Abstract
Advanced stage at diagnosis contributes to low breast cancer survival rates in sub-Saharan Africa. Living far from health services is known to delay presentation, but the effect of distance, the radius at which the effect sets in and the women most affected has not been quantified. In a peri-urban South African setting, we examined the effect of a GIS-measured straight-line distance, from a patient’s residence to diagnostic hospital, on stage at diagnosis in 1071 public-sector breast cancer patients diagnosed during 2006–12. Generalized linear models were used to estimate risk ratios for late stage (stage III/IV vs stage I/II) associated with distance, adjusting for year of diagnosis, age, race and socioeconomic indicators. Mean age of patients was 55 years, 90% were Black African, and diagnoses were at stages I (5%), II (41%), III (46%) and IV (8%). 62% of patients with distances >20 km (n=347) had a late stage at diagnosis compared to 50% with distances <20 km (n=724, p=0.02). Risk of late stage at diagnosis was 1.25-fold higher (95% CI: 1.09, 1.42) per 30 km. Effects were pronounced in an under-represented group of patients over age 70. This positive stage-distance association held to 40 km, and plateaued or slightly reversed in patients (9%) living beyond this distance. Studies of woman and the societal and healthcare-level influences on these delays and on the late stage at diagnosis distribution are needed to inform interventions that improve diagnostic stage and breast cancer survival rates in this and similar settings.
Keywords: breast neoplasms, early diagnosis, South Africa, disparities, stage at diagnosis, geographical
INTRODUCTION
In sub-Saharan Africa (SSA), breast cancer is the most commonly diagnosed cancer in women (2012 estimates) but its survival rates are poor, despite this cancer having a good prognosis in other settings.1 Within South Africa’s public health sector, first-world cancer treatment is available at little cost to breast cancer patients, thus diagnosis of this cancer at earlier stages could prevent deaths. Drivers of advanced stage at diagnosis and of low survival include poor existing healthcare infrastructure, lack of early-detection programs, and unavailability, inaccessibility and lack of adherence to treatment.2,3 Studies in the West (e.g. in the US and UK) and in Africa (South Africa, Egypt and Sudan) have found that urban versus rural residence, smaller travel burdens and geographical proximity to primary healthcare providers are associated with earlier diagnostic stage.4–8 In SSA, distances to treatment hospitals may be up to hundreds of kilometres and personal transport is uncommon, therefore, the effect of distance-to-hospital needs to be precisely quantified as it is likely to play a major role.
In post-apartheid South Africa, the public-sector health service, which serves approximately 75–80% of all patients, has a strong public health care approach at the community level, complimented by a hierarchical referral system through district hospitals.9 In this sector, a breast cancer patient typically first notices disease symptoms herself and proactively seeks help in primary healthcare clinics. She is then referred to a secondary hospital and subsequently to a tertiary hospital for diagnostic work-up and treatment. Such public-sector patients usually travel by ‘public’ transport (privately-run fare-charging minibuses) or by walking.10,11 Transport costs represent more than 10% of monthly household expenditure for the poorest quintile of the population.10 Transport, or other, barriers encountered prior to diagnosis may result in delays in diagnosis, especially for patients who reside far from the treating hospital, but they have not been quantified in this health system in relation to breast cancer.
In this study, we aimed to precisely quantify the association of GIS-measured distance from home to hospital with breast cancer clinical characteristics at diagnosis in South Africa, in particular stage at diagnosis. In over 1000 patients diagnosed at South Africa’s largest public referral hospital, the Chris Hani Baragwanath Academic Hospital (CHBAH), we investigated whether the effects of distance to hospital were present within a small radius (<60 km).
MATERIALS AND METHODS
Setting
CHBAH, situated in Soweto, is a tertiary public hospital with a catchment population of ~3 million people residing in Soweto and the surrounding southern areas of Gauteng Province up to 60 km away, including Katlehong, Orange Farm and Sebokeng. Situated adjacent to the financial capital of South Africa in Johannesburg, parts of these areas have transformed significantly during the political and economic changes of the past two decades. Their populations today are heterogeneous; Soweto and Katlehong contain both poor and affluent areas, informal and formal housing.12 Many residents however still live in substandard conditions and unemployment is high, e.g. formal employment in adults is 41–43% in Soweto and Katlehong.12 Further south of Soweto and of CHBAH, areas such as Orange Farm, Evaton and Sebokeng are, on average, of lower socioeconomic status. Formal employment rates are 36%12 and entrepreneurial informal employment is more common. Primary health clinics serving the community are located in all of these communities and refer to CHBAH.
Study population
CHBAH’s Breast Clinic, operational since 2000, receives referred and walk-in patients and, through diagnostic imaging, cytology and histology, diagnosed on average 11 breast cancer patients per month in 2006–07 which rose to an average of 20 cases per month in 2010–11.13 Most breast cancer patients are symptomatic at diagnosis as there is no population-wide breast cancer screening program. A small fee of R40 (≈$5) is waived if patients have no means to pay. From the date of first presentation at the Breast Clinic, this clinic aims to achieve a confirmatory diagnosis within 1–3 weeks and treatment commencement within one further week. Therapeutic options are chemotherapy (primary or neoadjuvant, in Johannesburg city centre, not at CHBAH), surgery, radiotherapy and hormonal therapy. Trastuzumab is not currently available in the public sector.14 All patients receive the same diagnostic, imaging and laboratory testing protocols at CHBAH.
