Abstract
Geminin is a nucleoprotein that can directly bind chromatin regulatory complexes to modulate gene expression during development. Geminin knockout mouse embryos are preimplantation lethal by the 32-cell stage, precluding in vivo study of Geminin's role in neural development. Therefore, here we used a conditional Geminin allele in combination with several Cre-driver lines to define an essential role for Geminin during mammalian neural tube (NT) formation and patterning. Geminin was required in the NT within a critical developmental time window (embryonic day 8.5–10.5), when NT patterning and closure occurs. Geminin excision at these stages resulted in strongly diminished expression of genes that mark and promote dorsal NT identities and decreased differentiation of ventral motor neurons, resulting in completely penetrant NT defects, while excision after embryonic day 10.5 did not result in NT defects. When Geminin was deleted specifically in the spinal NT, both NT defects and axial skeleton defects were observed, but neither defect occurred when Geminin was excised in paraxial mesenchyme, indicating a tissue autonomous requirement for Geminin in developing neuroectoderm. Despite a potential role for Geminin in cell cycle control, we found no evidence of proliferation defects or altered apoptosis. Comparisons of gene expression in the NT of Geminin mutant versus wild-type siblings at embryonic day 10.5 revealed decreased expression of key regulators of neurogenesis, including neurogenic bHLH transcription factors and dorsal interneuron progenitor markers. Together, these data demonstrate a requirement for Geminin for NT patterning and neuronal differentiation during mammalian neurulation in vivo.
Keywords: chromatin, neurogenesis, neuroepithelium, neural tube defect
Introduction
Neural tube defects (NTDs) are a common birth defect, occurring in approximately 1:1000 human births, and typically present as spina bifida or anencephaly in humans (Boulet et al., 2008). While folic acid supplementation has reduced the frequency of neural tube defects in clinical studies and myo-inositol can prevent some folate-resistant NTDs in mice, they remain a significant human health concern (Greene and Copp, 1997; MRC VITAMIN STUDY RESEARCH GROUP, 1991). The etiology of NTDs is complex involving both genetic susceptibilities and environmental factors and, as a result, the underlying mechanisms are not well understood. Over 200 genes have been found to cause or contribute to increased risk of NTDs in mouse or human (Harris and Juriloff, 2010). The large number of genetic defects that cause NTDs is due to the many steps that can be disrupted during the intricate process of neurulation.
Neurulation in mammals originates with specification of a flat neuroepithelium that rises at the lateral edges to form the neural tube. The dorsal tips of the neural tube extend filopodia and “fuse” through a process involving epithelial cell remodeling. Fusion of the neural tube initiates at the level of the 3rd somite (the future cervical/hindbrain boundary) at E8.5 and proceeds both cranially and caudally (Copp et al., 2003; Greene and Copp, 2009). Neural tube fusion in the head is completed by E9.5, while spinal neural tube fusion is completed by E10.5 (Copp et al., 2003). Failure of neural plate elevation, apposition of neural folds, or fusion results in NTDs. Another critical step of neural tube formation that can cause NTDs when perturbed is specification and differentiation of cells in the neural tube (Copp and Greene, 2010).
Specification and differentiation of neural progenitors is regulated by Shh signaling from the ventral notochord and floor plate (Chiang et al., 1996; Martí et al., 1995; Roelink et al., 1995) and Bmp/Wnt signaling from the dorsal roof plate (Lee et al., 2000; Liem et al., 1997, 1995). Dose-dependent signaling induces neurogenic transcription factors that specify distinct neural progenitors (dorsal progenitor domains 1–6, ventral progenitor domains 0–3, and motor neuron progenitors) (Briscoe et al., 2000; Ericson et al., 1997; Lee and Jessell, 1999). Specification of progenitors and subsequent differentiation occurs in ventral regions first, and then proceeds dorsally such that ventral neurons are produced prior to dorsal neurons at a given rostral-caudal position. In addition, there is a rostral to caudal progression of differentiation within the neural tube, such that rostral cells at a given dorsal-ventral position differentiate prior to cells at the same dorsal-ventral level that are located more caudally.
Both transcription factors and activities that modulate chromatin structure are required for neural tube closure, but the downstream pathways they control are largely unknown (Copp and Greene, 2010; Harris and Juriloff, 2007). Mutations in several epigenetic regulatory proteins that control histone modification states or remodel nucleosomes to alter gene expression are implicated in NTDs. For example, NTDs result from mutations in the histone acetyl transferases (HATs) Gcn5 (Bu et al., 2007; Lin et al., 2008), Ep300 (Yao et al., 1998), and CBP (Oike et al., 1999). Similar phenotypes are seen with proteins that interact with these HATs including Cited2 (Bamforth et al., 2001; Barbera et al., 2002) and Tfap2a (Schorle et al., 1996; Zhang et al., 1996). Mutation of several core components of the SWI/SNF chromatin remodeling complex also results in NTDs (Bultman et al., 2000; Harmacek et al., 2014) as does mutation of a chromatin remodeling complex-associated protein, Cecr2 (Banting et al., 2005; Davidson et al., 2007). In addition, folic acid, which prevents some NTDs, can exert downstream effects at the epigenetic level by altering histone H3 lysine27 (H3K27) methylation status at promoters of critical neural transcription factors (Hes1 and Ngn2) (Ichi et al., 2010). Together, these defects demonstrate that dysregulation of epigenetic controls can result in NTDs, although mechanisms through which this occurs remain poorly understood.
Another potential regulator of embryogenesis is Geminin, a small nuclear protein initially identified in both a screen for proteins degraded during mitosis and a screen for genes that expanded the neural plate in early Xenopus embryos (Kroll et al., 1998; McGarry and Kirschner, 1998). Geminin has multiple functions through interaction with chromatin binding proteins including preventing re-replication of DNA during the cell cycle through inhibitory binding to the pre-replication protein Cdt1 as well as controlling gene expression by binding to Brg1, the catalytic component of the SWI/SNF chromatin remodeling complex, and by functional cooperation with Polycomb repressive complexes to affect transcriptional activity (Lim et al., 2011; Luo et al., 2004; Ohtsubo et al., 2008; Wohlschlegel et al., 2000). In addition, Geminin can indirectly promote histone acetylation at neural genes during neural fate acquisition of embryonic stem cells in vitro, although the specific mechanism underlying this activity is not understood (Yellajoshyula et al., 2011). While Geminin can expand the neural plate in the developing amphibian embryo, its role during mammalian neurogenesis in vivo has not been investigated.
Homozygous Geminin null embryos are preimplantation lethal by the 32-cell stage, in part due to conversion of Oct4-positive inner cell mass cells to Cdx2-positive trophoblast giant cells (Gonzalez et al., 2006), precluding analysis of later in vivo requirements for Geminin during mammalian development. Conditional excision of Geminin was previously investigated in the context of neural stem cell maintenance and differentiation (Schultz et al., 2011; Spella et al., 2011), but has not been investigated during the initial formation and patterning of the mammalian neural tube. A recent study used a shRNA knockdown approach to reduce Geminin during gastrulation and identified a role for Geminin during gastrulation (Emmett and O'Shea, 2012). However, these embryos failed to survive to stages needed to determine whether Geminin plays a putative role in neural plate formation and patterning. Therefore, here we used multiple Cre lines in combination with a conditional Geminin allele to define requirements for Geminin during mammalian neural tube formation and patterning.
Materials & Methods
Animal husbandry
Animal studies were conducted under protocols approved by the Washington University Institutional Animal Care and Use Committee (Protocol #: 20130075). Geminin conditional knockout mice (CSD24729) were purchased from the Knockout Mouse Project (KOMP). The targeted Geminin allele contains a splice acceptor-βgeo-polyA sequence flanked by FRT sites (permitting excision by FLPe recombinase) inserted into intron 3 and loxP sites flanking exon 4 (permitting excision of exon 4 by Cre recombinase). Prior to FLPe excision of the splice acceptor-βgeo-polyA sequence, the targeted allele is predicted to be a null allele of Geminin. Excision by FLPe recombinase results in a targeted (floxed) allele that generates a wild-type mRNA. This floxed allele can be converted to a null allele in the presence of Cre, which excises exon4. Mox2-Cre (003755), Sox2-Cre (008454), Wnt1-Cre (009107), ACTFLPe (005703) and ROSA26R (003474) mice were purchased from The Jackson Laboratory. Pax3Pro-Cre (Pax3-Cre) (Li et al., 2000), Nestin-Cre (Tronche et al., 1999), and Dermo1-Cre (Šošić et al., 2003) mice were obtained from Drs. Jeffrey Miner, Naren Ramanan, and David Ornitz respectively. For timed matings, noon of the day of plug discovery was designated E0.5. Embryos between E8.5-E10.5 were staged more specifically by somite number (Copp et al., 2000).
Genotyping
DNA was extracted from embryonic membranes or tail tissue using the HotShot method for 15 minutes (Truett et al., 2000) and 2 μl DNA was used for PCR with Phusion polymerase (New England Biolabs). For Geminin and Cre PCRs, cycling conditions were: 98°C for 60 seconds followed by 33 cycles of (98°C 10 seconds, 65°C 10 seconds, 72°C 30 seconds). Primers for genotyping are in Supplementary Table S1.
