Abstract
Purpose
Krüppel-like factor 4 (KLF4) is a transcription factor and putative tumor suppressor. However, little is known about its effect on aerobic glycolysis in pancreatic tumors. Therefore, we investigated the clinical significance, biologic effects, and mechanisms of dysregulated KLF4 signaling in aerobic glycolysis in pancreatic cancer cells.
Experimental Design
Expression of KLF4 and lactate dehydrogenase A (LDHA) in 70 primary pancreatic tumors and 10 normal pancreatic tissue specimens was measured. Also, the underlying mechanisms of altered KLF4 expression and its impact on aerobic glycolysis in pancreatic cancer cells were investigated.
Results
We found a negative correlation between KLF4 and LDHA expression in pancreatic cancer cells and tissues and that their expression was associated with clinicopathologic features of pancreatic cancer. KLF4 underexpression and LDHA overexpression were correlated with disease stage and tumor differentiation. Experimentally, KLF4 overexpression significantly attenuated the aerobic glycolysis in and growth of pancreatic cancer cells both in vitro and in orthotopic mouse models, whereas knockdown of KLF4 expression had the opposite effect. Enforced KLF4 expression decreased LDHA expression, whereas small interfering RNA-mediated knockdown of KLF4 expression had the opposite effect. Mechanistically, KLF4 bound directly to the promoter regions of the LDHA gene and negatively regulated its transcription activity.
Conclusions
Dysregulated signaling in this novel KLF4/LDHA pathway significantly impacts aerobic glycolysis in and development and progression of pancreatic cancer.
Keywords: KLF4, LDHA, aerobic glycolysis, growth, pancreatic cancer
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in industrialized countries, with a 5-year survival rate of 6% in all patients. In 2012, physicians diagnosed 43,920 new cases of this cancer, and 37,390 patients died of it in the United States (1). Although surgery offers the best chance for a cure of pancreatic cancer, less than 20% of patients are eligible for potentially curative resection, as most cases have already spread locally or to distant organs at diagnosis precluding resection (2). The disappointing survival rates for pancreatic cancer even after margin-negative pancreatectomy indicate the aggressive nature of this disease. Therefore, a better understanding of the molecular mechanisms underlying pancreatic cancer initiation and progression is urgently needed (3, 4).
An increase in aerobic glycolysis, which is the continuous conversion of glucose to lactate in the presence of oxygen (Warburg effect), is a distinctive hallmark of solid tumors, including pancreatic cancer (5, 6). Because it is highly associated with increased progression and metastasis of cancer, aerobic glycolysis is considered a metabolic signature for invasive cancer (7, 8). Enzymatically functional lactate dehydrogenase (LDH) consists of four subunits grouped into two types: M (muscle-type, LDHA gene product) and H (heart-type, LDHB gene product). Five LDH isozymes have different substrate reactivity as a result of the five combinations of the two types of LDH subunits: LDH1 (H4), LDH2 (MH3), LDH3 (M2H2), LDH4 (M3H), and LDH5 (M4) (9). LDH5 in particular effectively catalyzes the conversion of pyruvate to lactate and is linked with metastatic cancer (10, 11). Therefore, regulation of expression of LDH, especially the LDHA subunit, plays a key role in aerobic glycolysis. This altered metabolism is maintained in cultured cells derived from pancreatic tumors when cultivated under normoxic conditions. This indicates that aerobic glycolysis is not just transiently produced by the tumor microenvironment in vivo but also may be constitutively upregulated via stable genetic or epigenetic changes (6, 7).
Krüppel-like factor 4 (KLF4) is a zinc-finger transcription factor, and KLF4 mRNA expression is found primarily in postmitotic, terminally differentiated epithelial cells in organs such as the skin and lungs and those in the gastrointestinal tract (12, 13). Authors have described accumulating clinical, experimental, and mechanistic evidence that KLF4 is a potential tumor suppressor in patients with various cancers, including pancreatic cancer (14–17). However, the precise role and underlying signaling cascade of KLF4 in aerobic glycolysis in pancreatic tumors remain unclear.
In the present study, we sought to determine the roles of regulation of KLF4 expression in aerobic glycolysis in pancreatic cancer cells, the effect of this regulation on aerobic glycolysis in these cells, and the underlying molecular mechanisms. We discovered that the novel KLF4/LDHA signaling pathway critically regulated this aerobic glycolysis.
Materials and Methods
Data mining
NCBI Gene Omnibus database provides insight into the biochemical function of dysregulated of KLF4. GSE21768 investigated the changes in gene expression of the KLF4-null MEFs. GSE3113 analyzed the changes in gene expression when RKO colon cancer cell line was treated for up to 24 hours with ponasterone A to induce expression of KLF4. Processing of gene expression data was according to the database’s guideline. In GSE21768 and GSE3113, the values of logFC represent the glycolysis related genes’ mRNA expression in KLF4-induced cells/KLF4-null MEFs versus in the control cells. The positive value of logFC means upregulation of the gene expression in KLF4-induced cells/KLF4-null MEFs than in control cells. Conversely, the negative value of logFC means downregulation of the gene expression in KLF4-induced cells/KLF4-null MEFs in a comparison with that in corresponding control cells. Statistical significance was set at P<0.05.
Cell lines and culture conditions
The human pancreatic adenocarcinoma cell lines PANC-1, PAU8902, AsPC-1, BxPC-3, and MiaPaCa-2 were purchased from the American Type Culture Collection. The pancreatic cancer cell line MDA Panc-28 was a gift from Dr. Paul J. Chiao (The University of Texas MD Anderson Cancer Center). The human pancreatic adenocarcinoma cell line FG was obtained from Michael P. Vezeridis (The Warren Alpert Medical School of Brown University) (18). All of these cell lines were maintained in plastic flasks as adherent monolayers in Eagle’s minimal essential medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution (Flow Laboratories). The cell lines obtained directly from the American Type Culture Collection, which performs cell line characterization or authentication using short tandem repeat profiling, and passaged in our laboratory fewer than 6 months after receipt.