In 2006, a clinical data system was established at the CHBAH Breast Clinic to systematically record core clinical and histological data for patients newly diagnosed with breast cancer. Between October 2006 and January 2012, data on 1104 consecutive female patients newly diagnosed with histologically-confirmed, invasive breast cancer were entered into the database. The current analysis is based on the 1071 (97%) women for whom both residential address and age at diagnosis were known. Data extracted for the present investigation include age at diagnosis, residential address, race (black African, white, Indian or Asian, coloured (i.e. mixed ancestry)), point of last referral before arriving at CHBAH, AJCC TNM stage15 included as stages I, II, III and IV (n=1051 (98% complete)) and Scarff-Bloom Richardson grade (1=well, 2=moderate or 3=poorly differentiated). Immunohistochemistry was performed for oestrogen (ER, n=944 (88% known)), progesterone (PR, n=941 (88%)) and HER2 (n=903 (84% complete)) receptors on pre-chemotherapy core biopsy specimens as per routine practice, to inform clinical management (further details provided in full elsewhere).16 Cut-offs of > 1% (score 1, 2 or 3) were considered ER+ and PR+ and for HER2 status, scores of 0, 1 and 2 were considered HER2-negative and score 3 as HER2-positive. A HIV test (ELISA method HIV test) was offered to all women, and is considered here as a social indicator and not a breast cancer risk factor, as we have previously shown that the proportion of HIV-positive breast cancer patients in this population is equal to that of the age-matched underlying catchment population13. The study was approved by the University of the Witwatersrand Human Research Ethics Committee (M130369, M130118).
GIS-based straight-line distance
The geo-coordinates of each patient’s reported residential address (physical street name and house number) were obtained through a manual geo-coding process using Google street map services through the iTouchmap.com web interface. Using these coordinates, the shortest straight-line distance to CHBAH from the woman’s residence was calculated using either GIS software (ESRI ARCGIS 9.3 with a Transverse Mercator projection system, central meridian of 29, Hartebeesthoek_1994 as Datum and WGS_1984 as Spheroid) or the Haversine formula below (where the patient’s residential address is (latitude φ1, longitude λ1) and CHBAH is at (φ2 = 26.26144S, λ2 = 27.94051E), coordinates were converted to radians and 6371 km used as the average radius of the earth); hereafter referred to as ‘distance’.
Availability of correlates of distance
In addition to individual-level data, we incorporated ward-level socio-economic indicators for women residing within the three most common residential areas: Soweto (patients originated from 44 wards), Orange Farm/Sebokeng (30 wards) and Katlehong municipalities (20 wards). In these areas, among the most densely populated area of South Africa, the median number of woman over age 20 in a ward was 9534 (range 2929–20615), with a median of 7851 households per ward. Each woman was assigned to the ward whose centroid was nearest to her residential address. Ward-level socioeconomic indicators (listed in table 2) were obtained from the 2011 South African census.12 Finally, to gauge travel burdens to CHBAH, we interviewed 73 women attending the Breast Clinic in March 2013, of which 92% used minibuses (16-seater commuter buses) to reach the hospital, 5% private car, 1% hospital transport and 1% walked. Median minibus costs for a one-way trip were R8 for journeys <0.5 hours, R11 for 0.5–1.0 and R22 for > 1 hour journeys (R20 ≈ $2.20 in 2013; average monthly income for women over the age of 20 ranges from R1250 in the Orange Farm/Sebokeng area to R2250 in Soweto, thus one return trip transport-costs are 1 to 3% of monthly income.
Table 2.
Distance to hospital in relation to woman-level and tumour characteristics of breast cancer patients
| Distance to hospital (straight-line distance, kilometres) | p-valuea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 5 | 5–9.9 | 10–19.9 | 20–29.9 | 30–39.9 | ≥40 | ||||||||
|
| |||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
|
| |||||||||||||
| Breast cancer cases (N) | 183 | 299 | 242 | 188 | 61 | 98 | |||||||
| Woman-level characteristics | |||||||||||||
| % of diagnoses in 2006–08 | 37.2 | 32.4 | 31.4 | 35.1 | 47.5 | 33.7 | 0.33 | ||||||
| Age at diagnosis (years) | |||||||||||||
| Mean (SD) | 57.5 (15.2) | 57.0 (14.7) | 54.4 (14.1) | 52.5 (12.9) | 53.9 (11.3) | 55.2 (14.8) | 0.002 | ||||||
| Race | |||||||||||||
| % Black African | 92.9 | 93.0 | 85.1 | 94.7 | 95.1 | 79.6 | <0.001 | ||||||
| Referral source, via: | |||||||||||||
| Primary health clinic | 55 | 66.3 | 86 | 61.2 | 62 | 52.1 | 23 | 26.6 | 7 | 31.8 | 11 | 23.9 | <0.001 |
| Secondary hospital | 2 | 2.4 | 5 | 3.7 | 11 | 9.2 | 47 | 52.2 | 12 | 54.6 | 30 | 65.2 | |
| Local doctor | 10 | 12.1 | 17 | 12.7 | 18 | 15.1 | 8 | 8.9 | 3 | 13.6 | 1 | 2.2 | |
| Self-referral | 16 | 19.3 | 26 | 19.4 | 28 | 23.5 | 21 | 13.3 | 0 | 0.0 | 4 | 8.7 | |
| Ward-level characteristics, meanb | |||||||||||||
| % primary education or less | 14.6 | 14.2 | 14.0 | 18.7 | 20.9 | - | <0.001 | ||||||
| % Annual household income <=R9600 | 29.4 | 27.4 | 24.7 | 32.9 | 27.0 | - | <0.001 | ||||||
| % Individual monthly income <=R800 | 50.3 | 51.2 | 49.3 | 61.0 | 58.0 | - | <0.001 | ||||||
| % Informal residential housing | 1.7 | 2.1 | 2.4 | 3.0 | 14.0 | - | <0.001 | ||||||
| Mean age of women in contributing wards over 30 | 46.3 | 46.4 | 45.5 | 45.0 | 48.2 | - | NAc | ||||||
| Tumour characteristics | |||||||||||||
| Stage (98% complete) | |||||||||||||
| Stage I | 10 | 5.5 | 17 | 5.8 | 15 | 6.3 | 2 | 1.1 | 2 | 3.4 | 7 | 7.1 | 0.02 |
| Stage II | 82 | 45.1 | 134 | 45.4 | 97 | 40.9 | 67 | 36.4 | 16 | 27.6 | 36 | 36.7 | |
| Stage III | 78 | 42.9 | 116 | 39.3 | 107 | 45.1 | 96 | 52.2 | 36 | 62.1 | 48 | 49.0 | |
| Stage IV | 12 | 6.6 | 28 | 9.5 | 18 | 7.6 | 19 | 10.3 | 4 | 6.9 | 7 | 7.1 | |
| % late stage (III/IV) | 49.5 | 48.8 | 52.7 | 62.5 | 69.0 | 56.1 | |||||||
| Receptor Status | |||||||||||||
| % ER+ and/or PR+ | 69.1 | 68.9 | 65.6 | 64.1 | 57.9 | 64.8 | 0.58 | ||||||
| % HER2+ | 23.8 | 28.9 | 21.7 | 25.8 | 33.9 | 27.7 | 0.37 | ||||||
| % Triple negative | 18.8 | 18.8 | 22.2 | 26.5 | 23.6 | 21.7 | 0.52 | ||||||
p-value from chi-squared test for associations with categorical age and race, and linear test for trend for continuous outcomes.