Immunohistochemistry (IHC)
Embryos were fixed in 4% paraformaldehyde overnight and processed to generate 10 μm frozen or 5 μm paraffin sections. Antigen retrieval using citrate buffer (10 mM Sodium citrate pH 6.0) and a pressure cooker for 10 minutes was used to treat paraffin sections prior to immunostaining. Transverse sections were stained with 1% toluidine blue to determine anterior-posterior location using anatomical landmarks including limb buds, the heart, and the gut. Immunohistochemistry was performed on transverse sections caudal to the forelimb (E9.5) and between the forelimb and hindlimb of E10.5, E11.5, and E12.5 embryos (caudal to the heart and containing gut). Anterior-posterior matched sections were used for immunohistochemistry analysis of wild-type and mutant embryos. Antibodies are listed in Supplementary Table S2. After antibody staining, immunohistochemical sections were counterstained with DAPI (represented in blue). Whole mount immunohistochemistry using 2H3 neurofilament antibody was performed as previously described (Joyner and Wall, 2008).
In Situ Hybridization
Embryos were dissected free from extraembryonic membranes and fixed in 4% paraformaldehyde overnight at 4°C. In situ hybridization using a probe corresponding to the Geminin open reading frame was performed as previously described (Wilkinson and Nieto, 1993).
Image processing
Immunofluorescence imaging was performed on a Zeiss Axio Imager Z1 at 10X or 20X magnification. Whole mount images of embryos at E18.5 were taken using a Zeiss Stereo Discovery V12 and individual images were stitched together using the Fiji Stitching plugin software as needed.
qRT-PCR
Embryos were dissected free of membranes and visceral organs. Dorsal tissue from the first to the last full somite was collected and RNA was extracted (Nucleospin RNAII kit; Clontech). cDNA was synthesized using random primers and Superscript II (Invitrogen). Fast Sybr Green Mastermix (Applied Biosystems) was used for qPCR with gene specific primers that amplified across exon-exon junctions (Supplementary Table S3). qPCR was performed on an ABI Fast 7500 using the cycling conditions 95°C 2 minutes, 40x (95°C 10 seconds, 60°C 45 seconds). Each qPCR was performed in triplicate, with normalization to Rpl19 expression level.
Skeletal Preparations
Embryos were dissected free of membranes, de-skinned, and fixed for 24 hours in 95% ethanol, followed by 24 hours in acetone. Embryos were stained overnight in 70% ethanol/5% acetic acid/0.015% Alcian Blue/0.005% Alizarin Red. Maceration was done in 1% potassium hydroxide / 20% glycerol until embryos were clear. After further clearing in 50% and 80% glycerol, embryos were photographed on a Zeiss Stereo Discovery V12 microscope.
Folate/Inositol supplementation
Folate (Sigma) supplementation was provided via drinking water at 0.2 mg/L. Myo-inositol (Sigma) was given in drinking water at 10 mg/mL. Dams were provided folate or myo-inositol supplemented water ad libitum for at least 3 weeks prior to mating and supplementation was continued until embryos were harvested.
Laser Capture Microdissection
Embryos were dissected and frozen immediately in OCT (Tissue-Tek). 30μm frozen sections were generated from somite-matched mutant (Mox2Cre;Gmnnflox/flox) and wild-type (Mox2Cre;Gmnn+/flox) sibling embryos, by transverse sectioning through the spinal neural tube between the forelimb and hindlimb bud regions at E10.5. Neural tube and surrounding paraxial tissue were isolated separately by laser capture microdissection using a Leica LMD7000. Examples of the regions acquired are in Supplementary Figure S1. All sections between the levels of the forelimb and hindlimb were combined and used to prepare RNA from laser captured material (Arcturus PicoPure RNA Isolation kit; Life Technologies) and quality was assessed by Agilent 2100 bioanalyzer (Agilent). cDNA was generated and hybridized to Agilent Mouse 8x60K microarrays by the Genome Technology Access Center (WUSM). Agilent Feature Extraction software was used to obtain background subtracted and spatially detrended ProcessedSignal intensities and features flagged as non-uniform outliers were excluded.
To define genes with differential expression between wild-type and mutant tissue, we excluded all probes that were not significantly expressed above background in at least one sample for each experimental pair (wild-type or mutant). We generated fold change ratios from ProcessedSignal intensities between mutant and wild-type paired samples. We further considered probesets where both independent experiments showed a >2-fold change in the same direction in mutant/wild-type ratio comparisons and where all probesets corresponding to a single gene showed a consistent difference between mutant and wild-type samples. These data were deposited into the Gene Expression Omnibus (GSE42052).
Results
Geminin deficient embryos generated by Mox2-Cre mediated excision are present in Mendelian ratios throughout gestation
Geminin (Gmnn) is strongly expressed in the neural tube at embryonic day 10.5 (E10.5) and continues to be expressed through E14.5, as detected both by in situ hybridization and immunohistochemistry (Fig. 1A). In transverse sections of the spinal neural tube, expression is strongest in the proliferating cells of the central ventricular zone (Fig. 1A), while expression is not seen in the ventral horns, which contain differentiating neurons. Staining is also present in some dividing cells of the paraxial mesenchyme.
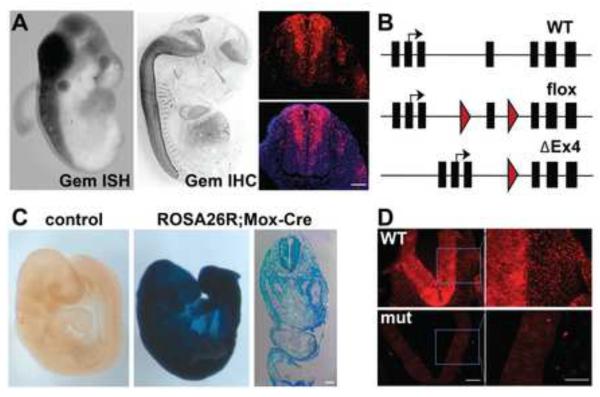
Figure 1. Geminin expression and excision by Mox2-Cre during embryogenesis.
(A) Geminin is predominantly expressed in the neural tube at E10.5 (in situ hybridization (ISH), left panel) and E14.5 (immunohistochemistry (IHC), center). IHC for Geminin on an E11.5 transverse section through the spinal neural tube demonstrates strong staining in the ventricular region of the neural tube (right). DAPI counterstain in blue. (B) Schematic of endogenous and conditional Geminin alleles. Exons are black boxes, introns are lines, red arrowhead indicates loxP sites, and black arrow indicates transcription start site. (C) Mox2-Cre is expressed throughout the E9.5 embryo. Transverse section shows broad β-galactosidase staining. (D) Geminin IHC shows efficient excision by Mox2-Cre in the spinal neural tube of mutant (Mox2Cre;Gmnnflox/flox) embryos by E10.5. Scale bars=100 μm.
To investigate the role of Geminin in neuroepithelium, we obtained Gmnn+/βgeo mice, a model in which exon 4 is flanked by loxP sites. We first excised the FRT-flanked βgeo cassette by breeding to constitutive FlpE-expressing mice to generate a floxed allele (Fig. 1B). Gmnnflox/flox mice are viable, fertile, and phenotypically indistinguishable from wild-type mice. We next bred Gmnnflox/flox female mice to Sox2Cre; Gmnn+/flox; male mice to generate a ΔEx4 allele (Fig. 1B). Heterozygous (Gmnn+/ΔEx4) mice were crossed and we obtained 13 Gmnn+/+ and 35 Gmnn+/ΔEx4 pups, while no GmnnΔEx4/ΔEx4 pups were found at postnatal day 1 (Fisher's Exact test p=0.001). This was consistent with homozygous deletion of Geminin causing lethality prior to birth, as expected based on preimplantation lethality of the Geminin null allele (Gonzalez et al., 2006). In addition, although we could detect a low level of ΔEx4 transcript in embryos, we could not detect the predicted carboxyl-terminally truncated protein by Western blot using an antibody recognizing the amino-terminal region of Geminin (Fig. S2A,B). This indicates that the ΔEx4 allele generated after Cre excision is a null or strong hypomorphic allele as predicted.
We used the Geminin floxed allele in combination with Mox2-Cre, which enables early excision (from ~E5.5) in the embryonic epiblast and its derivatives (Tallquist and Soriano, 2000). To lineage mark Mox2-Cre expressing cells, we generated Mox2Cre;ROSA26R mice. By day E9.5, most embryonic cells were β-galactosidase-positive, including cells throughout the neural tube and paraxial tissues (Fig. 1C). In Mox2Cre;Gmnnflox/flox embryos, Geminin is efficiently excised in the spinal neural tube, as indicated by loss of Geminin immunostaining (Fig. 1D). As Geminin null mice are preimplantation lethal at approximately E3.5 (Gonzalez et al., 2006), we initially performed timed matings to determine if mutant embryos (Mox2Cre;Gmnnflox/flox) were obtained at or after gastrulation stages (E7.5) and found the expected Mendelian ratios at this timepoint (Fig. S2C). Additionally, mutant embryos were morphologically indistinguishable from wild-type embryos. We subsequently analyzed timed matings from E7.5 to E18.5 and found that mutant embryos were obtained in expected numbers through E18.5 (Fig. S2C), but were never found alive after birth. We obtained 412 pups (P0) in matings of Gmnnflox/flox mice to Mox2Cre; Gmnn+/flox mice, but only found 2 stillborn Mox2Cre; Gmnnflox/flox pups compared to the expected 103 pups. This indicates that the Mox2Cre; Gmnnflox/flox genotype is lethal in the perinatal period.
