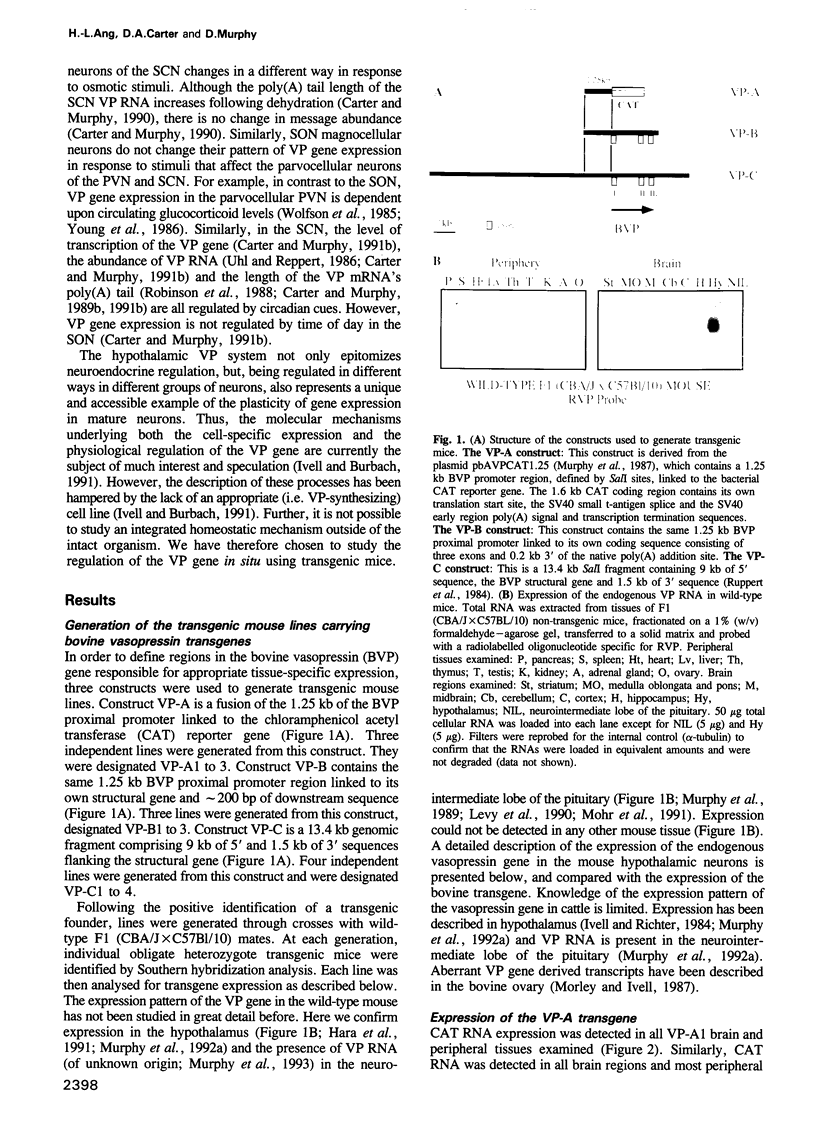
Abstract
We have used transgenic mice to analyse the regulation of the bovine vasopressin (BVP) gene. We find that the restriction of BVP gene expression to anatomically and functionally distinct hypothalamic neuronal groups is achieved, in part, by selective repression. The expression of a 1.25 kb BVP proximal promoter, which on its own confers general expression of a reporter to most peripheral and brain tissues, was limited by sequences in the BVP structural gene to neural cells in the adrenal medulla and brain. Transgene expression in the hypothalamus was shown to be regulated by the physiological stimulus of dehydration in parallel with the endogenous gene. The expression of a larger 13.4 kb BVP transgene, containing 9 kb of 5' upstream sequence, the VP structural gene and 1.5 kb 3' of the transcription unit, was even more restricted and resembles that of the endogenous mouse gene. Hypothalamic expression of the 13.4 kb BVP transgene was regulated appropriately in response to an osmotic challenge.