Restricted to wards from more densely populated areas namely Soweto, Katlehong and Orange Farm/Sebokeng.
Actual age of women used, not ward-level age.
Statistical Analyses
We used generalised linear models to assess associations with stage at diagnosis (primary outcome), treated as a binomial outcome of late (stage III/IV) versus early (I/III) stage, using a log link function to obtain risk ratios (RR, exp(β)) and 95% confidence intervals (95% CI) and an identity link function for absolute differences (100*β) in % late stage disease. The explanatory variable of interest, straight-line distance to hospital, was included using indicator categorical variables and as a continuous trend restricted to <50km beyond which data were sparse. Crude associations with distance were assessed first, and thereafter adjusted for age in 5-year age categories, race (black/non-black), year of diagnosis and referral source simultaneously and finally, adjusted for ward-level socioeconomic indicators. The ward-level socioeconomic indicators (for % with primary education only, % informal housing, % low household income) were divided into tertiles with 1 being the least deprived and 3 the most deprived and included as categorical variables. The same approach was used to investigate associations with the binary outcomes for ER, PR and HER2 status.
RESULTS
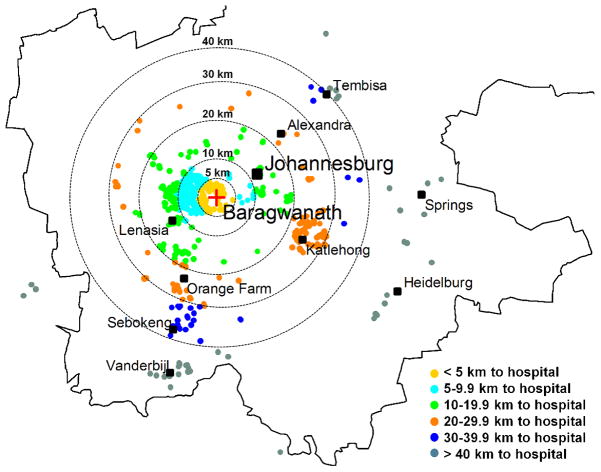
A total of 1071 breast cancer patients were included. Ninety percent were black African women and mean age at diagnosis was 55.4 years (SD 14.3). Overall, 54% of patients presented with late stage (stages III or IV) disease, 67% of tumours were ER and/or PR positive and 21% triple-negative (table 1). Residential locations of breast cancer patients in relation to CHBAH are shown in figure 1. Forty-five percent of patients resided within 10 km of CHBAH, and 95% within 50 km. Most patients (61%) resided in the Soweto area (average distance 7.5km (SD 4.0)). Patients’ residences also clustered near Katlehong (23.9 km away (SD 4.0)), Orange Farm and Sebokeng (28.5 km (6.0)) and Vanderbijlpark (47.5 km (2.7)) (table 1 and figure 1). Only 46% of patients identified their last referral point, but of these patients, 49% were referred from a primary healthcare clinic, 22% (primarily patients from beyond 20 km to the hospital) from a secondary hospital, 12% directly from a local doctor and 18% (mostly from the proximate area) were self-referrals (table 2).
Table 1.
Characteristics of 1071 breast cancer patients diagnosed at the Chris Hani Baragwanath Academic Hospital during October 2006–January 2012
| Demographic characteristicsa | N | Column % |
|---|---|---|
| Age at diagnosis (years) | ||
| < 40 | 157 | 14.7 |
| 40 – 49 | 253 | 23.6 |
| 50 – 59 | 274 | 25.6 |
| 60 – 69 | 200 | 18.7 |
| ≥ 70 | 187 | 17.5 |
| Year of diagnosis | ||
| 2006–07 | 167 | 15.6 |
| 2008–09 | 414 | 38.7 |
| 2010–2012 | 490 | 45.8 |
| Race | ||
| Black African | 964 | 90.0 |
| White | 43 | 4.0 |
| Coloured | 42 | 3.9 |
| Indian or Asian | 18 | 1.7 |
| Straight-line distance to hospital (km) | ||
| < 5 | 183 | 17.1 |
| 5 – 9.9 | 299 | 27.9 |
| 10–19.9 | 242 | 22.6 |
| 20–29.9 | 188 | 17.6 |
| 30–39.9 | 61 | 5.7 |
| ≥ 40 | 98 | 9.6 |
| Residential area | ||
| Nearest area Farther areas: | ||
| Soweto | 654 | 61.1 |
| Orange Farm & Sebokeng | 123 | 11.5 |
| Katlehong | 124 | 11.6 |
| Vanderbijlpark | 36 | 3.4 |
| Other | 134 | 12.5 |
| Referral route to hospital | ||
| Via: | ||
| Primary health clinic | 243 | 49.3 |
| Secondary hospitals | 107 | 21.7 |
| Local doctor | 57 | 11.6 |
| Self-referral | 86 | 17.5 |
| Clinicopathologic Characteristics | N | Column % |
|---|---|---|
| Stage | ||
| Stage I | 53 | 5.0 |
| Stage II | 431 | 41.0 |
| Stage III | 480 | 45.7 |
| Stage IV | 87 | 8.3 |
| Receptor Status | ||
| ER+ and/or PR+ | 628 | 66.6 |
| ER− and PR− | 315 | 33.4 |
| HER2+ | 235 | 26.0 |
| HER2− | 668 | 74.0 |
| ER−/PR−/HER2− | 193 | 21.5 |
| HIV status for 67% of women | ||
| HIV positive | 132 | 18.4 |
| HIV negative | 586 | 81.6 |
% (n) of each characteristic which was missing were 0% for age and distance (by definition of sample set) and for residential area and year of diagnosis, 1% (n=20) for stage, 12% (n=128 for ER and PR and 168 for HER2) for receptor status, 22% (n=231) for tumour size, 54% (n=578) for referral source, and 0.5% (n=4) for race.