Geminin deficient embryos have spinal neural tube defects
We assessed the phenotype of the Mox2Cre; Gmnnflox/flox (mutant) embryos. Mutant embryos were indistinguishable from wild-type littermates until E11.5 when they appeared slightly smaller. At E12.5, mutant embryos were present in Mendelian ratios, but were smaller and had complete penetrance of spinal neural tube defects (NTD), often exhibiting a kinked neural tube (Fig. 2A, arrowhead). While the neural tube is formed and the neural folds rise to contact each other in mutant embryos, the roof plate fails to remodel and is substantially thinner than that of wild-type embryos (Fig. 2B,C). At E14.5, mutant embryos continue to show kinked neural tubes (Fig. 2D,E, arrowhead). At E18.5, mutant embryos had complete penetrance of spinal NTDs (Fig. 2F–K), as well as incomplete penetrance of microphthalmia (~20%) (Fig. 2L,M) and mandible agenesis (<5%) (Fig. 2N,O). In addition, these embryos were alive, responded to touch, and had caudal NTDs combined with extensive edema and lack of muscular tone in the lower extremities. Mutant tails were often short and curled and were ~25% the length of wild-type tails, consistent with a failure of secondary neurulation (Fig. 2P–Q). Transverse sections through the affected region of the neural tube show a disrupted spinal neural tube with a juxtaposition of the dorsal neural tube and the skin (Fig S3). We further investigated the phenotype of the mutant embryos at E18.5 by performing bone and cartilage preparations. While we found the appendicular skeleton to be normal in mutant embryos, the axial skeleton was severely affected. Defects ranged from malformed vertebral bodies and neural arches to loss of ossified proximal ribs to total loss of all vertebrae and ribs (Fig. 2R–T). Overall, skeletal malformations were completely penetrant with only ~30% of mutant embryos having ribs (all ribs were malformed/residual). Vertebral body malformation was completely penetrant with all mutant embryos lacking a complete set of vertebral bodies and ~20% lacking any vertebral bodies. Most mutant embryos had some neural arches, but none had a complete set and the neural arches were often not symmetric.
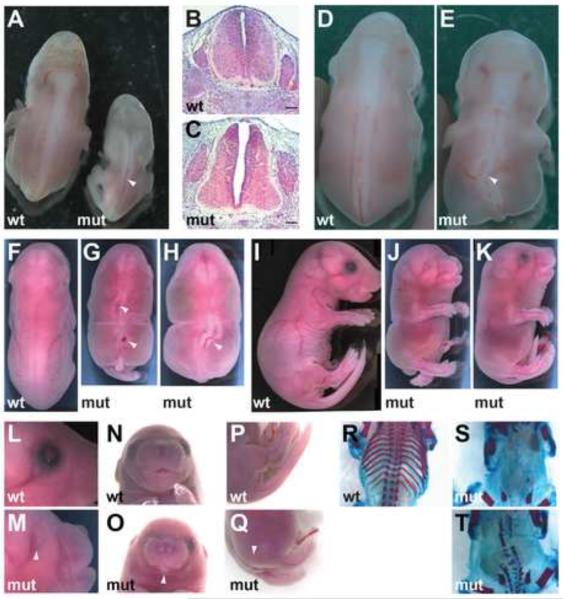
Figure 2. Geminin loss causes spinal neural tube defects and axial skeletal malformation.
(A) E12.5 mutant (Mox2Cre;Gmnnflox/flox) embryos have spinal NTDs. Arrowhead indicates spinal NTD. (B,C) Hematoxylin/eosin stained transverse section of embryos in (A) (scale bars=100 μm). (D,E) E14.5 embryos have a kinked spinal neural tube (arrowhead). (F–H) Dorsal view of E18.5 embryos demonstrating spinal neural tube defects (arrowheads). (I–K) Lateral view of E18.5 embryos. (L–Q) Arrowheads indicate microphthalmia (L,M), mandible agenesis (N,O), and malformed and curled tail (P,Q) in E18.5 mutant embryos. (R–T) E18.5 embryos stained for ossified bone (red) and cartilage (blue). Mutant embryos (S,T) have fused or absent neural arches and vertebral bodies as well as malformed or absent proximal and distal portions of the ribs.
Many neural tube defects can be prevented by folic acid or inositol supplementation prior to and during early pregnancy. We supplemented the drinking water of dams with folic acid or myo-inositol to determine if the spinal NTD resulting from Geminin deficiency could be prevented. However, neither folic acid nor myo-inositol supplementation prevented these NTDs, indicating either that defects caused by loss of Geminin are unrelated to pathways sensitive to these molecules or that Geminin acts downstream of processes requiring folate or myo-inositol (data not shown).
Alterations in neural tube patterning and differentiation in Geminin deficient embryos
In some contexts, Geminin contributes to cell cycle control, preventing re-licensing of DNA origins within S and G2 phase of the cell cycle by binding the pre-replication protein Cdt1. To determine if Geminin deficiency altered cell proliferation in this mouse model, we immunostained transverse sections of the E10.5 spinal neural tube with antibodies against Ki67, which marks all dividing cells, and phosphorylated-histone H3-Ser10 (pH3), which marks mitotic cells. Neural tubes from mutant embryos and their wild-type siblings had indistinguishable numbers of Ki67-positive and pH3-positive cells (Fig. 3A–C). We also assessed whether Geminin deficiency altered the number of cells undergoing apoptosis, by immunostaining spinal NT sections for activated Caspase3. There were very few activated Caspase3-positive cells outside of the dorsal root ganglia in both wild-type and mutant embryos and we did not detect a difference in apoptotic cell number between wild-type and mutant embryos (Fig. 3D). Together, these findings suggest that cell proliferation and apoptosis are not discernibly affected in these Geminin deficient embryos.
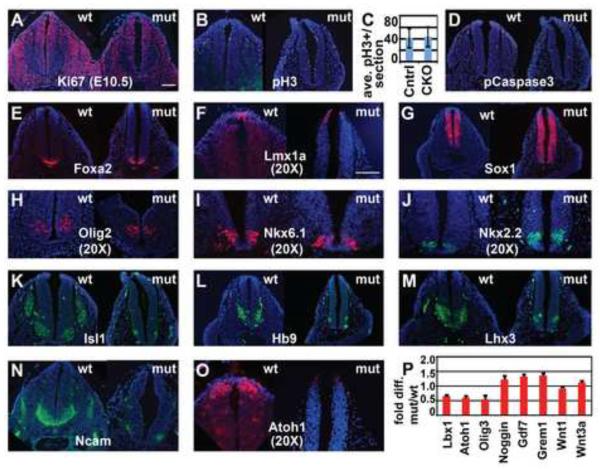
Figure 3. Geminin loss decreases expression of dorsal interneuron progenitor markers and ventral neuronal markers.
(A–C) Transverse sections of E10.5 spinal neural tube in wt and mutant (Mox2Cre;Gmnnflox/flox) embryos show no difference in proliferative markers. For (C), pH3+ cells/section were quantitated for 3 sections each from the caudal neural tubes of 6 mutant and 4 wild-type sibling control embryos (18 and 12 sections, respectively); data is expressed as pH3+ cells/section +/−std. dev. (D) Activated/phosphorylated Caspase3 (pCaspase3) shows very few apoptotic cells in wt and mutant. (E–O) Immunohistochemistry for regionally restricted markers of specific cell types in the neural tube. Embryos analyzed for immunohistochemistry (n=7). (P) qRT-PCR to detect altered expression of regionally restricted genes in spinal tissue (tissue dorsal of the dorsal aorta was dissected from the forelimb to the last full somite). Fold difference comparison between mutant and wild-type tissue shows decreased expression of dorsal interneuron progenitor genes (Lbx1, Atoh1, and Olig3), but not dorsal signaling molecules in the mutant; data is expressed as fold difference + std. dev. Scale bars=100 μm.
Dorsal-ventral patterning of the neural tube is determined by graded Shh, Wnt, and Bmp signaling, which establishes the ordered expression of distinct transcription factors (Jessell, 2000; Le Dréau and Martí, 2012; Liu and Niswander, 2005; Ribes and Briscoe, 2009; Wilson and Maden, 2005). Dorsal Bmp and Wnt signaling induces the roof plate and six dorsal interneuron progenitor cell populations (pd 1–6), while Shh produced by the notochord and floor plate is required for the formation of floor plate, motor neuron progenitors, and four other ventral neuron progenitor cell types (pv 0–3) (Alaynick et al., 2011; Briscoe et al., 2000; Ericson et al., 1997; Lee and Pfaff, 2001). To determine if Geminin loss affected dorsal-ventral patterning of the spinal neural tube at E9.5 and E10.5, we performed immunostaining for markers of dorsal and ventral progenitors and neuronal cell populations that differentiate from these progenitors. Expression of Nkx6.1 (which marks floor plate, v2–3, and motor neuron progenitors), Olig2 (which marks motor neuron progenitors), Nkx2.2 (which marks v3 progenitors), and the floor plate marker Foxa2 was unchanged in mutant embryos at E9.5, indicating that initial specification of ventral progenitors is not affected in mutant embryos (Fig. S4). At E10.5, both mutant and wild-type embryos strongly express Foxa2 (Fig. 3E), suggesting that short-range responses to Shh to generate the floor plate are unaffected in the mutant. Similarly, the roof plate in mutants still expresses Lmx1a, despite having altered morphology (Fig. 3F). Expression of the pan-neural progenitor marker Sox1 is unaffected in the mutant (Fig. 3G), as are markers of ventral progenitor identity: neither Olig2 expression nor Nkx6.1 is altered in the mutant (Fig. 3H–I), while Nkx2.2 has a slightly expanded domain (Fig. 3J). In contrast, markers of differentiated motor neurons, Isl1 and Hb9, are strongly reduced in mutant embryos, as is expression of Lhx3, which marks v2 progenitors and motor neuron progenitors (Fig. 3K–M). The neuronal marker Ncam is also strongly reduced in the ventral neural tube of mutants (Fig. 3N). These data suggest that ventral progenitor cells are specified correctly, but that their differentiation into neurons is severely affected in the mutant. This includes deficiencies in motor neuron differentiation, consistent with the lack of muscle flexion in the hindlimbs at later stages.