Full text
PDF

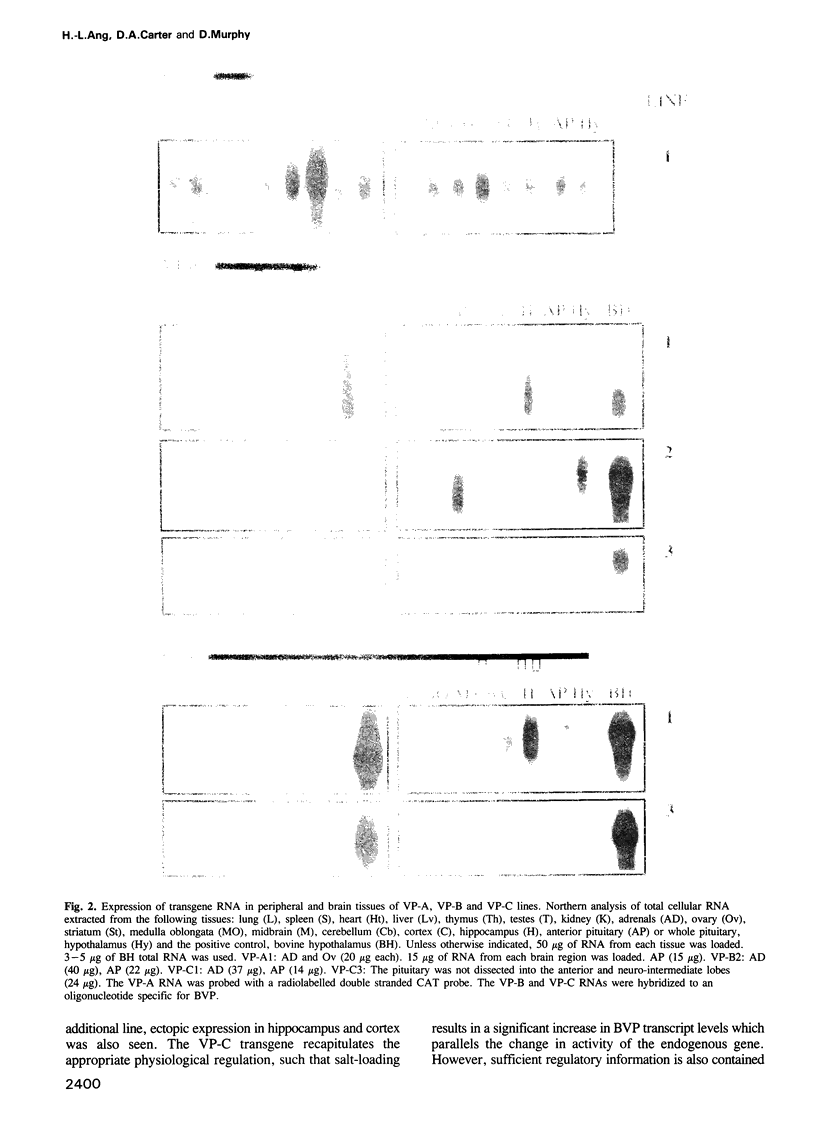
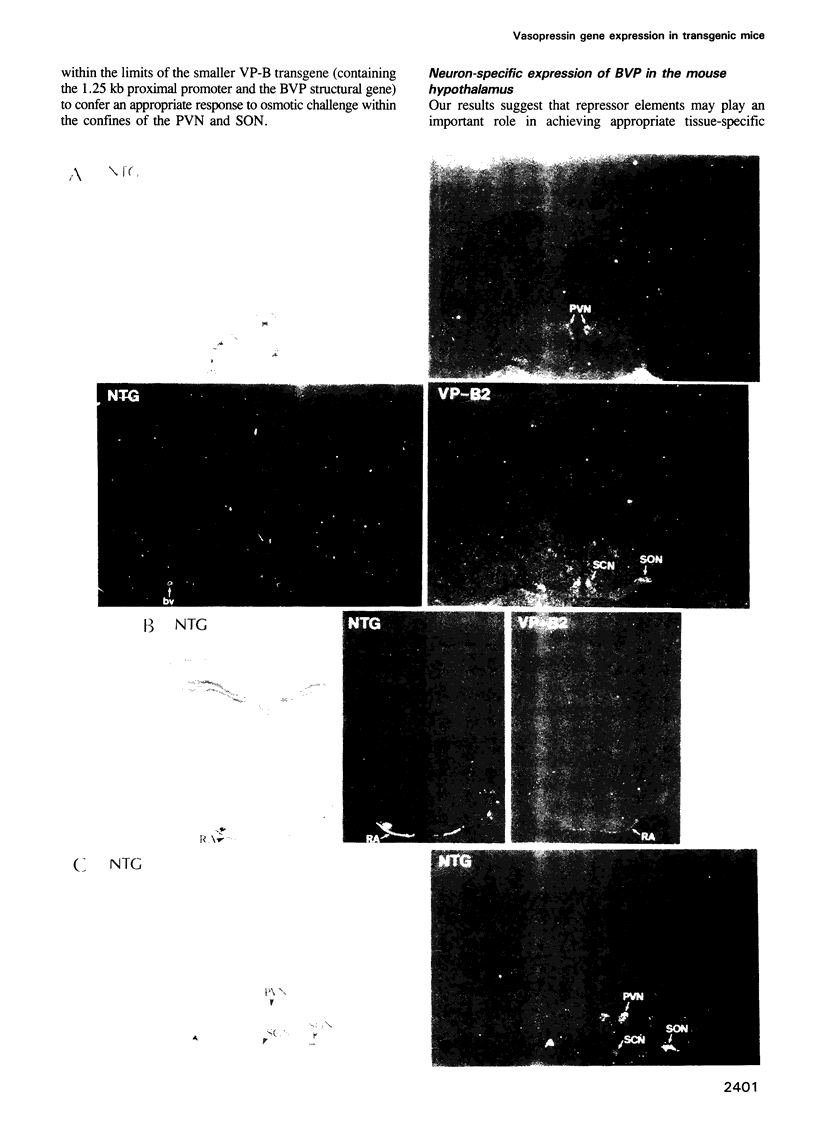
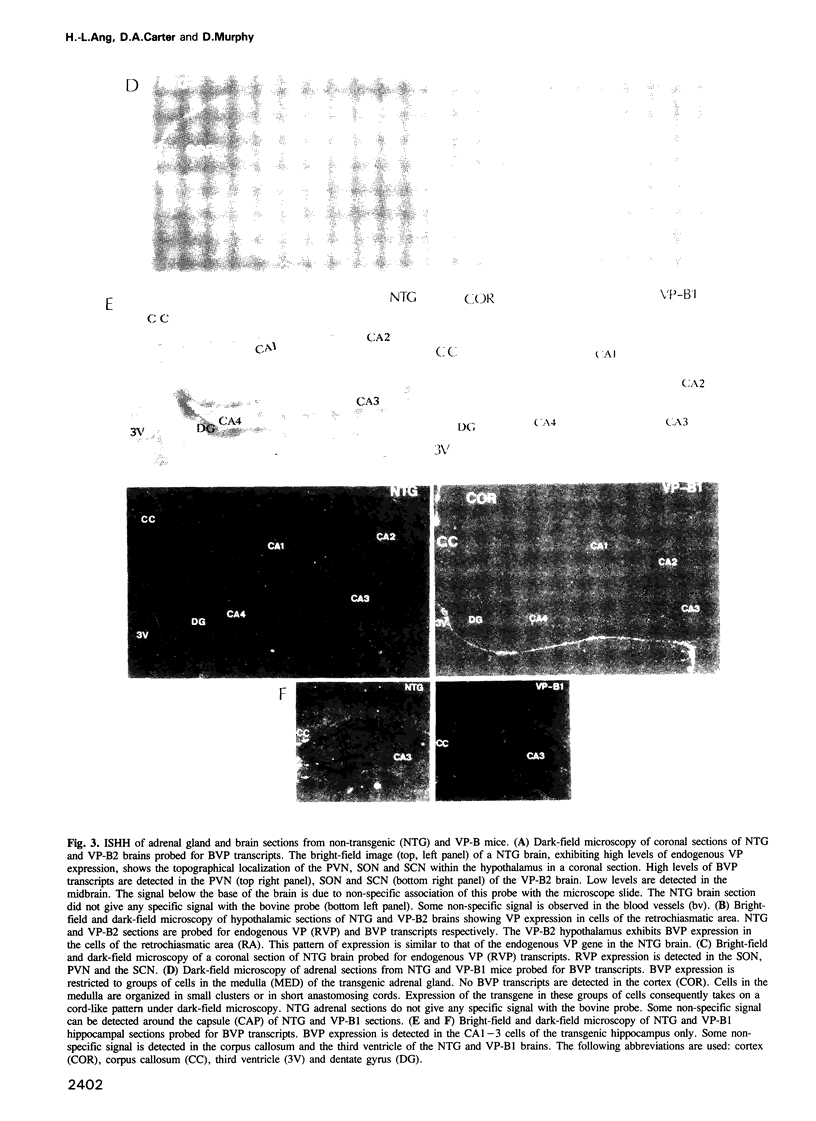

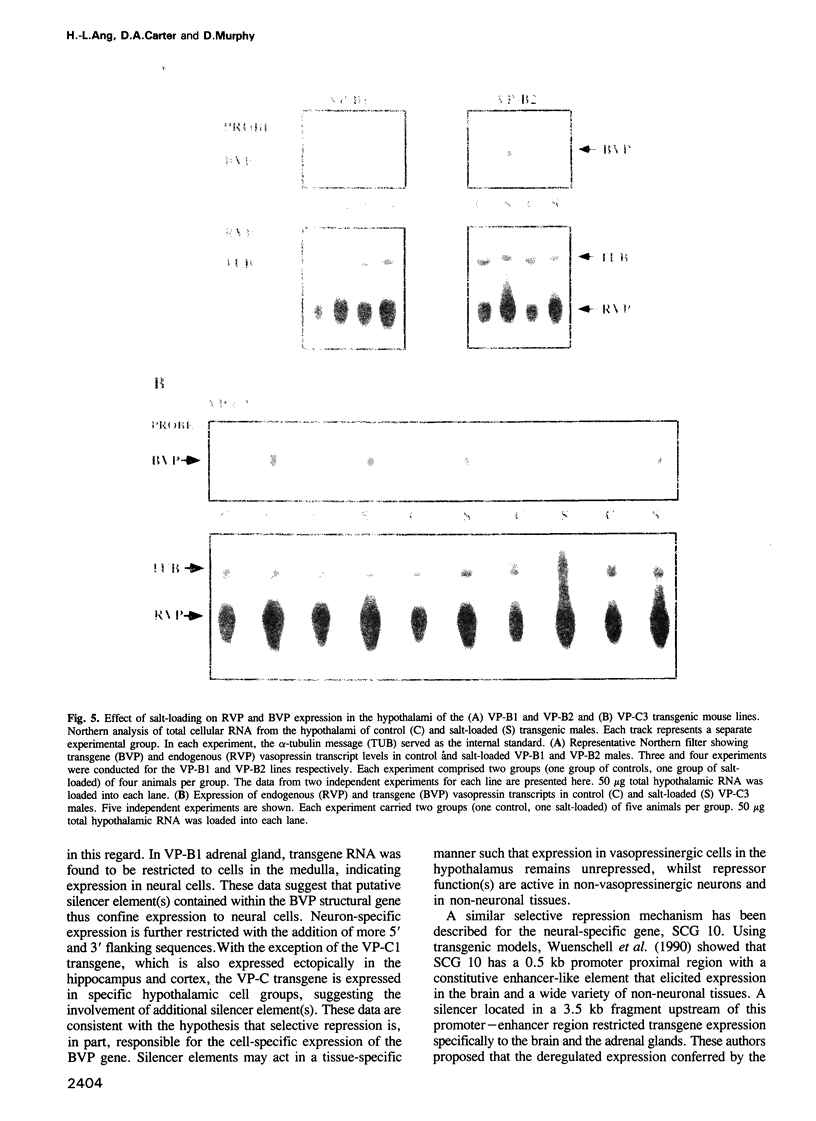



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang H. L., Ungefroren H., De Bree F., Foo N. C., Carter D., Burbach J. P., Ivell R., Murphy D. Testicular oxytocin gene expression in seminiferous tubules of cattle and transgenic mice. Endocrinology. 1991 Apr;128(4):2110–2117. doi: 10.1210/endo-128-4-2110. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Burbach J. P., De Hoop M. J., Schmale H., Richter D., De Kloet E. R., Ten Haaf J. A., De Wied D. Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinology. 1984 Dec;39(6):582–584. doi: 10.1159/000124040. [DOI] [PubMed] [Google Scholar]
- Carrazana E. J., Pasieka K. B., Majzoub J. A. The vasopressin mRNA poly(A) tract is unusually long and increases during stimulation of vasopressin gene expression in vivo. Mol Cell Biol. 1988 Jun;8(6):2267–2274. doi: 10.1128/mcb.8.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Cyclic nucleotide dynamics in the rat hypothalamus during osmotic stimulation: in vivo and in vitro studies. Brain Res. 1989 May 22;487(2):350–356. doi: 10.1016/0006-8993(89)90839-1. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Diurnal rhythm of vasopressin mRNA species in the rat suprachiasmatic nucleus: independence of neuroendocrine modulation and maintenance in explant culture. Brain Res Mol Brain Res. 1989 Dec;6(4):233–239. doi: 10.1016/0169-328x(89)90069-7. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Independent regulation of neuropeptide mRNA level and poly(A) tail length. J Biol Chem. 1989 Apr 25;264(12):6601–6603. [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Nuclear mechanisms mediate rhythmic changes in vasopressin mRNA expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1992 Feb;12(4):315–321. doi: 10.1016/0169-328x(92)90133-v. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Rapid changes in poly (A) tail length of vasopressin and oxytocin mRNAs form a common early component of neurohypophyseal peptide gene activation following physiological stimulation. Neuroendocrinology. 1991 Jan;53(1):1–6. doi: 10.1159/000125689. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Vasopressin mRNA in parvocellular neurons of the rat suprachiasmatic nucleus exhibits increased poly (A) tail length following water deprivation. Neurosci Lett. 1990 Feb 5;109(1-2):180–185. doi: 10.1016/0304-3940(90)90559-r. [DOI] [PubMed] [Google Scholar]
- Castel M., Morris J. F. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience. 1988 Mar;24(3):937–966. doi: 10.1016/0306-4522(88)90078-4. [DOI] [PubMed] [Google Scholar]
- Dierickx K. Immunocytochemical localization of the vertebrate cyclic nonapeptide neurohypophyseal hormones and neurophysins. Int Rev Cytol. 1980;62:119–185. doi: 10.1016/s0074-7696(08)61900-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Hara Y., Battey J., Gainer H. Structure of mouse vasopressin and oxytocin genes. Brain Res Mol Brain Res. 1990 Oct;8(4):319–324. doi: 10.1016/0169-328x(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Herman J. P., Schäfer M. K., Watson S. J., Sherman T. G. In situ hybridization analysis of arginine vasopressin gene transcription using intron-specific probes. Mol Endocrinol. 1991 Oct;5(10):1447–1456. doi: 10.1210/mend-5-10-1447. [DOI] [PubMed] [Google Scholar]
- Horn A. M., Robinson I. C., Fink G. Oxytocin and vasopressin in rat hypophysial portal blood: experimental studies in normal and Brattleboro rats. J Endocrinol. 1985 Feb;104(2):211–224. doi: 10.1677/joe.0.1040211. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. The gene for the hypothalamic peptide hormone oxytocin is highly expressed in the bovine corpus luteum: biosynthesis, structure and sequence analysis. EMBO J. 1984 Oct;3(10):2351–2354. doi: 10.1002/j.1460-2075.1984.tb02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Life with the new technologies. Nature. 