Mean tumour size for all women was 40 mm (n=863).
Figure 1.
Residential locations of breast cancer patients diagnosed at CHBAH breast clinic during 2006–2012, overlaid with circles showing straight-line distances to the breast clinic
The percentage of patients presenting with advanced stage at diagnosis increased from 50% for distances <20 km to 62% for distances of 20–29 km and 69% for distances of 30–39 km (table 2). In the 9% of patients who lived beyond 40km, the percentage of late stage tumours (56%) did not increase further. Stage-distance associations may have been confounded by age, race, or year at diagnosis, as these factors were associated with distance and stage at diagnosis, as explained below.
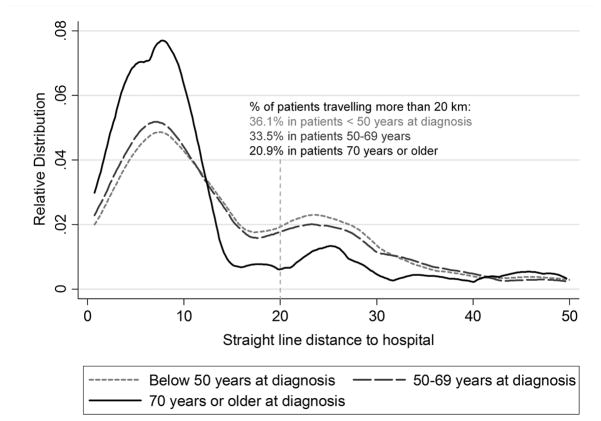
Patients with over 20 km distance to hospital were younger, by an average 2.7 years (mean age 53.5 years, SD=13.2) than those who lived within 20 km (mean 56.2, SD=14.7). This difference is partially, but not entirely accounted for by an older proximate at-risk population - 25% of over 30 year old women were aged >=50 in Soweto compared to 20% in more distal areas. After adjusting for differences in population structure, only 9% and 14% of breast cancer patients from distal areas (Katlehong and Orange Farm/Sebokeng respectively) were over age 70 compared to 21% of patients from the proximate area of Soweto. The distribution of the distances varied by age group; much fewer (21%) over 70 year old patients resided more than 20 km away, compared to 36% and 34% of patients under age 50 and 50–69 years respectively (figure 2). Distances were also associated with race, but not with year of diagnosis or HIV status (table 2). Indian/Asian patients were concentrated 10–20 km away, a distance which includes the predominantly Indian area of Lenasia, and a greater proportion of white women resided beyond 40 km. Ward-level socioeconomic indicators also revealed large differences in the at-risk populations. Areas beyond 20 km from CHBAH had a lower socio-economic status than proximate areas, as measured by a greater proportion of the female population whose highest educational level was primary school, lower household and individual incomes and by a greater proportion of informal housing (table 2). In this study population, late stage at diagnosis was also more common in older patients (RR 1.03 (95% CI: 0.99, 1.07) per 10 years for late vs early stage, not in tables) and in diagnoses prior to 2008 (RR 1.34 (95% CI: 1.17, 1.53) for diagnosis in 2006–07 vs 2008–12), but was not associated with race.
Figure 2.
Relative distribution of travel distances within 50 km of the CHBAH Breast Clinic, for breast cancer patients under 50 years (n=384), 50–69 years (n=437) and 70 years and older (n=176)
Risk ratios for late stage at diagnosis associated with distance are provided in table 3. The crude risk of a late stage tumour was 39% higher (95% CI: 11, 75) among women who lived 30–39 km from the hospital than for those who lived within 5 km. Across distances of up to 50 km, a statistically significant linear trend of more advanced stage with greater distance was observed (RR 1.24 per 30 km distance). These trends were present and as strong after adjustment for both demographic and ward-level socioeconomic indicators (table 3). When adjusting for last referral point, RRs did not change greatly. Further, there was no clear evidence that the distance-stage association differed by last point of referral (interaction test p=0.17). The distance-stage association was present in patients referred to CHBAH from clinics (RR 1.71 (1.35, 2.22), 22% of all patients), self-referrals (RR 1.48 (1.06, 2.07), 8% of all patients) or via other hospitals (RR 1.46 (0.74, 2.88), 10% of all patients). There was weak evidence of effect modification of the distance-stage association by age (p=0.10). The association was positive in all age groups, but was strongest in older women: RRs (95% CI) per 30 km were 1.26 (1.00, 1.59) under age 50 years, 1.08 (0.88, 1.33) at ages 50–69 years and 1.57 (1.14, 2.17) at ages 70 and over (not in tables).
Table 3.
Risk ratios for the association of breast tumour stage at diagnosis with residential distance of breast cancer patients from Chris Hani Baragwanath Academic Hospital
| Distance to hospital (km) | Risk Ratio for late stage (III/IV) breast cancer | |||||
|---|---|---|---|---|---|---|
| Crude | Adjusted for age, race, referral source, year | Adjusted for age, race, referral source and socio-economic indicatorsa | ||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| < 5 km | 1 | - | 1 | - | 1 | |
| 5 – 9.9 | 0.99 | 0.82, 1.19 | 0.98 | 0.82, 1.18 | 1.01 | 0.83, 1.23 |
| 10 – 19.9 | 1.06 | 0.88, 1.29 | 1.06 | 0.88, 1.28 | 1.08 | 0.88, 1.34 |
| 20 – 29.9 | 1.27 | 1.06, 1.53 | 1.31 | 1.08, 1.57 | 1.33 | 1.04, 1.68 |
| 30 – 39.9 | 1.39 | 1.11, 1.75 | 1.40 | 1.12, 1.75 | 1.33 | 0.93, 1.91 |
| 40 – 99 | 1.14 | 0.89, 1.46 | 1.17 | 0.92, 1.50 | - | |
| 100+ | 1.06 | 0.68, 1.67 | 1.12 | 0.71, 1.76 | - | |
| Trend per 30 kmb | 1.24 | 1.09, 1.41 | 1.25 | 1.09, 1.42 | 1.35 | 1.11, 1.65 |
Adjustment were carried out using continuous variables for age and year, and categories for race (black vs. non-black) and referral source (clinic, hospital, self, not known, GP).