We also assessed effects on the dorsal neural tube. While roof plate expression of Lmx1a was maintained (Fig. 3F), as described above, expression of the dorsal interneuron progenitor 1 marker Atoh1 was strongly reduced or absent in mutant embryos at E10.5 (Fig. 3O). Consistent with this result, Atoh1 mRNA levels are also reduced, as are mRNA levels of Olig3 (pd 1–3) and Lbx1 (pd 4–6) (Fig. 3P). These results suggest that dorsal neural progenitor cells that require BMP signaling for their induction are sensitive to Geminin loss in mutants. However, critical dorsal signaling ligands including Gdf7, Wnt1, and Wnt3a are expressed at normal levels, indicating that the defects may reflect neural cell intrinsic responses rather than loss of key dorsal signaling cues (Fig. 3P). Together, these results suggest that specification of some dorsal progenitor identities and differentiation of neuronal populations, particularly ventral motor neurons, is delayed or reduced in the Geminin deficient embryos.
To determine if these progenitors were delayed in their differentiation timing, we also stained E11.5 and E12.5 embryos with markers of progenitors and differentiated neurons. At E12.5, mutant embryos had an expanded Ki67-positive ventricular zone (Fig. S5B). Consistent with the perdurance of progenitors in mutant embryos, expression of dorsal and ventral progenitor markers Atoh1 and Nkx6.1 persisted in mutant embryos at E12.5, while these markers were absent or weakly expressed in wild-type embryos (Fig. S5D,F,H). Ncam and Isl1/2 are markers of differentiation and instead showed reduced expression in mutants at E12.5 (Fig. S5J,K). Together, these results suggest that Geminin is required for appropriate differentiation of neural progenitors, as these progenitors persist and show reduced and delayed differentiation in mutant embryos.
Loss of Geminin in the spinal neural tube results in defective progenitor specification and neurogenesis
To obtain a more comprehensive view of significant transcriptional differences between wild-type and mutant (Mox2Cre; Gmnnflox/flox)embryos, we performed microarray analysis on RNA captured from spinal neural tubes of wild-type and mutant embryos. Laser capture microdissection was used to collect neural tube tissue from sections of the trunk between the forelimb and hindlimb buds at E10.5 using somite-matched mutant and wild-type littermates. Genes showing significantly different expression between wild-type and mutant neural tube tissue in each of two independent experiments were defined as described in the Materials and Methods (Table S4).
Consistent with the immunohistochemistry data, many transcription factors that regulate neural plate patterning and neuronal differentiation showed strongly diminished expression in mutant neural tube tissue. These included genes whose expression marks differentiated ventral interneurons and/or motor neurons, including Hb9, Evx1, Isl1, Isl2, and Lhx5 (Fig. 4A). Expression of genes that mark dorsal interneuron progenitors including Atoh1 and transcription factors that promote neurogenesis and neural plate patterning, including Neurog3, Neurod1, Ebf1, Nhlh2, Heyl and Irx6, was also reduced. We also saw diminished expression of genes with roles in later neuronal function (e.g. axonal pathfinding proteins, including Robo1/3 and Semaphorins). Consistent with our prior work, there was not a strong presence of genes involved in cell proliferation or apoptosis in the list of differentially expressed genes, supporting our finding that the rate of cell death is not different between wild-type and mutant embryos (Table S4). Analysis of enriched gene ontology (GO) terms using the Metacore Suite indicated that top networks were involved in neurogenesis, with other top terms relevant to development/neural development (e.g. synaptic transmission) (Fig. 4B). While neither ventral Shh signaling nor dorsal Wnt signaling components appeared to be altered by Geminin deficiency, several components involved in mediating dorsal BMP signaling responses were diminished, including Smad1, Smad7, Rgmb, and Bmpr1b. Mutant neural tube tissue also had diminished expression of some genes whose loss results in NTDs in mouse models, including transcription factors and chromatin regulatory activities (e.g. Nap1l2, Zic5, Med12), suggesting that loss of these regulators might contribute to the failure to close the neural tube here.
Figure 4. Geminin loss causes diminished expression of transcription factors that control neurogenesis and neural tube patterning and of NTD-related genes.
Genes that showed significantly decreased (A) or increased (C) expression levels in mutant (Mox2Cre;Gmnnflox/flox) versus wt embryos were defined by microarray analysis of laser capture microdissected trunk neural tube. (B,D) Enriched gene ontology (GO) terms for these data sets were defined using MetaCore Suite/GeneGO software.
There were substantially fewer genes with increased expression in the mutant neural tube (Table S4). This gene set was enriched for genes involved in nucleosome and chromatin assembly, including histone genes, and genes that are expressed and function in neural crest (Cyp26b1, Phox2b, Dlx6) and epithelia (Dsp, Npnt) (Fig. 4C–D). These may also reflect a delay in other events that occur in the neural tube, such as delamination and migration of specific neural crest populations. Together, these findings support a requirement for Geminin in promoting the expression of other transcriptional and chromatin regulatory activities required for neurogenesis and neural plate patterning.
Mutant embryos have neural crest and axial skeleton defects
Since Mox2-Cre depletes Geminin function in paraxial tissues as well as in the neural tube, and later embryos showed deficiencies in the axial skeleton, we also assessed gene expression changes resulting from Geminin loss by E10.5 in paraxial tissues. We performed laser capture microdissection of paraxial tissues (Fig. S1) through the same trunk region from which neural tissue was captured and compared RNA from somite-matched wild-type and mutant siblings by microarray analysis in two independent experiments, as described in the Materials and Methods.
Genes with decreased expression in mutant paraxial tissue included many markers of neural crest (Foxd3, Sox9) and of mesenchymal tissue that contributes to paraxial mesoderm derivatives including dermomyotome (Pax7), myotome (Myog, Mylpf), and sclerotome (Pax1/Pax9) (Fig. 5A; Table S5). Consistent with this, the gene set with decreased expression in mutant paraxial tissues was strongly associated with GO terms related to muscle development (Fig. 5C).
Figure 5. Geminin loss in paraxial tissue results in decreased expression of muscle/mesenchymal genes and increased expression of cell adhesion related and intermediate filament genes.
Genes with significantly decreased (A) or increased (B) expression in mutant (Mox2Cre;Gmnnflox/flox) versus wt embryos were defined by microarray analysis of trunk paraxial tissue. (C,D) Top GO terms were defined using Metacore Suite/GeneGo for these data sets. (E,F) Neurofilament immunostaining of wt (E) or mutant (F) embryos. (E′,F′) boxed regions from (E,F). For several somites, white arrows highlight neurofilament staining and black arrowheads denote somite boundaries. In the mutant, staining for neural crest is normal in the rostral trunk, but differentiated neural crest is absent in the caudal region of the trunk.
Both neural crest migration and somite segmentation into dermomyotome, myotome and sclerotome to form the musculature and axial skeleton depend upon de-epithelialization. By contrast with decreased expression of genes associated with neural crest cells (NCC) and mesodermal fate acquisition, genes with increased expression in mutant trunk paraxial tissue included many genes with roles in epithelial adhesion (Fig. 5B; Table S5). Most notably there was increased expression of genes involved in cell adhesion, including tight junctions (Cldn4 and 27) and desmosomes (Dsg2 and Dsp). Multiple keratins (Krt8/18/19) were also overexpressed. In addition to genes that function directly in cell adhesion, the transcription factor Grhl2 is overexpressed in mutant paraxial tissue. Grhl2 inhibits epithelial to mesenchymal transitions and its mutation has previously been shown to cause NTDs. Epcam and Dsp expression decreases in Grhl2 mutant mice, while increasing in our model, potentially through increased Grhl2 (Pyrgaki et al., 2011). Consistent with these examples, top GO terms were associated with cell adhesion and cell-cell junctions (Fig. 5D).
Given that neural crest-associated gene expression decreased in mutant paraxial tissue, we also evaluated the presence of differentiated NCCs in day E10.5 embryos by using neurofilament staining. In the rostral region of the spinal neural tube, NCCs appear to migrate and differentiate appropriately. However, caudally we observed a lack of differentiated NCCs as visualized by neurofilament staining, indicating either a defect in migration or differentiation (Fig. 5E,F). Together, these findings suggest that loss of Geminin in the Mox2-Cre model also delays or blocks differentiation of paraxial structures, consistent with the later findings of perturbed axial skeletal development in mutant embryos (Fig. 2S,T).