Human tissue specimens and immunohistochemical analysis
Expression of KLF4, LDHA, and LDHB was analyzed using tissue microarrays (TMA; US Biomax) containing both human pancreatic tumor specimens and normal pancreatic tissue specimens (Supplementary Table S1). Use of the tissue specimens was approved by the MD Anderson Institutional Review Board. Standard immunohistochemical staining was performed using anti-KLF4 (Santa Cruz Biotechnology), anti-LDHA (Epitomics), and anti-LDHB (Epitomics) antibodies. The staining results were scored by two investigators blinded to the clinical data as described previously (19).
Measurement of intracellular glucose utilization, LDH activity and lactate concentration in a culture medium
To estimate the intracellular glucose utilization, LDH activity and lactate concentration in pancreatic cancer cells, a glucose colorimetric assay kit II (BioVision), lactate dehydrogenase activity assay kit (Sigma-Aldrich) and lactate assay kit (Sigma-Aldrich) were used according to the manufacturer’s protocols. The glucose utilization was estimated using a standard glucose calibration curve prepared under the same condition and reported in micrograms per microliter. The LDH activity was measured by comparing the initial rate with a calibration curve for a known concentration of standard LDH created at the same time. The LDH activity was reported in milliunits per milliliter. The lactate concentration was estimated using a standard lactate calibration curve prepared under the same condition and reported in nanograms per microliter.
Reverse transcription-polymerase chain reaction
Total RNA extraction from pancreatic tumor cells was performed using the TRIzol reagent (Invitrogen). Next, 2 µg of total RNA was reverse-transcribed using a First Strand cDNA synthesis kit (Promega) to synthesize cDNA samples. Subsequently, 2 µL of cDNA products was subjected to polymerase chain reaction (PCR) amplification with Taq DNA polymerase (Qiagen) using a thermal cycler with the following primers: KLF4, sense strand 5'-cccacatgaagcgacttccc-3' and antisense strand 5'-caggtccaggagatcgttgaa-3'; LDHA, sense strand 5'-ttgacctacgtggcttggaag-3' and antisense strand 5'-ggtaacggaatcgggctgaat-3'; and α-Tubulin, sense strand 5'-gcagccaacaactatgcccg-3' and antisense strand 5'-cttccgtatgcggtccagca-3'. The PCR products were loaded onto 2% agarose gels and visualized using ethidium bromide staining under ultraviolet light.
Western blot analysis
Standard Western blotting was carried out using whole-cell protein lysates and primary antibodies against KLF4 (Santa Cruz Biotechnology) and LDHA (Epitomics) and a secondary antibody (anti-rabbit IgG; Santa Cruz Biotechnology). Equal protein sample loading was monitored using an anti-α-Tubulin antibody (Santa Cruz Biotechnology).
Transient transfection of pancreatic cancer cells
For overexpression of KLF4 in PANC-1 cells, they were transduced with adenoviral KLF4 (Ad-KLF4) or adenoviral enhanced green fluorescent protein (EFGP) as described previously (14). To inhibit KLF4 expression in BxPC-3 cells, they were transfected with a pool of KLF4 small interfering RNA (siRNA) oligonucleotides or control siRNA oligonucleotides (Santa Cruz Biotechnology; 50 nmol/L). For overexpression of KLF4 and LDHA in PANC-1 cells, the cells were transduced with Ad-KLF4 and pLDHA expression vector (OriGene, Rockville, MD). LDH inhibitor Oxamate sodium (Oxa) with a concentration of 20 mmol/L was added into the BxPC-3 cells transfected with KLF4 siRNA for further rescue experiments. Cells treated with Oligofectamine (Life Technologies) alone were included as mock transfection controls.
Construction of LDHA promoter reporter plasmids and mutagenesis
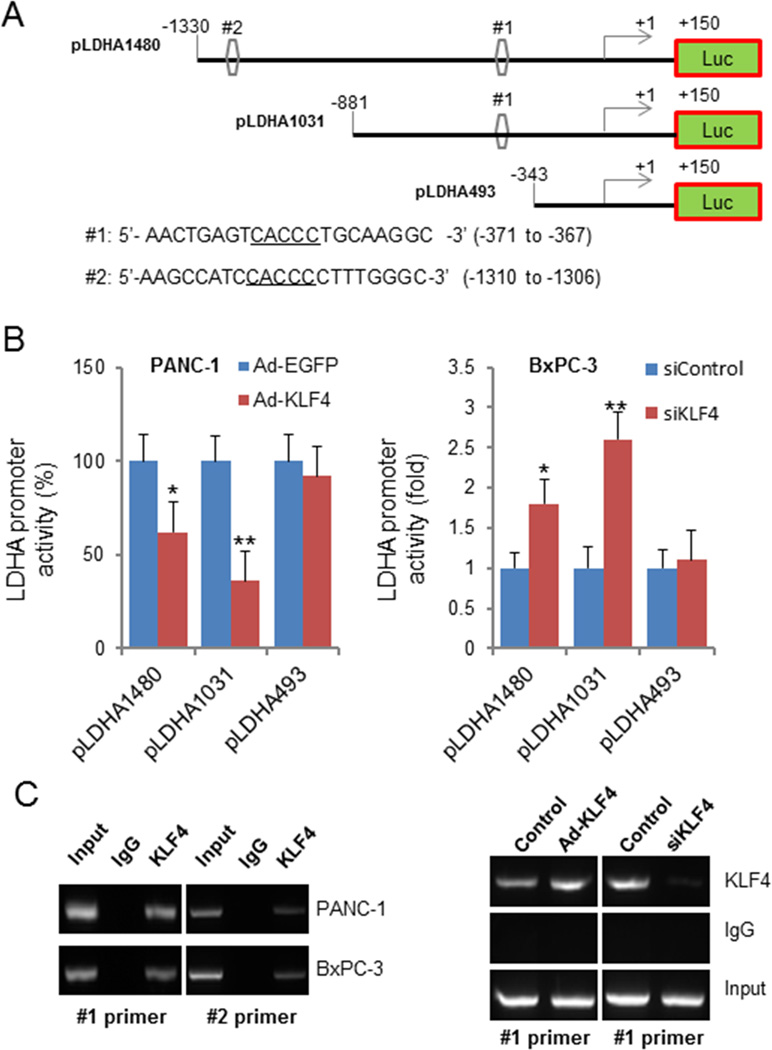
A 1.48-kb fragment containing LDHA 5' sequences from −1330 to +150 bp relative to the transcription initiation site was subcloned into the pGL3-basic vector (Promega). The resulting full-length reporter plasmid, which contained multiple KLF4-binding sites, was designated pLDHA1480. Deletion mutation reporters pLDHA1031 and pLDHA493 for this plasmid were then generated from the pLDHA1480. All constructs were verified by sequencing the inserts and flanking regions of the plasmids.
