although higher Hb could contribute to pain, it is also possible that patients are transfused more frequently in response to chronic pain or other complications. Nevertheless, it is likely that pain is multifactorial.
Use of pain medications/treatments varied from 25–35% over the transfusion cycle (15–18% for those aged 18–29 years, 35–51% aged 30+ years; 14–32% for those transfused frequently, 40–50% transfused infrequently).
Furthermore, midway through the transfusion cycle, pain severity was greater among subjects with frequent pain (5.1/10 vs. 0/10, p<0.001). Additionally, these subjects reported using pain medications more often: 75% compared to 0% of subjects without frequent pain (p<0.001).
Limitations to this pilot study must be acknowledged. The sample size was small due to the exploratory nature of this study. Additionally, we relied exclusively on pre-transfusion laboratory values. Despite these shortcomings, our work demonstrates that pain prevalence is reduced following transfusion, especially for longer transfusion cycles. Furthermore, a subset of patients experience frequent pain, which is more severe, requires more pain medication and probably impacts quality of life. Further work is needed to understand pain aetiology and develop optimal treatments.
Acknowledgments
This work was supported by the following NIH-NHLBI cooperative agreements: U01-HL65232 and NIH/NCRR UL1-RR-024134 to the Children’s Hospital of Philadelphia, U01-HL72291 and by Harvard Catalyst CTSC U-01RR025758 to Children’s Hospital, Boston, U01-HL65233 to University Health Network Toronto General Hospital, U01-HL65239 to Children’s Hospital & Research Center Oakland, U01-HL65244 and CTSC UL1-RR024996 to Weill Medical College of Cornell University, and U01-HL65238 to New England Research Institutes. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI.
The authors would also like to express their gratitude to all of the thalassaemia patients who participated in this study.
Appendix 1
The Thalassaemia Clinical Research Network of the National Heart, Lung and Blood Institute is a clinical research network funded by the National Institutes of Health, composed of five core centres in North America, 13 clinical satellites, and a data-coordinating centre.
The following institutions and researchers contributed to the Thalassaemia Clinical Research Network Assessment of Pain Substudy data reported in this paper.
Children’s Healthcare of Atlanta: Leann Schilling, MPH, Study Coordinator, Principal Investigator; Baylor College of Medicine: Bogden Dino, Study Coordinator; Weill Medical College of Cornell University: Dorothy Kleinert, RN, Research Nurse, Patricia Giardina, MD; The Children’s Hospital of Philadelphia: Alan Cohen, MD, Janet Kwiatkowski, MD, Marie Martin, RN, Research Nurse, Principal Investigator, Sage Green, Study Coordinator; Children’s Memorial Hospital, Chicago, IL: Alexis Thompson, MD, Janice Beatty, RN, Research Nurse, Diane Calamaras, RN, CPNP, Research Nurse, Pauline Hess, Study Coordinator; Children’s Hospital & Research Center Oakland: Dru Haines, CPNP, Research Nurse, Principal Investigator, Olivia Oliveros, Study Coordinator, Elliott Vichinsky, MD; Children’s Hospital of Los Angeles: Thomas Coates, MD, Principal Investigator, Susan Carson, RN, Research Nurse, Principal Investigator, Ani Dongelyan, Study Coordinator, Tatiana Hernandez, Study Coordinator; Toronto General Hospital, Toronto, Ontario, Canada: Nancy Oliveri, MD, Cecilia Kim, BS, Study Coordinator; NHLBI oversight: Kathryn Hassell, MD; Data Coordinating Center: New England Research Institutes: Sonja McKinlay, PhD, Principal Investigator, Lisa Virzi, RN, MS, MBA, Project Director, Felicia Trachtenberg, PhD, Senior Statistician, Eric Gerstenberger, MS, Statistician.
Footnotes
All the authors listed have read and approve of this submission. Their contributions are as follows: S.T.G performed data collection, assisted with analysis and authored the manuscript; M.B.M designed the study, assisted with analysis and reviewed the manuscript; D. H designed the study, assisted with analysis and reviewed the manuscript; S.C designed the study, assisted with analysis and reviewed the manuscript, T. C designed the study and reviewed the manuscript; O.O performed data collection, assisted with analysis and reviewed the manuscript; F.T and E. G performed the statistical analysis and reviewed the manuscript; J.L.K designed the study and edited the manuscript
All authors state that they have no conflict of interest.
References
- Cleeland C. User Guide. The University of Texas M. D. Anderson Cancer Center; Houston, TX: 2009. The Brief Pain Inventory; p. 63. [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Immpact Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Haines D, Martin M, Carson S, Oliveros O, Green S, Coates T, Eile J, Schilling L, Dinu B, Mendoza T, Gerstenberger E, Trachtenberg F, Vichinsky E Thalassaemia Clinical Research N. Pain in thalassaemia: the effects of age on pain frequency and severity. British Journal of Haematology. 2013;160:680–687. doi: 10.1111/bjh.12177. [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clinical Journal of Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. Journal of Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Trachtenberg F, Foote D, Martin M, Carson S, Coates T, Beams O, Vega O, Merelles-Pulcini M, Giardina PJ, Kleinert DA, Kwiatkowski J, Thompson AA, Neufeld EJ, Schilling L, Thayalasuthan V, Pakbaz Z, Yamashita R Thalassaemia Clinical Research N. Pain as an emergent issue in thalassaemia. American Journal of Haematology. 2010;85:367–370. doi: 10.1002/ajh.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm IA, Lane J, Schneider R, Fleisher M, Grady RW, Peterson CC, Giardina PJ Thalassaemia Clinical Research N. Bone disease in thalassaemia: a frequent and still unresolved problem. Journal of Bone and Mineral Research. 2009;24:543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]