Ward level socio-economic indicators restricted to patients residing in Soweto (number of patients n=634), Orange Farm/Sebokeng (n=145) and Katlehong (n=125).
Restricted to women residing within 50 km of CHBAH
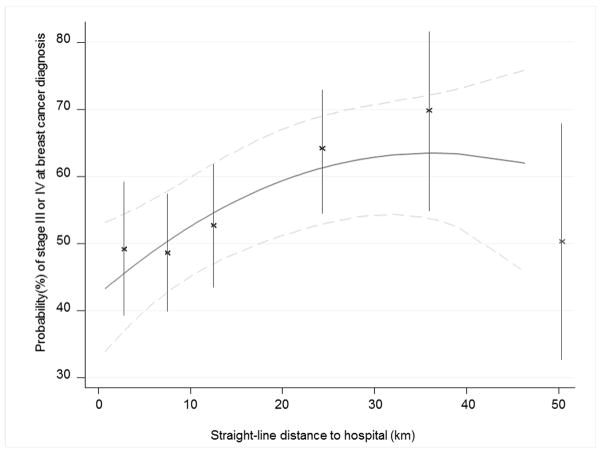
Figure 3 shows the absolute differences in the probabilities of having a late-stage tumour as a function of distance to diagnosing hospital, predicted for a 50-year old black African patient, generated from a model adjusting for race, age, and referral pattern. Model predictions based on both categorical (points and 95% CI error bars) and continuous (solid line with 95% CI represented by dotted lines) distance using splines are consistent; distal patients have up to a 15 percentage point absolute increase in the probability of a late stage tumour compared to proximate patients. Further than 40 km from the breast clinic, the stage-distance association plateaued somewhat, with some suggestion that it reversed slightly, but with few patients residing at those distances, confidence intervals were very wide.
Figure 3.
Probability (95% confidence interval) of a woman with breast cancer being diagnosed at late stages (stage III or IV) according to her straight-line distance from home to CHBAH using both categorical and continuous variables
There was no association of distance to hospital with tumour hormone receptor (ER, PR or HER2) status (table 2). This crude association did not materially alter after adjustment for potential confounders (supporting-table 1), despite black patients having a greater risk of ER-negative tumours compared to non-black women (RR 1.66 (95% CI: 1.12, 2.43), not in tables).
DISCUSSION
This study showed public-sector breast cancer patients diagnosed at CHBAH who live farther from the hospital were diagnosed at later stages than those who lived nearer. The association was detectable beyond 20 km of the hospital and was of a clinically meaningful magnitude. The effect was stronger in older patients, and an underrepresentation of such patients at greater distances may be a continuation of this phenomenon, i.e. older women residing far from CHBAH being vulnerable to both more advanced diagnostic stage and to never reaching the tertiary hospital for treatment.
Distance-stage association
CHBAH is a public sector tertiary hospital and as such is the third tier in the hierarchical referral structure. Private healthcare in South Africa is available to those who can afford medical insurance, and thus exhibits great socio-economic and racial disparities, with 7% of black Africans having private medical insurance, compared to ~70% of whites.17 More advanced stage at breast cancer diagnosis in distally located patients was likely to arise from several patient- and health system-level factors. For the latter, primary healthcare facilities are often underutilized, sometimes being perceived as suboptimal by patients.10 Some of the clinics are short-staffed9 and nursing staff at these clinics may not be adequately trained in symptom recognition and clinical breast examinations, leading to unnecessary delays in referral. Some patients (one fifth of proximate patients in the present study) choose to bypass the referral system and go directly to the tertiary hospitals.11 Alternatively, women residing further from the hospital may be less aware of the breast clinic, and less ‘breast aware’ in general, being exposed to fewer health awareness campaigns in the media. The financial burdens associated with travelling to the tertiary hospital are an added barrier.3 A round-trip to the hospital may cost 5% to 14% of a woman’s weekly income for a domestic worker on the minimum wage, in addition to the loss of work hours. Many women, including older women, have responsibilities as family care-givers; they may need to organise child/grand-child minding in order to visit the clinic or hospital. Distance-linked diagnostic delays appeared to affect older patients to a greater extent, possibly due to a concentration of barriers in this group. It is unlikely that competing risks in rural areas would have explained the lack of older patients, as several dominant causes of premature deaths (HIV/AIDS, tuberculosis, lower respiratory infections18) would disproportionately affect lower socioeconomic groups who have lower breast cancer risks. In particular HIV, a dominant cause of premature mortality in South Africa, is not a risk factor for breast cancer and we have already demonstrated that the HIV-prevalence in this breast cancer patient group matches that of the background population; HIV status of breast cancer patients was not associated with stage.13 There is a need for specifically addressing the barriers in this patient population, and whether some older patients at lower levels of the health system may not be referred.
Late stage at diagnosis
The late stage distribution, even in patients living adjacent to CHBAH who have more immediate access to the hospital, is suggestive of further barriers to presentation other than distance-associated factors. Locally-residing patients included some who were diagnosed when palliation was the only care possible. The 50% stage III/IV disease observed in patients living within 20 km is a worse stage distribution than that seen in other unscreened populations or unscreened age groups in which less than 20% stage III/IV was reported.19–21 Further, worse stage distributions (60–90% stage III/IV) exist in certain African settings.22–24 Although the stage distribution at CHBAH is late, we previously documented how it improved from 70% to 50% stage III/IV in just 5 years.16 Unfortunately, we do not have accurate measures of the time-delays associated with differences in stage at diagnosis between proximal and distally residing patients, but instead we used growth tumour models to provide an approximate order of magnitude of the time delays.19 Assuming mean tumour sizes of 10 mm for T1, 35 mm for T2 and, for T3 and T4 a range from the mean ± 10 mm tumour size obtained from SEER database (2010 breast cancers), the stage distributions translate to a potential 2 to 4 month diagnostic delay associated with distal (≥ 20km) compared to proximally residing (<20 km) patients. Stage for stage, tumour sizes may have wider ranges in the South African setting compared to SEER populations, thus this time-delay estimate is approximate.