1984 Mar 1;308(5954):1–2. doi: 10.1038/308001a0. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol. 1987 Dec;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight R. A., Shamay A., Sankaran L., Wall R. J., Hennighausen L. Matrix-attachment regions can impart position-independent regulation of a tissue-specific gene in transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6943–6947. doi: 10.1073/pnas.89.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker R. B., Greenwood R. S., Hayward J. N. Vasopressin mRNA expression in individual magnocellular neuroendocrine cells of the supraoptic and paraventricular nucleus in response to water deprivation. Neuroendocrinology. 1991 Sep;54(3):236–247. doi: 10.1159/000125881. [DOI] [PubMed] [Google Scholar]
- Mercer E. H., Hoyle G. W., Kapur R. P., Brinster R. L., Palmiter R. D. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991 Nov;7(5):703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- Mohr E., Bahnsen U., Kiessling C., Richter D. Expression of the vasopressin and oxytocin genes in rats occurs in mutually exclusive sets of hypothalamic neurons. FEBS Lett. 1988 Dec 19;242(1):144–148. doi: 10.1016/0014-5793(88)81003-2. [DOI] [PubMed] [Google Scholar]
- Mohr E., Fehr S., Richter D. Axonal transport of neuropeptide encoding mRNAs within the hypothalamo-hypophyseal tract of rats. EMBO J. 1991 Sep;10(9):2419–2424. doi: 10.1002/j.1460-2075.1991.tb07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E., Schmitz E., Richter D. A single rat genomic DNA fragment encodes both the oxytocin and vasopressin genes separated by 11 kilobases and oriented in opposite transcriptional directions. Biochimie. 1988 May;70(5):649–654. doi: 10.1016/0300-9084(88)90249-0. [DOI] [PubMed] [Google Scholar]
- Moore R. Y. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983 Aug;42(11):2783–2789. [PubMed] [Google Scholar]
- Morley S. D., Ivell R. Vasopressin gene transcripts in the bovine corpus luteum are defective. Mol Cell Endocrinol. 1987 Oct;53(3):255–258. doi: 10.1016/0303-7207(87)90182-1. [DOI] [PubMed] [Google Scholar]
- Murphy D., Ang H. L., Zeng Q., Ho M. Y., Funkhouser J., Carter D. Neuropeptide gene expression in transgenic animals. Prog Brain Res. 1992;92:77–96. doi: 10.1016/s0079-6123(08)61166-8. [DOI] [PubMed] [Google Scholar]