Geminin is required in neuroepithelium from embryonic day 8.5 through 10.5
Since Mox2-Cre is broadly expressed in cells that give rise to the neural tube and axial skeleton, this model did not enable us to define temporal or spatial requirements for Geminin activity for neural tube closure, patterning and differentiation, or for axial skeletal development. To address this question, we next crossed the Geminin floxed allele to several temporally- and tissue-restricted drivers. We began by using the Dermo1-Cre line where Cre is expressed in mesenchymal cells but not in neural tissues from approximately E10 (Fig. S6A,A′) (Yokoyama et al., 2009). Dermo1Cre;Gmnnflox/flox animals were viable and fertile and embryos with this genotype had neither spinal neural tube defects nor axial skeleton defects (data not shown). These data indicate that Geminin is not required after E10.5 in paraxial mesenchyme for later aspects of formation of the axial skeleton, but do not preclude roles for Geminin in mesodermal derivatives prior to E9.5–10.5, when expression of Dermo1-Cre begins. We also found that NestinCre;Gmnnflox/flox mice, where Geminin was excised predominantly in the ventral spinal neural tube from E9.5–E10.5 (Fig. S6 B,B′–E,E′), were viable and fertile with no apparent alterations in nervous system development, consistent with a prior study (Schultz et al., 2011). These data suggest that Geminin activity is likely to be dispensable for embryonic patterning in both neural and axial mesenchymal tissues after ~E10–E10.5.
To address whether neural deficiencies observed in our Mox2-Cre model reflected a neural tissue autonomous defect, we next asked whether conditional deletion of Geminin by two Cre lines that drive spatially restricted expression in the nervous system at earlier developmental time points than Nestin-Cre would result in NTDs. To determine if Geminin was required specifically in the spinal neural tube or if Geminin was also required in the cranial neural tube, we used Wnt1-Cre to excise Geminin in the dorsal midbrain beginning at E8.5. Expression of Wnt1-Cre is restricted to the brain prior to E9.5 and progresses caudally such that expression also encompasses the dorsal spinal neural tube by ~E10.5 (Stottmann and Klingensmith, 2011). Wnt1-Cre mutant embryos (Wnt1Cre;Gmnnflox/flox) have small midbrains at E10.5, which results in complete penetrance of exencephaly in later stage embryos (Fig. 6A–D). However, the later loss of Geminin activity in the dorsal spinal neural tube (compared to the Mox2-Cre driver) resulted in embryos with normal caudal development with neither spinal NTDs nor axial skeleton defects (Fig. 6A–D).
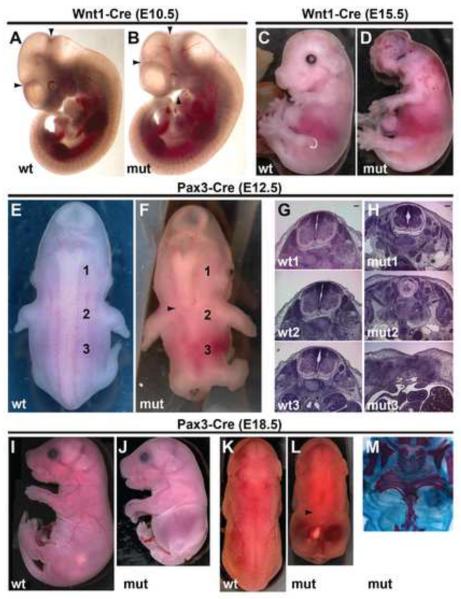
Figure 6. Wnt1-Cre and Pax3-Cre mediated Geminin deletion cause distinct neural tube defects.
(A,B) Lateral view of E10.5 wt and mutant (Wnt1Cre;Gmnnflox/flox) embryos. Mutant embryo (B) has a reduced midbrain (region between arrowheads) and diminished pharyngeal arches (arrowhead). (C,D) At E15.5, Wnt1-Cre mutant embryos exhibit exencephaly and lack mandibles. (E,F) Dorsal view of wt and mutant (Pax3Cre;Gmnnflox/flox) embryos at E12.5 shows degeneration of the spinal neural tube in mutant embryos. Arrowhead in F indicates the caudal extent of the neural tube. (G,H) Hematoxylin/eosin stained sections taken at the rostral-caudal levels indicated in (E,F). Lateral (I,J) and dorsal (K,L) views of E18.5 mutant (Pax3Cre;Gmnnflox/flox) embryos show a loss of the spinal neural tube. Arrowhead in L indicates the caudal extent of the neural tube. (M) Skeletal and cartilage preparations of E18.5 mutant (Pax3Cre;Gmnnflox/flox) embryos. Embryo has a compressed torso with malformed ribs and fused or absent vertebrae. Scale bars=100 μm.
We also used a line carrying a Pax3-Cre transgene, which is expressed in the dorsal half of the neural tube and neural crest, but not in the somites, beginning as early as E8.5 (Stottmann and Klingensmith, 2011). Pax3-Cre is expressed only in the caudal two-thirds of the embryo, with expression beginning near the level of the otic vesicle (Jarad and Miner, 2009; Stottmann and Klingensmith, 2011). Consistent with the expression profile of the Pax3-Cre transgene, by E9.5 Geminin protein is not detectable in the spinal neural tube of mutant embryos (Pax3Cre;Gmnnflox/flox), but remains in non-neural paraxial cells (Fig. S6F,G). At E10.5, mutant embryos showed normal rostral spinal neural tube and somites, but the dorsal neural tube appeared to be sunken in caudal regions of the embryo. By E12.5, mutants exhibited severe spinal neural tube defects (Fig. 6E,F). We dissected and sectioned E12.5 embryos and upon gross examination and hematoxylin/eosin staining, it was evident that they lacked a caudal neural tube from the same rostral-caudal levels where the Pax3-Cre transgene is expressed (Fig. 6G,H). This resulted in E18.5 mutant embryos that lacked a neural tube and also had axial skeleton defects, specifically abnormal or missing ribs and fused or absent vertebrae (Fig. 6I–M). However, we never saw microphthalmia or mandible loss in Pax3-Cre mutant embryos, consistent with lack of rostral Cre expression from the Pax3-Cre transgene.
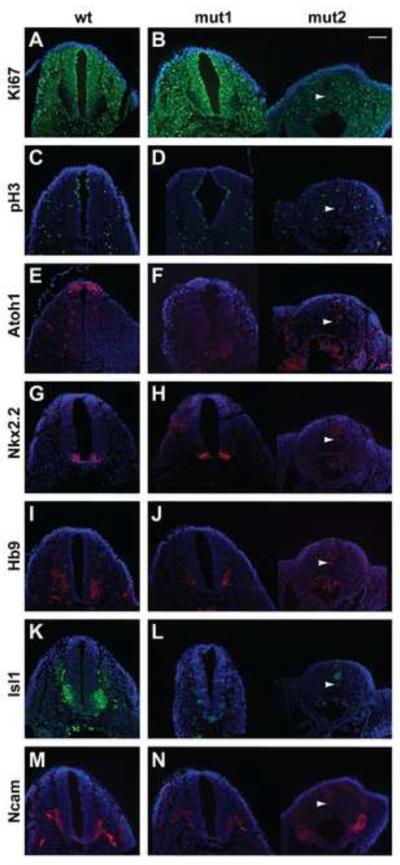
We examined the neural tubes of Pax3-Cre mutant embryos (Pax3Cre;Gmnnflox/flox) at E10.5 and found that, even within litters, there was variable morphology. Within a litter, we observed mutant embryos with grossly normal neural tubes (Fig. 7, mut1) to mutants that lacked caudal neural tubes (Fig. 7, mut2). The most severely affected mutant embryos (mut2) had strongly reduced Ki67 and lacked expression of all progenitor and differentiation markers, consistent with degeneration of the neural tube at this time (Fig. 7). The proliferative markers Ki67 and pH3 showed no differences between wt and mutant embryos with grossly normal neural tube structures (mut1) at E10.5 (Fig. 7A–D). However, when we examined transcription factor expression in the spinal neural tube, expression of the dorsal neural tube marker Atoh1 was lost in mutants (Fig. 7E,F), similar to what we found in Mox2-Cre mutant embryos. Also, similar to Mox2-Cre mutant embryos, Nkx2.2 expression was not affected in mut1 (Fig 7G,H), while Hb9, Isl1, and Ncam expression were greatly reduced in all Pax3-Cre mutant embryos, indicating a defect in motor neuron differentiation (Fig. 7I–N). Therefore, these data suggest a neural tissue autonomous requirement for Geminin in the neural plate during the time window when neural plate patterning and neural tube closure occur (E8.5–E10.5).
Figure 7. Pax3-Cre mediated Geminin deletion decreases expression of dorsal interneuron progenitor markers and ventral neuronal markers.
A wt and two mutant (Pax3Cre;Gmnnflox/flox) E10.5 littermate embryos (mut1,2) are shown to present the range of phenotypes observed at this time. mut2 lacks dorsal and ventral progenitor marker expression, consistent with the degenerated neural tube structure. In comparisons of wt and mut1, (A–D) transverse sections of E10.5 spinal neural tube show no difference in proliferative markers Ki67 and pH3. (E–H) Mutant embryos have diminished expression of the dorsal progenitor marker Atoh1, but not the ventral progenitor marker Nkx2.2. (I–N) Differentiation markers Hb9, Isl1, and, Ncam are decreased in mutant embryos. Arrowheads indicate the notochord for mut2 images. Embryos analyzed for immunohistochemistry (n=5). Scale bar=100 μm.