Further factors contributing to delayed diagnosis may include lack of education concerning where to go to seek help, poor knowledge of symptoms, lack of breast awareness, fear and beliefs held on the causes of cancer and whether it is curable. Low utilisation of healthcare services by women with non-communicable diseases in general has been documented in this same Sowetan setting.25 In some African settings, traditional healers are consulted; conventional western treatments are thought to interfere with indigenous remedies and cancer is believed to be a death sentence.3,26–28 In South Africa, especially within rural communities, cancer is sometimes believed to be caused by witchcraft and traditional healers are consulted to reverse this sorcery before a patient presents for treatment at a hospital.6 In other disease areas, traditional medicine is also often used in conjunction with Western medicine in the hope to offset the side-effects of treatment.29
Previous studies in sub-Saharan Africa have observed more advanced disease in rural compared to urban patients.6–8 In Ethiopia rural breast cancer patients were more impoverished than their urban counterparts and, as they reside further from treatment centres, they presented at later stages and were less compliant with treatment.30 However, few previous studies were able to quantify associations with distance to diagnosing hospital precisely. In other settings, such as the United States, studies in low-income groups found associations between late stage at diagnosis and both distance from screening and diagnostic facilities and lack of health insurance.31–34 Diagnosis based on symptoms, the principle means of breast cancer detection in low- and middle-income countries, rather than on screening, is also associated with delays in diagnosis.35–39
Strengths and weaknesses
This study benefits from its large sample size and the fact that it was based on a consecutive case series in a public hospital setting. The sample represents women who reach this stage of the health system, but the series is not a complete population-based sample. Notably, as evidenced in the probable underrepresentation of older patients, some patients may never reach the tertiary facility and furthermore private sector cases are not included. In the absence of a population-based cancer registry covering Gauteng, it is difficult to accurately evaluate the selection biases introduced, but it is unlikely that they would give rise to the stage-distance association observed as cancer is not treated in secondary hospitals and CHBAH is the tertiary referral hospital. We used calculated straight-line distance to the hospital rather than actual individual-level travel time and cost. However, straight line distance has been shown to approximate true travel distance and any errors introduced by using this measurement would have been non-differential in nature.40 We used self-reported place of residence which was not able to be confirmed. However, any patients temporarily residing within Soweto solely for the purpose of treatment at CHBAH would, if anything, have led to an attenuation of our results because of a greater misclassification of later stage distal patients. Additionally, we did not have optimal individual-level data with which to fully investigate the effect pathways but as a proxy we used ward-level indicators for adjustment and we only had data on the last referral point, not on the entire referral path. Our correlates of socio-economic status are strengthened by the fact that we made use of both educational and income related variables in our analysis.41 However these ecological measures incur measurement error which attenuates associations to a greater extent as area size increases. Previous work in the UK has demonstrated the validity of using ecological data at the electoral ward-level, although associations were weaker than those observed using the smaller enumeration district level.41 Average ward size in SA is larger than the UK electoral ward, so the attenuation would be greater in our study. Thus, measurement errors in the distance calculation and in socioeconomic factors are most likely random in nature and as such, the magnitude of the associations presented here would be underestimated.
Implications
As part of an increasing non-communicable disease burden affecting ageing and westernizing populations in general, the cancer burden of many middle income countries such as South Africa, is expanding, and cancer control efforts need gearing up to tackle this problem.42 Understanding barriers to early presentation, diagnosis and treatment commencement are essential for cancer in particular, as diagnosis and treatment centres are usually located in a few specialized referral hospitals, and early diagnosis combined with prompt treatment can halt disease spread and metastases. Earlier diagnosis is particularly important for breast cancer as this tumour has the potential of excellent prognosis39,43, especially in South Africa where diagnostic and therapeutic facilities are available, which is not the case in all sub-Saharan African countries. Stage at diagnosis is a strong predictor of breast cancer survival, with 5-year survival rates decreased from 90% for stage I breast cancer to 65% at stage II, 33% at stage III and only 6% at stage IV (Indian data).44 Equivalent recent statistics are needed for South Africa, but are at least likely to have a similar trend. To improve the stage-at-diagnosis distribution, research is needed in two areas: first, to identify context-specific barriers that drive later diagnostic stage in general and specifically in distally-residing patients; and second, to identify interventions to achieve earlier diagnosis and treatment and ultimately improve breast cancer survival rates.
Guidelines for breast cancer management in such settings are provided by the Breast Health Global Initiative.39,45 Appropriate early-presentation and diagnosis programs for these contexts need to consider the current state of the breast cancer burden including its relatively young age, low incidence rates, advanced symptomatic disease presentation and context-specific health infrastructure.45–47 There are several relevant research efforts in this area. In Sudan and India, initial results from both a cluster-randomized trial of triennial clinical breast examination (India) and the training of female volunteers in the detection of breast abnormalities (Sudan) have demonstrated improved stage at diagnosis.48,49 Similar interventions at the primary healthcare level in South Africa may also have the desired effects of earlier presentation and faster referrals and need investigation. We have focussed on late diagnosis, but the distance to the tertiary hospitals is likely to affect post-diagnosis treatment access and adherence, further impacting on survival. Cycles of chemo- and radiotherapy require repeated hospital visits for which travel-associated barriers may be amplified as patients become weaker.50 Additionally, later stage at diagnosis, present at a 20 km radius of the hospital, may worsen for rural patients travelling greater distances (often up to hundreds of kilometres) to tertiary treatment centres.
Supplementary Material
Novelty and Impact.