Measurement of promoter reporter activity by using dual luciferase assay
Pancreatic cancer cells were transfected with the indicated LDHA promoter reporters, siRNAs, or specific gene expression plasmids. The LDHA promoter activity in these cells was normalized by co-transfecting a β-actin/Renilla luciferase reporter containing a full-length Renilla luciferase gene (20). The luciferase activity in the cells was quantified using a dual luciferase assay system (Promega) 24 hours after transfection (21).
Chromatin immunoprecipitation assay
Pancreatic tumor cells (2×106) were prepared for a chromatin immunoprecipitation (ChIP) assay using a ChIP assay kit (Millipore) according to the manufacturer’s protocol. The resulting precipitated DNA samples were analyzed using PCR to amplify a 207-bp region of the LDHA promoter with the primers 5'-caagccactgacagttcttg-3' (sense) and 5'-acctaagtcgagtgacctcc-3' (antisense) and a 306-bp region of the LDHA promoter with the primers 5'-gtgctattttggagctgaggtt-3' (sense) and 5'-agcccttgagtatgccaaaat-3' (antisense). The PCR products were resolved electrophoretically on a 2% agarose gel and visualized using ethidium bromide staining.
In vitro cell growth assay
PANC-1 cells were transduced with Ad-KLF4 or with Ad-KLF4 plus pLDHA, and BxPC-3 cells were treated with siKLF4 or with siKLF4 plus 20 mmol/L oxamate for 48 hours. A total of 5000 cells/well in 100 µl medium from each treatment were plated in 96-well plates. After 24 hours incubation, viable cells were determined by standard MTT assay.
Animal experiments
Female pathogen-free athymic nude mice were purchased from the National Cancer Institute. The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the current regulations and standards of the U.S. Department of Agriculture and U.S. Department of Health and Human Services. Pancreatic tumor cells (1×106) in 0.1 mL of Hank’s balanced salt solution were injected subcutaneously into the right scapular region of each mouse. The tumor-bearing mice were killed when they became moribund or on day 35 after inoculation, and their tumors were removed and weighed.
Statistical analysis
The significance of the patient specimen data was determined using the Pearson correlation coefficient. The significance of the in vitro and in vivo data was determined using the Student t-test (two-tailed), Mann-Whitney U test (two-tailed), or one-way analysis of variance. P values less than 0.05 were considered significant. The SPSS software program (version 17.0; IBM Corporation) was used for statistical analysis.
Results
Dysregulated KLF4 impacts the expression of multiple glycolysis-related genes
In order to investigate whether dysregulated KLF4 could impact the expression of glycolysis related-genes, we analyzed the glycolysis related gene expression data which were deposited in the NCBI Gene Omnibus database (KLF4 null MEFs: GSE21768; KLF4 induced: GSE3113). The expression levels of many glycolysis-related genes were altered upon the alterations of KLF4 expression. Among all the expression alterations of glycolysis-related genes in these two databases, PFKP, PKM2, and LDHA were upregulated in KLF4-null MEFs cells, while downregulated in KLF4-induced RKO cells (Supplementary Table S2 and S3). This initial data mining study indicated that KLF4 play an important role in glycolysis. Given that LDHA was the most altered genes upon the regulation of KLF4, we chose LDHA as the target gene and try to investigate whether KLF4-LDHA signaling pathway could impact glycolysis in pancreatic cancer.
Direct association of LDHA but not LDHB overexpression with clinicopathologic features of pancreatic cancer
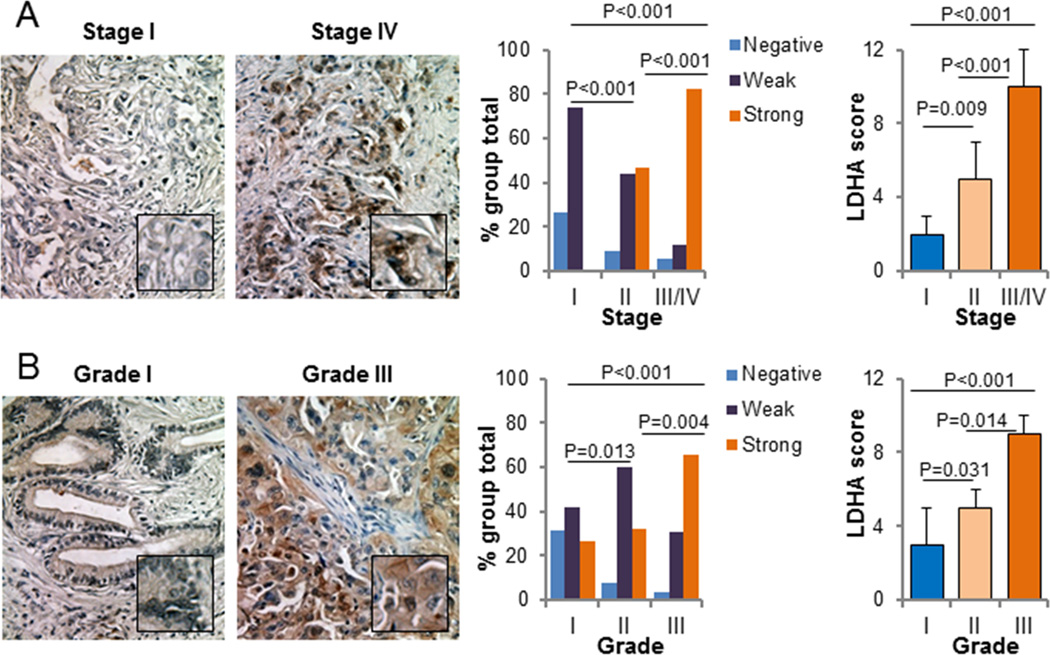
We first investigated the expression of LDHA and LDHB in the 70 primary pancreatic tumor and 10 normal pancreatic tissue specimens in the TMA. We observed LDHA-positive staining in the cytoplasm of the tumor cells but LDHA-negative or weakly LDHA-positive staining in the cytoplasm of normal pancreatic cells. In contrast, we observed LDHB-negative staining in the cytoplasm of tumor cells but weakly LDHB-positive or LDHB-positive staining in the cytoplasm of normal pancreatic cells (Supplementary Fig. S1). LDHA expression was positively correlated with disease stage, indicating that LDHA expression was upregulated in late-stage pancreatic tumors. This association between stage I, II versus stage III/IV tumors was significant (P<0.001) (Fig. 1A; Supplementary Table S4). Moreover, increased LDHA expression correlated with decreased tumor differentiation (P=0.008) (Fig. 1B; Supplementary Table S4). Nevertheless, LDHB expression was not related to clinicopathologic features of pancreatic cancer (Supplementary Fig. S2; Supplementary Table S5). These findings indicated that LDHA but not LDHB overexpression plays a critical role in pancreatic cancer development and progression.