Together, these models demonstrate a strict requirement for Geminin in differentiating neuroepithelium. Lack of Geminin in the neural tube results in either exencephaly or caudal NTDs depending on the rostral-caudal region where Geminin is deleted. In addition, these data demonstrate a critical time window when Geminin is required tissue autonomously in the neural tube between E8.5–E10.5. Marker analysis and comprehensive comparisons of gene expression changes resulting from Geminin deficiency suggest that failure to activate programs of gene expression that drive dorsal neural specification and neuronal differentiation may contribute to the neural tube defects that occur in these embryos.
Discussion
Here we have identified a critical role for Geminin during neural tube formation. We used a floxed Geminin allele combined with various Cre drivers to define the temporal and spatial requirements for Geminin during neurulation. Mox2-Cre mutant embryos have completely penetrant caudal NTDs. In these embryos, ventral interneuron progenitors are specified, but differentiation is impaired, while dorsal interneuron progenitors are not specified or are drastically reduced in number. Similar results were obtained upon using a Pax3-Cre driver, which excised Geminin specifically in caudal neural tissues from ~E8.5, while deletion of Geminin from E8.5 in the midbrain using Wnt1-Cre instead caused exencephaly. Wnt1-Cre mediated deletion of Geminin in the spinal neural tube (from E10.5) did not result in caudal NTDs. Excision of Geminin in neural tissues from E10–10.5 with Nestin-Cre or in paraxial mesenchyme using Dermo1-Cre resulted in normal nervous system development and embryos developed into viable, fertile adults. Together, these data suggest that Geminin activity is required in neural plate tissue during the time window when specification of interneuron progenitors and other aspects of neural plate patterning, initiation of neuronal differentiation, and neural tube closure occur.
Many genes, when mutated in the mouse, can result in NTDs (Harris and Juriloff, 2010, 2007). These genes can be grouped into several categories according to the cellular processes affected. These include defects in anterior-posterior patterning of the embryo, in the signaling systems that drive dorsal-ventral patterning of the neural tube, and in multiple other processes required for proper neural development, including planar cell polarity/cell orientation, formation of neural hinge points, cell proliferation, apoptosis, and differentiation (Copp and Greene, 2010; Pyrgaki and Niswander, 2013). NTDs caused by defects in anterior-posterior patterning are typically secondary sequelae of mispatterning the anterior-posterior axis, resulting in defects that present by E8.5–E9.5 and often result in lethality by E9.5 (Harris and Juriloff, 2007). Mox2-Cre and Pax3-Cre mutant embryos do not show overt defects until E10.5, survive until birth, and anterior-posterior patterning of the body axis is intact, suggesting a different etiology for the NTDs in these models.
NTDs can also be caused by mutation of genes in the planar cell polarity (PCP) pathway (Juriloff and Harris, 2012; Wallingford et al., 2013). These genes affect cell shape/polarity and movements that drive convergent extension during gastrulation to control lengthening of the A-P body axis. PCP pathway genes are also required for apical constriction during hinge point formation in the neural tube, a necessary step of neural tube closure, and mutation of PCP pathway genes causes the most severe type of NTD, craniorachichisis, where both the rostral and caudal aspects of the NT fail to close (Copp et al., 2013). Hinge point formation appears to be normal in Mox2-Cre mutant embryos and we never observed craniorachichisis in our model, nor did we see PCP pathway genes differentially expressed in mutant neural tubes in our array analysis, suggesting this is also not the basis of the NTD seen upon Geminin deficiency.
Changes in either cell proliferation or apoptosis can also result in neural tube defects (Copp and Greene, 2010; Pyrgaki and Niswander, 2013). Geminin is one of several activities that regulate re-licensing of DNA origins of replication through inhibitory binding to Cdt1, to prevent re-replication of the genome within a cell cycle. Therefore, we assayed for changes in proliferation and apoptosis in Geminin deficient embryos. We do not see changes in the mitotic marker pH3, nor do we see changes in activated Caspase3, an apoptotic marker. We cannot rule out the possibility that loss of Geminin caused proliferative or apoptotic changes that we were unable to detect. However, we did not detect changes in these pathways in our microarrays and could not visually distinguish wild-type from mutant embryos prior to the time of neural tube closure, suggesting that these effects, if present, are not a major contributor to the phenotype. Other mechanisms for inhibiting Cdt1 activity by proteolysis may compensate for the loss of Geminin here, as is seen in other normal cell contexts where Geminin loss does not result in altered cell proliferation (Nishitani et al., 2006; Zhu and Depamphilis, 2009).
NTDs can also be caused through perturbation of differentiation in the neural tube (Copp and Greene, 2010). Investigation of neural tube differentiation in mutant (Mox2Cre; Gmnnflox/flox) embryos identified reduced expression of neurogenic transcription factors that function in discrete dorsal-ventral cell domains. Dorsal-ventral patterning of the neural tube results from integration of growth factor signals, particularly ventral Shh and dorsal Bmp and Wnt signals. Disruption of these signaling pathways can dorsalize (for Shh) or ventralize (for Bmp/Wnt) the neural tube, leading to NTDs (Alvarez-Medina et al., 2008; Chiang et al., 1996; Le Dréau and Martí, 2013; Lee and Jessell, 1999). We did not observe dorsalization or ventralization of the neural tube and our microarrays did not indicate changes in Wnt or Shh signaling pathway components or BMP growth factors. However, we observed decreased expression of several components that mediate the dorsal BMP growth factor response (decreased expression of Smad1, Smad7, and Bmpr1b). Furthermore, genes whose expression was strongly affected in the mutant neural tube are neurogenic transcription factors required for specification, patterning and differentiation of the neural tube, including Neurod1, Neurog3, Nhlh2, Atoh1, Hb9, and Isl1. This is in agreement with our immunohistochemistry results, demonstrating decreased expression of Atoh1, Hb9, and Isl1. Atoh1 controls specification of dorsal interneuron progenitors (pd1) (Helms and Johnson, 1998) and is among the most strongly downregulated genes in Geminin mutant neural tubes. During neural cell specification from embryonic stem cells in vitro, we previously observed that Geminin could directly bind and/or promote increased histone acetylation at the promoters of several of these genes (Atoh1, Neurod1, Zic5), promoting their expression (Yellajoshyula et al., 2011). These findings suggest that some effects of Geminin in promoting expression of regulators of neural specification and neurogenesis may be direct. Geminin loss in the mutant also resulted in decreased expression of several transcription factors or chromatin regulators whose loss in other mouse models results in NTDs, including Nap1l2, Med12, and Zic5 (Attia et al., 2007; Inoue et al., 2004; Rocha et al., 2010; Rogner et al., 2000).
Geminin has essential functions in cell fate specification mediated through its interactions with the Polycomb repressor and SWI/SNF chromatin remodeling complexes (PRC and SWI/SNF) and with Hox and Six family homeodomain transcription factors (Del Bene et al., 2004; Luo et al., 2004; Seo et al., 2005). Previously, we demonstrated that Geminin has a role in regulating the epigenetic status, expression, or activity of critical transcription factors during primary neurogenesis in Xenopus and also during in vitro mouse ES cell differentiation towards neuroectoderm (Lim et al., 2011; Seo et al., 2005; Yellajoshyula et al., 2012, 2011). However, analyzing Geminin's in vivo function in mammals was hampered due to the preimplantation lethality of Geminin null embryos (Gonzalez et al., 2006).
Here, we have demonstrated a functional requirement for Geminin during neuroepithelium formation and neural tube patterning in vivo between E8.5–E10.5. Loss of Geminin altered expression of transcriptional and epigenetic regulators that are important for neural tube closure. Mutation of chromatin regulators or changes in chromatin modifications due to environmental factors (low dietary folic acid intake or gestational diabetes) can also cause NTDs (Copp and Greene, 2010; Pinney and Simmons, 2012; Salbaum and Kappen, 2010). Together, our findings indicate that Geminin is essential during neural tube formation for specification and differentiation of neural tube progenitors, and suggest that this may occur through regulation of transcription or functional interactions with chromatin regulators to control the epigenetic status and expression of genes that drive neurogenesis and neural plate patterning.
Supplementary Material
(A) Hematoxylin stained section of trunk neural tube prior to laser capture microdissection (LCM). (B)Same section after LCM. 1 marks the area taken for the neural tube RNA samples and 2 marks the areas taken for the paraxial tissue RNA samples that were further analyzed by microarray.Scale bar=100μm.
(A)RT-PCR with primers spanning Exon 4 shows low expression of the Δexon4 allele at the mRNA level in E10.5 trunk (tissue dorsal of the dorsal aorta was dissected) in Mox2Cre;Gmnn+/floxembryos. (B) No protein of the predicted size is detected on a Western blot. The low level of full length Geminin protein is from cells that have not excised Geminin. (C)Enumeration of embryos found upon dissection at various embryonic days showing survival of mutant embryos until E18.5.
(A) Wild-type E18.5 embryo. (B) Mutant Mox2-Cre embryos at E18.5 have completely penetrant caudal neural tube defects. (C,D) Hematoxylin/eosin stained transverse sections at the levels indicated by dashed lines in A,B. Scale bar=500μm.
Immunohistochemistry on transverse sections of E9.5 spinal neural tube in wt and mutant (Mox2Cre;Gmnnflox/flox) embryos shows no difference in expression of the ventral progenitor markers (A) Nkx6.1, (B) Olig2, (C) Nkx2.2, and (D) Foxa2. Embryos analyzed for immunohistochemistry (n=3). Scale bar=100μm.