Disease diagnosis at earlier stages is a priority for breast cancer control in sub-Saharan Africa. In 1071 public hospital South African breast cancer patients, stage at diagnosis increased with greater residential distance of the patient to hospital. The effect was sizeable (25% absolute difference in late stage tumours), present within a short distance (20–40 km) and particularly affected older women. Targeting these women could improve prognosis for, and potentially avert deaths from, this treatable cancer.
Acknowledgments
The work reported in this paper is being undertaken during the tenure of a Postdoctoral Fellowship from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions – People – Co-funding of regional, national and international programmes (COFUND). Data collection was supported in part by the Columbia University – South Africa Training Program for Research on AIDS-related Malignancies through the National Cancer Institute, NIH (grant # 1D43CA153715-03). The study design, analysis and manuscript writing were undertaken by the International Agency for Research on Cancer.
Footnotes
Conflicts of Interest: None
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. [Internet] [DOI] [PubMed] [Google Scholar]
- 2.Vento S. Cancer control in Africa: which priorities? Lancet Oncol. 2013;14:277–9. doi: 10.1016/S1470-2045(13)70022-6. [DOI] [PubMed] [Google Scholar]
- 3.Wright SV. An investigation into the causes of absconding among black African breast cancer patients. S Afr Med J. 1997;87:1540–3. [PubMed] [Google Scholar]
- 4.Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel times to health care and survival from cancers in Northern England. Eur J Cancer. 2008;44:269–74. doi: 10.1016/j.ejca.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, McLafferty S, Escamilla V, Luo L. Late-Stage Breast Cancer Diagnosis and Health Care Access in Illinois. Prof Geogr. 2008;60:54–69. doi: 10.1080/00330120701724087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorobiof DA, Sitas F, Vorobiof G. Breast cancer incidence in South Africa. J Clin Oncol. 2001;19:125S–7S. [PubMed] [Google Scholar]
- 7.Elgaili EM, Abuidris DO, Rahman M, Michalek AM, Mohammed SI. Breast cancer burden in central Sudan. Int J Womens Health. 2010;2:77–82. doi: 10.2147/ijwh.s8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapleton JM, Mullan PB, Dey S, Hablas A, Gaafar R, Seifeldin IA, Banerjee M, Soliman AS. Patient-mediated factors predicting early- and late-stage presentation of breast cancer in Egypt. Psychooncology. 2011;20:532–7. doi: 10.1002/pon.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coovadia H, Jewkes R, Barron P, Sanders D, McIntyre D. The health and health system of South Africa: historical roots of current public health challenges. Lancet. 2009;374:817–34. doi: 10.1016/S0140-6736(09)60951-X. [DOI] [PubMed] [Google Scholar]
- 10.Harris B, Goudge J, Ataguba JE, McIntyre D, Nxumalo N, Jikwana S, Chersich M. Inequities in access to health care in South Africa. J Public Health Policy. 2011;32 (Suppl 1):S102–S123. doi: 10.1057/jphp.2011.35. [DOI] [PubMed] [Google Scholar]
- 11.Nteta TP, Mokgatle-Nthabu M, Oguntibeju OO. Utilization of the primary health care services in the Tshwane Region of Gauteng Province, South Africa. PLoS One. 2010;5:e13909. doi: 10.1371/journal.pone.0013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics South Africa 2011 National Census. Feb 4, 2013. [Google Scholar]
- 13.Cubasch H, Joffe M, Hanisch R, Schuz J, Neugut AI, Karstaedt A, Broeze N, van den BE, McCormack V, Jacobson JS. Breast cancer characteristics and HIV among 1,092 women in Soweto, South Africa. Breast Cancer Res Treat. 2013;140:177–86. doi: 10.1007/s10549-013-2606-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Bogaert LJ. Breast cancer molecular subtypes as identified by immunohistochemistry in South African black women. Breast J. 2013;19:210–1. doi: 10.1111/tbj.12090. [DOI] [PubMed] [Google Scholar]
- 15.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–36. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 16.McCormack VA, Joffe M, van den BE, Broeze N, dos SSI, Romieu I, Jacobson JS, Neugut AI, Schuz J, Cubasch H. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res. 2013;15:R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban M, Banks E, Egger S, Canfell K, O’Connell D, Beral V, Sitas F. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South african women: case-control study. PLoS Med. 2012;9:e1001182. doi: 10.1371/journal.pmed.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillay V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Matzopoulos R, Prinsloo M, Nojilana B, Nannan N, Gwebushe N, Vos T, Somdyala N, et al. Second National Burden of Disease Study South Africa: national and subnational mortality trends, 1997–2009. Lancet. 2013;381:S113. [Google Scholar]
- 19.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10:R41. doi: 10.1186/bcr2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyratzopoulos G, Abel GA, Brown CH, Rous BA, Vernon SA, Roland M, Greenberg DC. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol. 2013;24:843–50. doi: 10.1093/annonc/mds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Fidalgo JA, Miranda J, Chirivella I, Ibanez J, Bermejo B, Pons C, Melchor I, Santaballa A, Martinez-Ruiz F, Lluch A, Salas D. Impact of a mammography screening programme on the breast cancer population of the Region of Valencia (Spain) Clin Transl Oncol. 2008;10:745–52. doi: 10.1007/s12094-008-0281-y. [DOI] [PubMed] [Google Scholar]
- 22.Traore B, Keita M, Diane S, Dankoro A, Kabba IS, Keita N. Clinicopathological study of Breast Diseases presenting to the Surgical Oncology Unit of Donka University Hospital in Conakry, Guinea. West Afr J Med. 2012;31:227–31. [PubMed] [Google Scholar]
- 23.Adisa AO, Lawal OO, Adesunkanmi AR. Paradox of wellness and nonadherence among Nigerian women on breast cancer chemotherapy. J Cancer Res Ther. 2008;4:107–10. doi: 10.4103/0973-1482.42640. [DOI] [PubMed] [Google Scholar]
- 24.Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183–8. doi: 10.1007/s10549-007-9694-5. [DOI] [PubMed] [Google Scholar]