Figure 1.
LDHA expression in pancreatic tumor specimens and its association with clinicopathologic features of pancreatic cancer patients. A, Positive correlation of LDHA expression with disease stage. Representative images of stage I and IV tumors are shown (200× magnification). B, Positive correlation of LDHA expression with tumor differentiation. Representative images of grade 1 and 3 tumors are shown (200× magnification).
Decreased KLF4 expression and its direct association with clinicopathologic features of pancreatic cancer
We further evaluated the expression of KLF4 protein in the TMA specimens. Typically, we observed KLF4-negative or weakly KLF4-positive staining in the tumor cells and KLF4-positive staining in the normal pancreatic cells (Supplementary Fig. S1). Furthermore, we analyzed the relationship between clinicopathologic features and KLF4 expression in the pancreatic cancer cases. We found that KLF4 expression was negatively correlated with disease stage, demonstrating that KLF4 expression was downregulated in late-stage tumors. This association between stage I, II versus stage III/IV tumors was significant (P<0.001) (Supplementary Fig. S3; Supplementary Table S6). Moreover, decreased KLF4 expression correlated with decreased tumor differentiation (P=0.004) (Supplementary Fig S3; Supplementary Table S6). These findings strongly indicated that loss of KLF4 expression plays a critical role in pancreatic cancer development and progression and is a valuable biomarker for this disease.
Association of decreased KLF4 expression with LDHA overexpression in pancreatic cancer cells
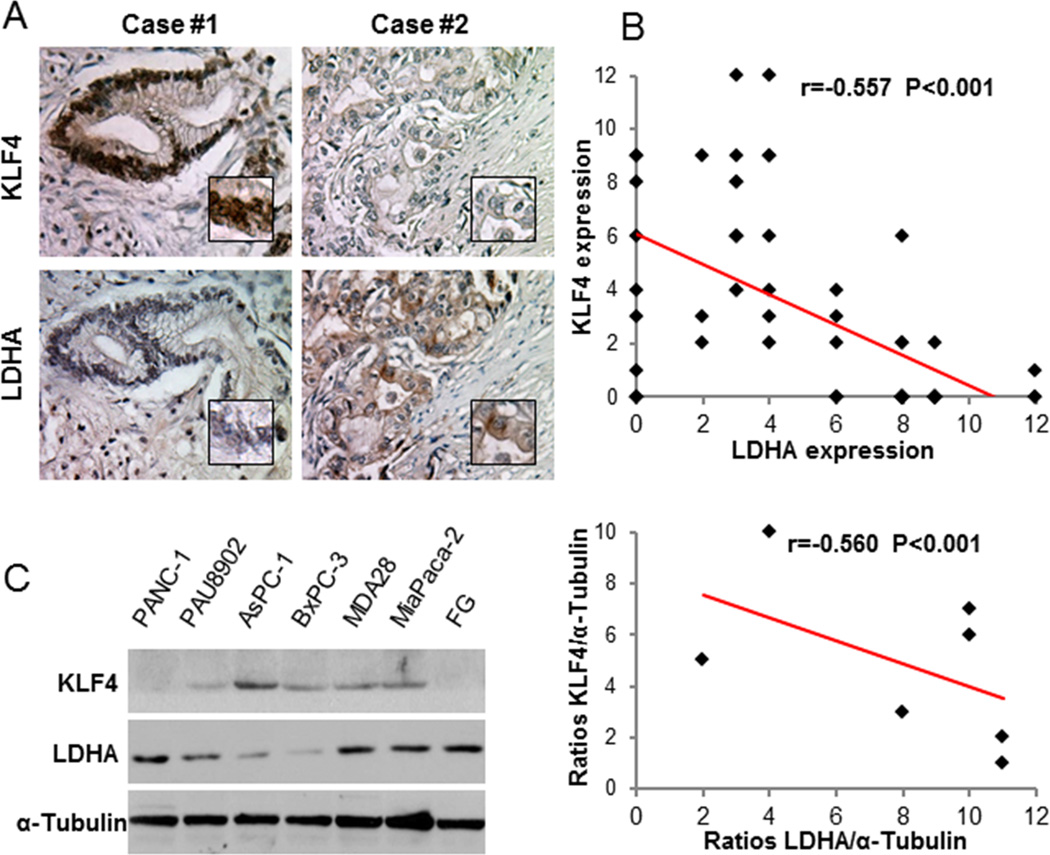
Given that KLF4 expression loss and LDHA overexpression are closely related to the clinicopathologic features of patients with pancreatic cancer, we sought to evaluate the relationship between KLF4 expression and LDHA expression in pancreatic cancer cells. First, we analyzed the expression of KLF4 and LDHA in the TMA specimens (Fig. 2A). KLF4 expression was negatively correlated with LDHA expression at a statistically significant level (r = −0.557, P < 0.001) (Fig. 2B). Consistently, expression of KLF4 was negatively correlated with expression of LDHA in pancreatic cancer cell lines (Fig. 2C). These data were clinical evidence that KLF4 inactivation is associated with increased LDHA expression.
Figure 2.
Negative association of KLF4 expression with LDHA expression in pancreatic cancer cells. A, Immunohistochemical staining of pancreatic tumor specimens for KLF4 and LDHA. Shown are representative photographs (200× magnification) of KLF4 and LDHA staining in two pancreatic cancer cases. B, Negative correlation of LDHA expression with KLF4 expression (n = 70; Pearson correlation test: r=−0.557; P<0.001). C, Western blot analysis of KLF4 and LDHA expression in pancreatic cancer cell lines (left panel), and quantitative Western blot analysis results obtained using densitometric analysis and standardized according to α-Tubulin, and negative correlation between KLF4 expression and LDHA expression in pancreatic cancer cell lines (right panel, Pearson correlation test: r=−0.560, P<0.001).