Transverse sections of E11.5 and E12.5 wt and mutant (Mox2Cre;Gmnnflox/flox) embryos. Mutant embryos show an expanded Ki67 positive proliferative progenitor zone (A,B) and persistentexpression ofthe progenitor markers Atoh1 (C–F) and Nkx6.1 (G,H) at E12.5. The differentiation marker Ncam (I,J) is decreased in E11.5 and E12.5 mutant embryos and Isl1/2 (K) is decreased in E12.5 mutant embryos.Embryos analyzed for immunohistochemistry (E11.5; n=4) and (E12.5; n=6). Scale bars=100μm.
(A,A′) Transverse section of a Dermo1-Cre; R26R E10.5 embryo at the forelimb level demonstrates Cre activity (blue) in paraxial mesenchyme, but not in the neural tube (Nuclear Fast Red counterstain). Scale bar forA=250μm; A′=100μm. (B–E)Whole mount images of Nestin-Cre; R26R embryos at E9.5 (B) or E10.5 (C–E). (B′–E′) Transverse sections of embryos in B–E demonstrate that Nestin-Cre is only active in the ventral neural tube in the rostral portion of the spinal neural tube (blue). In panels B′–E′, spinal neural tube showing ventral expression of Nestin-Cre is oriented to the left and the tail neural tube lacking Nestin-Cre expression is orientedto the right in the images. Dashed lines in B–E indicate the plane of the section in B′–E′. Scale bar forB′=100μm; C′=250μm.(F–G) Transverse sections of E9.5 Pax3-Cre (F) wt and (G) mutant (Pax3Cre;Gmnnflox/flox) embryos. Geminin and Sox1 immunohistochemistry and DAPI staining of nuclei are shown. Nuclear staining for Geminin is visible in the wild-type neural tube, but is not detected in the neural tube of the mutant embryo. Nuclear Geminin staining is visible in non-neural tissue in the mutant (G, arrowhead), consistent with neural tube-specific excision by the Pax3-Cre driver. Embryos analyzed for immunohistochemistry (n=2). Scale bar for G=100 μm.
Table S1. Primers used for genotyping
Table S2. Antibody clone/catalog numbers and dilutions used.
Table S3. Primers used forqRT-PCR.
Table S4. Genes with more than 2-fold difference in expression in mutant versus wild-type neural tube tissue, as defined by microarray of LCM captured material (Materials and Methods). Negative values indicate decreased expression (fold change) in mutant embryos, relative to wild-type siblings, while positive values indicate increased expression in mutant embryos relative to wild-type.
Table S5. Genes with >2-fold difference in expression in mutant paraxial tissue relative to wild-type (see Materials and Methods).
Highlights.
Geminin is required in the mammalian neural tube between E8.5 and E10.5
Loss of Geminin in neuroectoderm causes completely penetrant neural tube defects
Geminin deletion causes neural progenitor specification and differentiation defects
Neurogenic transcription factor expression is reduced in the neural tube of mutants
Loss of Geminin in the neural tube also results in axial skeleton malformations
Acknowledgements
We thank Dr. Lilianna Solnica-Krezel for use of her microscope, Dr. Jane Johnson for the Atoh1 antibody, and Dr. Russell Addis and Dr. Stacey Rentschler for critical reading of the manuscript. Drs. Jeffrey Miner, Naren Ramanan, and David Ornitz provided the Pax3Pro-Cre, Nestin-Cre, and Dermo1-Cre mice, respectively. This work was supported by grants from the March of Dimes (FY13-413) and NIH (GM66815) to K.L.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions E.S.P.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; L.E.W.: collection and assembly of data, and data analysis and interpretation; K.L.K.: conception and design, data analysis and interpretation, manuscript writing, and financial support.
References
- Alaynick W. a, Jessell TM, Pfaff SL. SnapShot: spinal cord development. Cell. 2011;146:178–178.e1. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Martí E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–47. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Attia M, Rachez C, De Pauw A, Avner P, Rogner UC. Nap1l2 promotes histone acetylation activity during neuronal differentiation. Mol. Cell. Biol. 2007;27:6093–102. doi: 10.1128/MCB.00789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Bragança J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 2001;29:469–74. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- Banting GS, Barak O, Ames TM, Burnham AC, Kardel MD, Cooch NS, Davidson CE, Godbout R, McDermid HE, Shiekhattar R. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum. Mol. Genet. 2005;14:513–24. doi: 10.1093/hmg/ddi048. [DOI] [PubMed] [Google Scholar]
- Barbera JPM, Rodriguez T. a, Greene NDE, Weninger WJ, Simeone A, Copp AJ, Beddington RSP, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum. Mol. Genet. 2002;11:283–93. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, Meyer R, Canfield M. a, Mulinare J. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res. A. Clin. Mol. Teratol. 2008;82:527–32. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani a, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Bu P, Evrard Y. a, Lozano G, Dent SYR. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol. Cell. Biol. 2007;27:3405–16. doi: 10.1128/MCB.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam a, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell. 2000;6:1287–95. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Copp A, Cogram P, Fleming A, Gerrelli D, Henderson D, Hynes A, Kolatsi-Joannou M, Murdoch J, Ybot-Gonzalez P. Neurulation and neural tube closure defects. Methods Mol. Biol. 2000;136:135–60. doi: 10.1385/1-59259-065-9:135. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene NDE. Genetics and development of neural tube defects. J. Pathol. 2010;220:217–30. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Stanier P, Greene NDE. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. doi: 10.1016/S1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CE, Li Q, Churchill G. a, Osborne LR, McDermid HE. Modifier locus for exencephaly in Cecr2 mutant mice is syntenic to the 10q25.3 region associated with neural tube defects in humans. Physiol. Genomics. 2007;31:244–51. doi: 10.1152/physiolgenomics.00062.2007. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Tessmar-Raible K, Wittbrodt J. Direct interaction of geminin and Six3 in eye development. Nature. 2004;427:745–9. doi: 10.1038/nature02292. [DOI] [PubMed] [Google Scholar]
- Emmett LSD, O'Shea KS. Geminin is required for epithelial to mesenchymal transition at gastrulation. Stem Cells Dev. 2012;21:2395–409. doi: 10.1089/scd.2011.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb. Symp. Quant. Biol. 1997;62:451–66. [PubMed] [Google Scholar]
- Gonzalez MA, Tachibana KK, Adams DJ, van der Weyden L, Hemberger M, Coleman N, Bradley A, Laskey RA. Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev. 2006;20:1880–4. doi: 10.1101/gad.379706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene ND, Copp AJ. Inositol prevents folate-resistant neural tube defects in the mouse. Nat. Med. 1997;3:60–6. doi: 10.1038/nm0197-60. [DOI] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ. Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn. 2009;29:303–11. doi: 10.1002/pd.2206. [DOI] [PubMed] [Google Scholar]
- Harmacek L, Watkins-Chow DE, Chen J, Jones KL, Pavan WJ, Salbaum JM, Niswander L. A unique missense allele of BAF155, a core BAF chromatin remodeling complex protein, causes neural tube closure defects in mice. Dev. Neurobiol. 2014;74:483–97. doi: 10.1002/dneu.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. A. Clin. Mol. Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res. A. Clin. Mol. Teratol. 2010;88:653–69. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- Helms a W., Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–28. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Ichi S, Costa FF, Bischof JM, Nakazaki H, Shen Y-W, Boshnjaku V, Sharma S, Mania-Farnell B, McLone DG, Tomita T, Soares MB, Mayanil CSK. Folic acid remodels chromatin on Hes1 and Neurog2 promoters during caudal neural tube development. J. Biol. Chem. 2010;285:36922–32. doi: 10.1074/jbc.M110.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hatayama M, Tohmonda T, Itohara S, Aruga J, Mikoshiba K. Mouse Zic5 deficiency results in neural tube defects and hypoplasia of cephalic neural crest derivatives. Dev. Biol. 2004;270:146–62. doi: 10.1016/j.ydbio.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Jarad G, Miner JH. The Pax3-Cre transgene exhibits a rostrocaudal gradient of expression in the skeletal muscle lineage. Genesis. 2009;47:1–6. doi: 10.1002/dvg.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–9. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Joyner A, Wall N. CSH Protoc. 2008. Immunohistochemistry of whole-mount mouse embryos. 2008, pdb.prot4820. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res. A. Clin. Mol. Teratol. 2012;94:824–40. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Salic AN, Evans LM, Kirschner MW. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development. 1998;125:3247–3258. doi: 10.1242/dev.125.16.3247. [DOI] [PubMed] [Google Scholar]
- Le Dréau G, Martí E. Dorsal-ventral patterning of the neural tube: a tale of three signals. Dev. Neurobiol. 2012;72:1471–81. doi: 10.1002/dneu.22015. [DOI] [PubMed] [Google Scholar]
- Le Dréau G, Martí E. The multiple activities of BMPs during spinal cord development. Cell. Mol. Life Sci. 2013;70:4293–305. doi: 10.1007/s00018-013-1354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–40. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 1999;22:261–94. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat. Neurosci. 2001;4(Suppl):1183–91. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Li J, Chen F, Epstein J. a. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis. 2000;26:162–4. doi: 10.1002/(sici)1526-968x(200002)26:2<162::aid-gene21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–38. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–79. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Lim J-W, Hummert P, Mills JC, Kroll KL. Geminin cooperates with Polycomb to restrain multi-lineage commitment in the early embryo. Development. 2011;138:33–44. doi: 10.1242/dev.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhang Z, Srajer G, Chen YC, Huang M, Phan HM, Dent SYR. Proper expression of the Gcn5 histone acetyltransferase is required for neural tube closure in mouse embryos. Dev. Dyn. 2008;237:928–40. doi: 10.1002/dvdy.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat. Rev. Neurosci. 2005;6:945–54. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Luo L, Yang X, Takihara Y, Knoetgen H, Kessel M. The cell-cycle regulator geminin inhibits Hox function through direct and polycomb-mediated interactions. Nature. 2004;427:749–53. doi: 10.1038/nature02305. [DOI] [PubMed] [Google Scholar]