- 25.Lopes Ibanez-Gonzalez D, Norris SA. Chronic non-communicable disease and healthcare access in middle-aged and older women living in Soweto, South Africa. PLoS One. 2013;8:e78800. doi: 10.1371/journal.pone.0078800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekortarl A, Ndom P, Sacks A. A study of patients who appear with far advanced cancer at Yaounde General Hospital, Cameroon, Africa. Psychooncology. 2007;16:255–7. doi: 10.1002/pon.1144. [DOI] [PubMed] [Google Scholar]
- 27.Sighoko D, Kamate B, Traore C, Malle B, Coulibaly B, Karidiatou A, Diallo C, Bah E, McCormack V, Muwonge R, Bourgeois D, Gormally E, et al. Breast cancer in pre-menopausal women in West Africa: Analysis of temporal trends and evaluation of risk factors associated with reproductive life. Breast. 2013 doi: 10.1016/j.breast.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Ukwenya AY, Yusufu LM, Nmadu PT, Garba ES, Ahmed A. Delayed treatment of symptomatic breast cancer: the experience from Kaduna, Nigeria. S Afr J Surg. 2008;46:106–10. [PubMed] [Google Scholar]
- 29.Lotika AA, Mabuza LH, Okontak HI. Reasons given by hypertensive patients for concurrently using traditional and Western medicine at Natalspruit Hospital in the Gauteng Province, South Africa. African Journal of Primary Health Care & Family Medicine. 2013:5. [Google Scholar]
- 30.Kantelhardt EJ, Zerche P, Mathewos A, Trocchi P, Addissie A, Aynalem A, Wondemagegnehu T, Ersumo T, Reeler A, Yonas B, Tinsae M, Gemechu T, et al. Breast cancer survival in Ethiopia: A cohort study of 1,070 women. Int J Cancer. 2013 doi: 10.1002/ijc.28691. [DOI] [PubMed] [Google Scholar]
- 31.Celaya MO, Berke EM, Onega TL, Gui J, Riddle BL, Cherala SS, Rees JR. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998–2004) Rural Remote Health. 2010;10:1361. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang B, Dignan M, Han D, Johnson O. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. J Rural Health. 2009;25:366–71. doi: 10.1111/j.1748-0361.2009.00245.x. [DOI] [PubMed] [Google Scholar]
- 33.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279:1801–7. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 34.Scoggins JF, Fedorenko CR, Donahue SM, Buchwald D, Blough DK, Ramsey SD. Is distance to provider a barrier to care for medicaid patients with breast, colorectal, or lung cancer? J Rural Health. 2012;28:54–62. doi: 10.1111/j.1748-0361.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermiah E, Abdalla F, Buhmeida A, Larbesh E, Pyrhonen S, Collan Y. Diagnosis delay in Libyan female breast cancer. BMC Res Notes. 2012;5:452. doi: 10.1186/1756-0500-5-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Partridge AH, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Tamimi RM. The effect of age on delay in diagnosis and stage of breast cancer. Oncologist. 2012;17:775–82. doi: 10.1634/theoncologist.2011-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S, White E. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91:2020–8. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 38.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Partridge AH. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res Treat. 2012;136:813–21. doi: 10.1007/s10549-012-2304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip CH, Cazap E, Anderson BO, Bright KL, Caleffi M, Cardoso F, Elzawawy AM, Harford JB, Krygier GD, Masood S, Murillo R, Muse IM, et al. Breast cancer management in middle-resource countries (MRCs): consensus statement from the Breast Health Global Initiative. Breast. 2011;20 (Suppl 2):S12–S19. doi: 10.1016/j.breast.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Celaya MO, Rees JR, Gibson JJ, Riddle BL, Greenberg ER. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States) Cancer Causes Control. 2006;17:851–6. doi: 10.1007/s10552-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 41.Woods LM, Rachet B, Coleman MP. Choice of geographic unit influences socioeconomic inequalities in breast cancer survival. Br J Cancer. 2005;92:1279–82. doi: 10.1038/sj.bjc.6602506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sylla BS, Wild CP. A million africans a year dying from cancer by 2030: what can cancer research and control offer to the continent? Int J Cancer. 2012;130:245–50. doi: 10.1002/ijc.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson BO, Cazap E, El Saghir NS, Yip CH, Khaled HM, Otero IV, Adebamowo CA, Badwe RA, Harford JB. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12:387–98. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 44.Nair MK, Sankaranarayanan R, Nair KS, Amma NS, Varghese C, Padmakumari G, Cherian T. Overall survival from breast cancer in Kerala, India, in relation to menstrual, reproductive, and clinical factors. Cancer. 1993;71:1791–6. doi: 10.1002/1097-0142(19930301)71:5<1791::aid-cncr2820710513>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 45.Harford JB, Otero IV, Anderson BO, Cazap E, Gradishar WJ, Gralow JR, Kane GM, Niens LM, Porter PL, Reeler AV, Rieger PT, Shockney LD, et al. Problem solving for breast health care delivery in low and middle resource countries (LMCs): consensus statement from the Breast Health Global Initiative. Breast. 2011;20 (Suppl 2):S20–S29. doi: 10.1016/j.breast.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Harford JB. Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all. Lancet Oncol. 2011;12:306–12. doi: 10.1016/S1470-2045(10)70273-4. [DOI] [PubMed] [Google Scholar]
- 47.Panieri E. Breast cancer screening in developing countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:283–90. doi: 10.1016/j.bpobgyn.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Abuidris DO, Elsheikh A, Ali M, Musa H, Elgaili E, Ahmed AO, Sulieman I, Mohammed SI. Breast-cancer screening with trained volunteers in a rural area of Sudan: a pilot study. Lancet Oncol. 2013;14:363–70. doi: 10.1016/S1470-2045(12)70583-1. [DOI] [PubMed] [Google Scholar]
- 49.Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Prabhakar J, Augustine P, Venugopal M, Anju G, Mathew BS. Clinical breast examination: preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103:1476–80. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- 50.Goudge J, Gilson L, Russell S, Gumede T, Mills A. Affordability, availability and acceptability barriers to health care for the chronically ill: longitudinal case studies from South Africa. BMC Health Serv Res. 2009;9:75. doi: 10.1186/1472-6963-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.