Effects of altered KLF4 expression on aerobic glycolysis and growth in pancreatic cancer cells in vitro
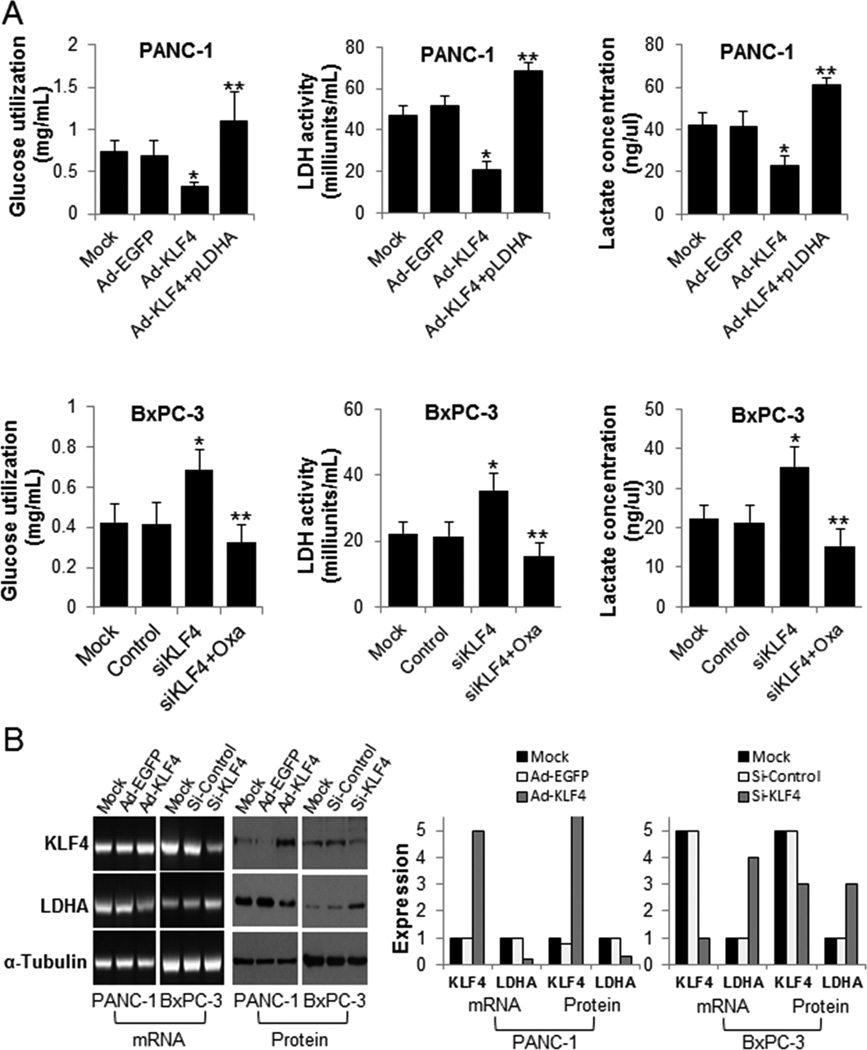
To determine the effect of altered KLF4 expression on aerobic glycolysis in and growth of pancreatic cancer cells, we transfected PANC-1 and BxPC-3 cells with Ad-KLF4 or with Ad-KLF4 plus pLDHA expression vector; or treated with KLF4 siRNA or with KLF4 siRNA plus Oxamate sodium, respectively, for 48 hours. We calculated the glucose utilization, LDH activity, lactate concentrations and MTT assay of the treated cells. Restored KLF4 expression strongly decreased the glucose utilization, LDH activity, lactate concentrations in and growth of PANC-1 cells, whereas this inhibitory effect was attenuated by overexpression of LDHA (Fig. 3A and Supplementary Fig S4). Downregulation of KLF4 expression increased the glucose utilization, LDH activity, lactate concentrations in and growth of BxPC-3 cells. Furthermore, when we added Oxamate sodium to the BxPC-3 cells transfected with KLF4-siRNA, oxamate sodium could rescue these effects which caused by knockdown of KLF4 (Fig. 3A and Supplementary Fig S4). We confirmed these results using reverse transcription-PCR and Western blot analysis, which demonstrated that the levels of LDHA mRNA and protein expression in Ad-KLF4–transfected PANC-1 cells were significantly lower than those in control cells, whereas LDHA mRNA and protein expression was upregulated in KLF4 siRNA-transfected BxPC-3 cells (Fig. 3B). Thus, our data clearly established that KLF4 could regulate aerobic glycolysis in and the growth of pancreatic cancer by impacting LDHA expression.
Figure 3.
The effect of KLF4 expression on aerobic glycolysis in pancreatic cancer cells. A, PANC-1 cells were transfected with Ad-KLF4 or with Ad-KLF4 plus pLDHA. BxPC-3 cells were transfected with KLF4 siRNA or KLF4 siRNA plus 20mmol/L Oxamate sodium (Oxa) for 48 hours, respectively. The glucose utilization, LDH activity and lactate concentrations were determined as described in Materials and Methods. The data represent the mean ± standard deviation results of experiments performed in triplicate. *P < 0.05 in a comparison between Ad-KLF4– or KLF4 siRNA-transfected cells and mock or control cells. **P<0.05 in a comparison between cells transfected with Ad-KLF4 and pLDHA and cells transfected with Ad-KLF4; or between groups treated with KLF4 siRNA plus Oxamate sodium and groups treated with KLF4 siRNA. B, Reverse transcription-PCR (mRNA) and Western blot (Protein) analysis of KLF4 and LDHA expression in PANC-1 and BxPC-3 cells transfected with Ad-KLF4 and KLF4 siRNA for 48 hours, respectively. Total RNA and protein lysates were harvested for measurement of the expression (left panels). Quantitative results also were shown (right panels).