- Martí E, Bumcrot DA, Takada R, McMahon AP. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–5. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–53. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- MRC VITAMIN STUDY RESEARCH GROUP Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama K, Fujita M, Lygerou Z, Nishimoto T. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–36. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Yasunaga S, Ohno Y, Tsumura M, Okada S, Ishikawa N, Shirao K, Kikuchi A, Nishitani H, Kobayashi M, Takihara Y. Polycomb-group complex 1 acts as an E3 ubiquitin ligase for Geminin to sustain hematopoietic stem cell activity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10396–401. doi: 10.1073/pnas.0800672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike Y, Takakura N, Hata a, Kaname T, Akizuki M, Yamaguchi Y, Yasue H, Araki K, Yamamura K, Suda T. Mice homozygous for a truncated form of CREB-binding protein exhibit defects in hematopoiesis and vasculo-angiogenesis. Blood. 1999;93:2771–9. [PubMed] [Google Scholar]
- Pinney SE, Simmons RA. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr. Diab. Rep. 2012;12:67–74. doi: 10.1007/s11892-011-0248-1. [DOI] [PubMed] [Google Scholar]
- Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev. Biol. 2011;353:38–49. doi: 10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgaki C, Niswander L. Neural Circuit Development and Function in the Heathy and Diseased Brain. Elsevier Inc.; 2013. 27 - Neural-Tube Defects; pp. 503–519. [Google Scholar]
- Ribes V, Briscoe J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb. Perspect. Biol. 2009;1:a002014. doi: 10.1101/cshperspect.a002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha PP, Bleiss W, Schrewe H. Mosaic expression of Med12 in female mice leads to exencephaly, spina bifida, and craniorachischisis. Birth Defects Res. A. Clin. Mol. Teratol. 2010;88:626–32. doi: 10.1002/bdra.20693. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter J. a, Chiang C, Tanabe Y, Chang DT, Beachy P. a, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–55. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rogner UC, Spyropoulos DD, Le Novère N, Changeux JP, Avner P. Control of neurulation by the nucleosome assembly protein-1-like 2. Nat. Genet. 2000;25:431–5. doi: 10.1038/78124. [DOI] [PubMed] [Google Scholar]
- Salbaum JM, Kappen C. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Res. A. Clin. Mol. Teratol. 2010;88:601–11. doi: 10.1002/bdra.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–8. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- Schultz KM, Banisadr G, Lastra RO, McGuire T, Kessler J. a, Miller RJ, McGarry TJ. Geminin-deficient neural stem cells exhibit normal cell division and normal neurogenesis. PLoS One. 2011;6:e17736. doi: 10.1371/journal.pone.0017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Herr A, Lim J-W, Richardson G. a, Richardson H, Kroll KL. Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 2005;19:1723–34. doi: 10.1101/gad.1319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šošić D, Richardson J. a, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–80. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Spella M, Kyrousi C, Kritikou E, Stathopoulou A, Guillemot F, Kioussis D, Pachnis V, Lygerou Z, Taraviras S. Geminin regulates cortical progenitor proliferation and differentiation. Stem Cells. 2011;29:1269–82. doi: 10.1002/stem.678. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Klingensmith J. Bone morphogenetic protein signaling is required in the dorsal neural folds before neurulation for the induction of spinal neural crest cells and dorsal neurons. Dev. Dyn. 2011;240:755–65. doi: 10.1002/dvdy.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–5. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52, 54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013;339:1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–73. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev. Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–12. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- Yao TP, Oh SP, Fuchs M, Zhou ND, Ch'ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–72. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- Yellajoshyula D, Lim J, Thompson DM, Witt JS, Patterson ES, Kroll KL. Geminin regulates the transcriptional and epigenetic status of neuronal fate-promoting genes during mammalian neurogenesis. Mol. Cell. Biol. 2012;32:4549–60. doi: 10.1128/MCB.00737-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellajoshyula D, Patterson ES, Elitt MS, Kroll KL. Geminin promotes neural fate acquisition of embryonic stem cells by maintaining chromatin in an accessible and hyperacetylated state. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3294–9. doi: 10.1073/pnas.1012053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Ito Y, Ueno-Kudoh H, Shimizu H, Uchibe K, Albini S, Mitsuoka K, Miyaki S, Kiso M, Nagai A, Hikata T, Osada T, Fukuda N, Yamashita S, Harada D, Mezzano V, Kasai M, Puri PL, Hayashizaki Y, Okado H, Hashimoto M, Asahara H. A systems approach reveals that the myogenesis genome network is regulated by the transcriptional repressor RP58. Dev. Cell. 2009;17:836–48. doi: 10.1016/j.devcel.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–41. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- Zhu W, Depamphilis ML. Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 2009;69:4870–7. doi: 10.1158/0008-5472.CAN-08-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Hematoxylin stained section of trunk neural tube prior to laser capture microdissection (LCM). (B)Same section after LCM. 1 marks the area taken for the neural tube RNA samples and 2 marks the areas taken for the paraxial tissue RNA samples that were further analyzed by microarray.Scale bar=100μm.
(A)RT-PCR with primers spanning Exon 4 shows low expression of the Δexon4 allele at the mRNA level in E10.5 trunk (tissue dorsal of the dorsal aorta was dissected) in Mox2Cre;Gmnn+/floxembryos. (B) No protein of the predicted size is detected on a Western blot. The low level of full length Geminin protein is from cells that have not excised Geminin. (C)Enumeration of embryos found upon dissection at various embryonic days showing survival of mutant embryos until E18.5.
(A) Wild-type E18.5 embryo. (B) Mutant Mox2-Cre embryos at E18.5 have completely penetrant caudal neural tube defects. (C,D) Hematoxylin/eosin stained transverse sections at the levels indicated by dashed lines in A,B. Scale bar=500μm.
Immunohistochemistry on transverse sections of E9.5 spinal neural tube in wt and mutant (Mox2Cre;Gmnnflox/flox) embryos shows no difference in expression of the ventral progenitor markers (A) Nkx6.1, (B) Olig2, (C) Nkx2.2, and (D) Foxa2. Embryos analyzed for immunohistochemistry (n=3). Scale bar=100μm.
Transverse sections of E11.5 and E12.5 wt and mutant (Mox2Cre;Gmnnflox/flox) embryos. Mutant embryos show an expanded Ki67 positive proliferative progenitor zone (A,B) and persistentexpression ofthe progenitor markers Atoh1 (C–F) and Nkx6.1 (G,H) at E12.5. The differentiation marker Ncam (I,J) is decreased in E11.5 and E12.5 mutant embryos and Isl1/2 (K) is decreased in E12.5 mutant embryos.Embryos analyzed for immunohistochemistry (E11.5; n=4) and (E12.5; n=6). Scale bars=100μm.
(A,A′) Transverse section of a Dermo1-Cre; R26R E10.5 embryo at the forelimb level demonstrates Cre activity (blue) in paraxial mesenchyme, but not in the neural tube (Nuclear Fast Red counterstain). Scale bar forA=250μm; A′=100μm. (B–E)Whole mount images of Nestin-Cre; R26R embryos at E9.5 (B) or E10.5 (C–E). (B′–E′) Transverse sections of embryos in B–E demonstrate that Nestin-Cre is only active in the ventral neural tube in the rostral portion of the spinal neural tube (blue). In panels B′–E′, spinal neural tube showing ventral expression of Nestin-Cre is oriented to the left and the tail neural tube lacking Nestin-Cre expression is orientedto the right in the images. Dashed lines in B–E indicate the plane of the section in B′–E′. Scale bar forB′=100μm; C′=250μm.(F–G) Transverse sections of E9.5 Pax3-Cre (F) wt and (G) mutant (Pax3Cre;Gmnnflox/flox) embryos. Geminin and Sox1 immunohistochemistry and DAPI staining of nuclei are shown. Nuclear staining for Geminin is visible in the wild-type neural tube, but is not detected in the neural tube of the mutant embryo. Nuclear Geminin staining is visible in non-neural tissue in the mutant (G, arrowhead), consistent with neural tube-specific excision by the Pax3-Cre driver. Embryos analyzed for immunohistochemistry (n=2). Scale bar for G=100 μm.
Table S1. Primers used for genotyping
Table S2. Antibody clone/catalog numbers and dilutions used.
Table S3. Primers used forqRT-PCR.
Table S4. Genes with more than 2-fold difference in expression in mutant versus wild-type neural tube tissue, as defined by microarray of LCM captured material (Materials and Methods). Negative values indicate decreased expression (fold change) in mutant embryos, relative to wild-type siblings, while positive values indicate increased expression in mutant embryos relative to wild-type.
Table S5. Genes with >2-fold difference in expression in mutant paraxial tissue relative to wild-type (see Materials and Methods).