Altered KLF4 expression inhibits the tumorigenicity of pancreatic cancer cells in vivo by downregulating LDHA expression
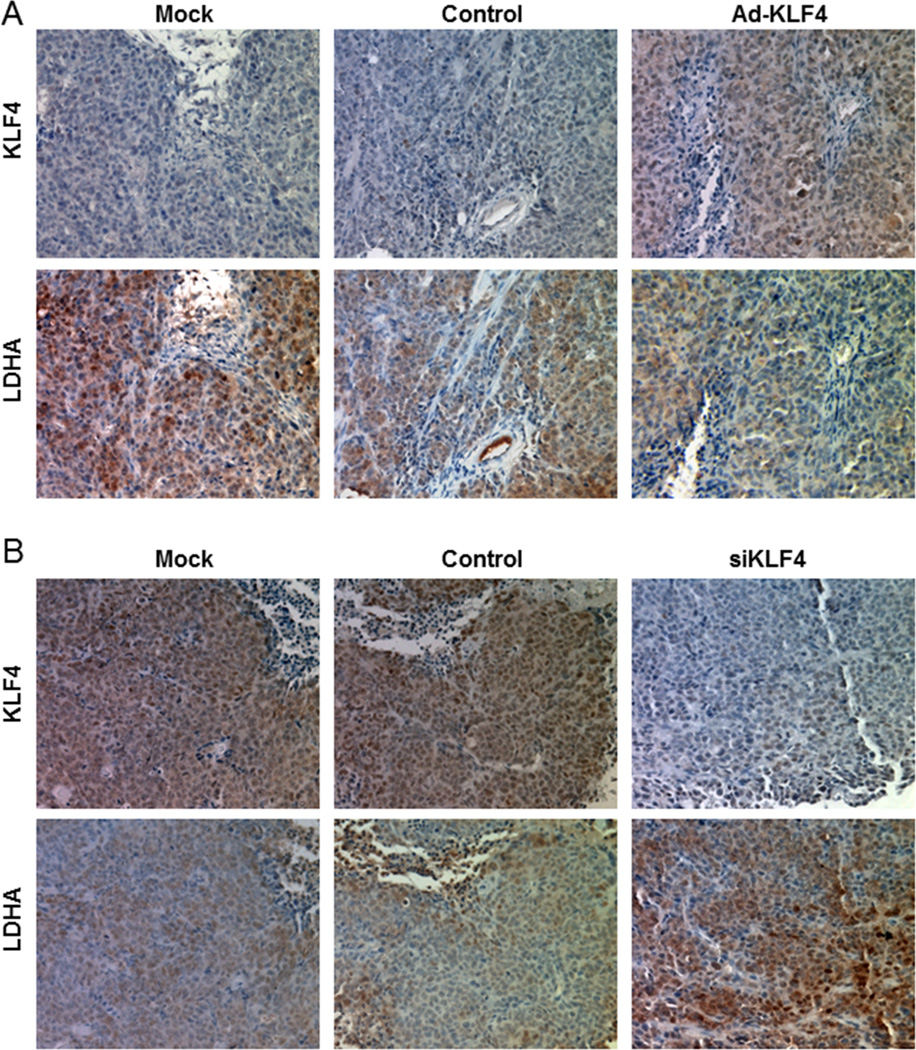
To determine whether KLF4 regulates LDHA expression in pancreatic cancer cells in vivo, we injected KLF4-overexpressing PANC-1 cells and KLF4 siRNA-transfected BxPC-3 cells into the pancreas of nude mice. Overexpression of KLF4 significantly inhibited tumor growth (P<0.001), while knockdown KLF4 promoted tumor growth (P=0.011, Supplementary Fig. S5). Immunohistochemical staining of tumor xenografts indicated that LDHA expression was inhibited by KLF4 overexpression (Fig. 4A). Conversely, we observed LDHA overexpression in xenografts after downregulation of KLF4 expression (Fig. 4B). These results were consistent with the effect of altered KLF4 expression on glucose utilization, LDH activity, lactate concentrations in and the growth of pancreatic cancer cells in vitro as described above, and suggested that KLF4 inhibited pancreatic cancer by downregulation of LDHA expression.
Figure 4.
Influence of KLF4 expression on pancreatic tumor growth. A, Overexpression of KLF4 by gene transduction using Ad-KLF4 and B, knockdown of KLF4 expression by using siRNA against KLF4 (siKLF4). Presented were representative photographs (200× magnification) of KLF4 and LDHA staining in mouse pancreatic tumor xenografts.
Transcriptional inhibition of LDHA expression by KLF4 in pancreatic cancer cells
Our study demonstrated a negative correlation between KLF4 and LDHA expression in pancreatic cancer cell lines. To further explore the molecular mechanisms of regulation of LDHA expression by KLF4, we first analyzed the LDHA promoter sequence for the presence of potential KLF4-binding elements by using the KLF4 consensus sequence 5'-CACCC-3'. We identified two putative KLF4-binding elements (referred to as DNA sequences #1 and #2) in the LDHA promoter region. According to these two elements, we generated an LDHA promoter (pLDHA1480) and two deletion mutation LDHA promoters (pLDHA1031 and pLDHA493). The pLDHA1480 contains potential KLF4-binding sites #1 and #2, pLDHA1031 contains potential KLF4-binding site #1 only, and pLDHA493 contains no potential KLF4-binding sites (Fig. 5A). Transfection of pancreatic cancer cells with KLF4 greatly decreased the LDHA promoter activity (pLDHA1480 and pLDHA1031), whereas knockdown of KLF4 expression in the cells increased this activity. Altered KLF4 expression had no effect on LDHA promoter activity (pLDHA493). These results suggested that the KLF4-binding sites were negative regulatory elements in the LDHA promoter (Fig. 5B).
Figure 5.
Transcriptional inactivation of LDHA expression by KLF4 in pancreatic cancer cells. A, Sequences and positions of putative KLF4-binding elements on the LDHA promoter #1 and #2). B, Relative LDHA promoter activity in PANC-1 and BxPC-3 cells transfected with the LDHA promoter and with Ad-KLF4 and KLF4 siRNA, respectively, in triplicate. The relative LDHA promoter activity was measured 24 hours after transfection. Statistical significanc: *P<0.05; **P<0.01. C, ChIP assay. Chromatins were isolated from PANC-1 and BxPC-3 cells, and binding of KLF4 to the LDHA promoter in these cells was examined using a specific anti-KLF4 antibody and oligonucleotides flanking the LDHA promoter regions containing putative KLF4-binding sites as described in Materials and Methods. Similar ChIP assays were conducted using chromatins isolated from PANC-1, Ad-KLF4–transfected PANC-1, BxPC-3, and KLF4 siRNA-transfected BxPC-3 cells.
In addition, to determine whether KLF4 interacts directly with the LDHA promoter in pancreatic cancer cells, we conducted ChIP assays using chromatins prepared from PANC-1 and BxPC-3 cells and two primer sets flanking the 207-bp (−471 to −265 bp; #1 site) and 306-bp (−1427 to −1122 bp; #2 site). We amplified both of these DNA fragments from PANC-1 and BxPC-3 cell precipitates using anti-KLF4 antibodies but not control IgG, suggesting that endogenous KLF4 bound to the regions of the LDHA promoter from −471 to −265 bp and from −1427 to −1122 bp in these pancreatic cancer cells. We confirmed these results by engineering overexpression of KLF4, which led to increased KLF4 recruitment to the LDHA promoter, and by knocking down KLF4 expression, which led to decreased KLF4 recruitment to the LDH promoter. The altered KLF4 recruitment was consistent with changes in LDHA promoter activity (Fig. 5C). Collectively, these findings demonstrated that KLF4 bound to the LDHA promoter primarily at positions −371 to −367 bp and −1310 to −1306 bp and negatively regulated LDHA transcription in pancreatic cancer cells.
Discussion
In the present study, we determined the critical roles of KLF4 and LDHA expression in aerobic glycolysis in pancreatic cancer cells and the underlying mechanism. We found that KLF4 transcriptionally repressed the expression of the LDHA gene, constituting a novel signaling pathway that directly impacted aerobic glycolysis, and that alterations informed the clinicopathologic features of patients with pancreatic cancer.
Aberrant expression and activity of metabolic enzymes are among the hallmarks of cancer. Advances in understanding the biology of tumor progression and metastasis have clearly highlighted the importance of aberrant tumor metabolism, which supports not only tumor cells’ energy requirements but also their enormous biosynthetic needs. Such metabolic alterations modulate glucose, amino acid, and fatty acid-dependent metabolite biosynthesis and energy production. Researchers are only just beginning to understand these metabolic alterations in pancreatic cancer cells, which have vast implications for diagnosis of and therapy for this disease (22).
In the 1920s, Warburg (5) made the striking discovery that even in the presence of ample oxygen, cancer cells prefer to metabolize glucose via glycolysis. In contrast, normal cells produce ATP mainly via mitochondrial oxidative phosphorylation. LDH catalyzes the final step of glycolysis, in which pyruvate is directly converted to lactate or vice versa. An isozyme shift toward increased LDH5 expression potentiates lactate production (23, 24). In pancreatic cancer, it has been reported that the expression of LDHA is elevated in both clinical samples and cell lines, and forced expression of LDHA promoted the growth and tumorigenicity of pancreatic cancer cells (25). However, the mechanism underlying LDHA overexpression in pancreatic cancer remains unclear.
Aerobic glycolysis in cancer cells has long been regarded as a phenotype acquired in response to environmental constraints such as intermittent hypoxia in premalignant lesions (6, 26). However, this metabolic change is currently thought of as not simply an adaptation to a hypoxic environment but rather an active cellular strategy that confers a significant proliferative and malignancy advantage to cancer cells (27). From a metabolic standpoint, cancer-related gene signaling networks may alter the energy profiles of cancer cells and enhance the production of biosynthetic intermediates, which are required for generation of the building blocks necessary for sustaining the growth and survival in unfavorable microenvironments (22). Thus, investigation of the molecular mechanisms leading to this phenotype and their contributions to pancreatic cancer development is urgently needed (28).
KLF4, also known as gut-enriched Krüppel-like factor, is a member of the KLF family of zinc-finger transcription factors. Investigators have observed inactivation or silencing of KLF4 in cases of a number of human cancers, including gastric, colorectal, pancreatic, esophageal, lung, prostate, and hepatocellular cancer (14, 16, 17, 29–33). Deletion of KLF4 in mouse models have led to the formation, abnormal differentiation, and increased proliferation of intestinal adenomas in the colon and gastric epithelia (34–36). Also, upregulation of expression of KLF4 inhibits cell proliferation, colony formation, and cancer cell migration and invasion; promotes cell-cycle arrest; and induces apoptosis in vitro and suppresses carcinogenesis and metastasis in vivo (16, 37). Moreover, many lines of clinical evidence demonstrate that KLF4 functions as a tumor suppressor and has potential prognostic value for lymph node metastasis (14, 32). These observations are evidence that KLF4 has a putative tumor suppressor function for a variety of malignancies. Furthermore, KLF4 expression is increased in primary breast ductal carcinoma and oral and dermal squamous cell carcinoma cells, and functioned as an oncogenic transcription factor (38–40). All of these results suggest that KLF4 expression plays a pivotal role in tumor development and progression. Recently, a study shows that KLF4 activates the transcription of PFKP gene and plays a role in the maintenance of high glycolytic metabolism in breast cancer cells (41). In pancreatic cancer, KLF4 functions as a tumor suppressor, and appears to be oncogenic in breast cancer. Therefore, the regulatory roles and mechanisms of KLF4 in aerobic glycolysis in pancreatic cancer cells needs further study.
Herein we provide evidence that KLF4 expression affects aerobic glycolysis in pancreatic cancer cells. We assessed the glucose utilization, LDH activity and lactate concentrations, which reflected aerobic glycolysis, in PANC-1 and BxPC-3 cells transfected with Ad-KLF4 and KLF4 siRNA, respectively. Restoration of KLF4 expression strongly attenuated the LDH activity and lactate concentrations in PANC-1 cells, whereas downregulation of KLF4 expression promoted them in BxPC-3 cells.
Given the important role of KLF4 in aerobic glycolysis in pancreatic tumors, we further examined the underlying mechanisms responsible for this role. We found that KLF4 regulated aerobic glycolysis in pancreatic cancer cells via transcriptional regulation of LDHA. Specifically, 1) KLF4 staining loss and LDHA overexpression in primary pancreatic tumors were highly correlated with clinicopathologic features of the patients, and their negative correlation was statistically significant and consistent in pancreatic cancer cells; 2) overexpression of KLF4 led to decreased expression of LDHA, whereas knockdown of expression of KLF4 had the opposite effect both in vitro and in vivo; and 3) KLF4 bound directly to the promoter regions of the LDHA gene and inactivated LDHA gene transcription. Therefore, our findings provided clinical and mechanistic evidence supporting the existence of a novel KLF4/LDHA signaling pathway and its critical contribution to aerobic glycolysis in pancreatic cancer cells.
In summary, this study provided both clinical and mechanistic evidence supporting that KLF4 regulates LDHA expression and that the KLF4/LDHA signaling pathway has a critical role in aerobic glycolysis in pancreatic cancer cells. Our study not only identified a novel molecular mechanism of this aerobic glycolysis but also identified aberrant KLF4/LDHA signaling as a promising new molecular target for the design of novel therapeutic modalities to control pancreatic cancer development and progression.
Supplementary Material
Translational Relevance.
We used a pancreatic tumor tissue microarray, molecular biology, and animal models to evaluate the inactivation and function of the Krüppel-like factor 4 (KLF4)/lactate dehydrogenase A (LDHA) pathway in pancreatic cancer cells. Our clinical and mechanistic findings indicated that LDHA is a direct transcriptional target of KLF4 and that dysregulation of KLF4 expression, which occurs frequently, leads to aberrant LDHA expression. Moreover, KLF4 negatively regulated aerobic glycolysis in and growth of pancreatic cancer cells, suggesting a novel molecular basis for the critical role of KLF4 inactivation in pancreatic tumor metabolism. It also suggests that dysregulated KLF4/LDHA signaling is a promising new molecular target for designing novel preventive and/or therapeutic strategies to control this malignancy. Therefore, our findings may have a major effect on clinical management of pancreatic cancer.
Acknowledgments
The authors thank Don Norwood for editorial comments.
Financial Support: Supported by grants R01-CA129956, R01-CA148954, R01-CA152309, and R01-CA172233 from the National Cancer Institute, National Institutes of Health (to K. Xie); and grant 81272917 and 81172022 (to Y.G.) from National Natural Science Foundation of China.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 3.Pliarchopoulou K, Pectasides D. Pancreatic cancer: current and future treatment strategies. Cancer Treat Rev. 2009;35:431–436. doi: 10.1016/j.ctrv.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 6.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 7.Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 8.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 9.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 10.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 11.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 13.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 15.Kanai M, Wei D, Li Q, Jia Z, Ajani J, Le X, et al. Loss of Kruppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12:6395–6402. doi: 10.1158/1078-0432.CCR-06-1034. [DOI] [PubMed] [Google Scholar]
- 16.Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008;68:4631–4639. doi: 10.1158/0008-5472.CAN-07-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Peng Z, Tang H, Wei P, Kong X, Yan D, et al. KLF4-mediated negative regulation of IFITM3 expression plays a critical role in colon cancer pathogenesis. Clin Cancer Res. 2011;17:3558–3568. doi: 10.1158/1078-0432.CCR-10-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vezeridis MP, Tzanakakis GN, Meitner PA, Doremus CM, Tibbetts LM, Calabresi P. In vivo selection of a highly metastatic cell line from a human pancreatic carcinoma in the nude mouse. Cancer. 1992;69:2060–2063. doi: 10.1002/1097-0142(19920415)69:8<2060::aid-cncr2820690810>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–6380. [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, et al. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68:8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong X, Li L, Li Z, Le X, Huang C, Jia Z, et al. Dysregulated Expression of FOXM1 Isoforms Drives Progression of Pancreatic Cancer. Cancer Res. 2013;73:3987–3996. doi: 10.1158/0008-5472.CAN-12-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh PK, Brand RE, Mehla K. MicroRNAs in pancreatic cancer metabolism. Nat Rev Gastroenterol Hepatol. 2012;9:334–344. doi: 10.1038/nrgastro.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3' enhancer. Proc Natl Acad Sci U S A. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D, et al. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523–1530. doi: 10.1007/s13277-013-0679-1. [DOI] [PubMed] [Google Scholar]
- 26.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(Suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 27.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Kim EL, Lee YK, Park CB, Kim BW, Wang HJ, et al. Decreased lactate dehydrogenase B expression enhances claudin 1-mediated hepatoma cell invasiveness via mitochondrial defects. Exp Cell Res. 2011;317:1108–1118. doi: 10.1016/j.yexcr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, Yang VW. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23:395–402. doi: 10.1038/sj.onc.1207067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Goldstein BG, Chao HH, Katz JP. KLF4 and KLF5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol Ther. 2005;4:1216–1221. doi: 10.4161/cbt.4.11.2090. [DOI] [PubMed] [Google Scholar]
- 31.Hu W, Hofstetter WL, Li H, Zhou Y, He Y, Pataer A, et al. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin Cancer Res. 2009;15:5688–5695. doi: 10.1158/1078-0432.CCR-09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE, et al. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 2010;70:10182–10191. doi: 10.1158/0008-5472.CAN-10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Gao Y, Jia Z, Mishra L, Guo K, Li Z, et al. Dysregulated Kruppel-like factor 4 and vitamin D receptor signaling contribute to progression of hepatocellular carcinoma. Gastroenterology. 2012;143:799–810. e1–e2. doi: 10.1053/j.gastro.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, Yang VW. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Yoon HS, Yang VW. Requirement of Kruppel-like factor 4 in preventing entry into mitosis following DNA damage. J Biol Chem. 2004;279:5035–5041. doi: 10.1074/jbc.M307631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, et al. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–6495. [PubMed] [Google Scholar]
- 39.Huang CC, Liu Z, Li X, Bailey SK, Nail CD, Foster KW, et al. KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia. Cancer Biol Ther. 2005;4:1401–1408. doi: 10.4161/cbt.4.12.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster KW, Ren S, Louro ID, Lobo-Ruppert SM, McKie-Bell P, Grizzle W, et al. Oncogene expression cloning by retroviral transduction of adenovirus E1A-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zinc finger protein GKLF. Cell Growth Differ. 1999;10:423–434. [PubMed] [Google Scholar]
- 41.Moon JS, Kim HE, Koh E, Park SH, Jin WJ, Park BW, et al. Kruppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. J Biol Chem. 2011;286:23808–23816. doi: 10.1074/jbc.M111.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.