- Murphy D., Bishop A., Rindi G., Murphy M. N., Stamp G. W., Hanson J., Polak J. M., Hogan B. Mice transgenic for a vasopressin-SV40 hybrid oncogene develop tumors of the endocrine pancreas and the anterior pituitary. A possible model for human multiple endocrine neoplasia type 1. Am J Pathol. 1987 Dec;129(3):552–566. [PMC free article] [PubMed] [Google Scholar]
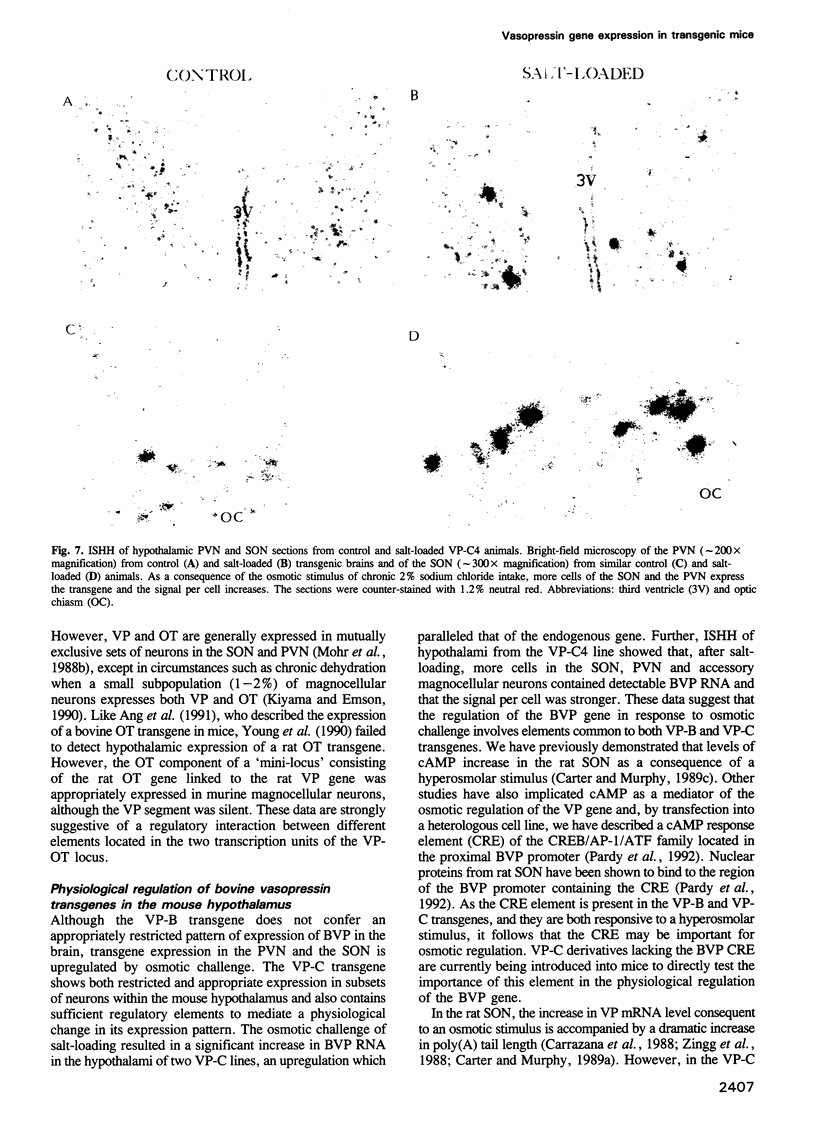
- Murphy D., Carter D. Vasopressin gene expression in the rodent hypothalamus: transcriptional and posttranscriptional responses to physiological stimulation. Mol Endocrinol. 1990 Jul;4(7):1051–1059. doi: 10.1210/mend-4-7-1051. [DOI] [PubMed] [Google Scholar]
- Murphy D., Levy A., Lightman S., Carter D. Vasopressin RNA in the neural lobe of the pituitary: dramatic accumulation in response to salt loading. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9002–9005. doi: 10.1073/pnas.86.22.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardy K., Adan R. A., Carter D. A., Seah V., Burbach J. P., Murphy D. The identification of a cis-acting element involved in cyclic 3',5'-adenosine monophosphate regulation of bovine vasopressin gene expression. J Biol Chem. 1992 Oct 25;267(30):21746–21752. [PubMed] [Google Scholar]
- Rehbein M., Hillers M., Mohr E., Ivell R., Morley S., Schmale H., Richter D. The neurohypophyseal hormones vasopressin and oxytocin. Precursor structure, synthesis and regulation. Biol Chem Hoppe Seyler. 1986 Aug;367(8):695–704. doi: 10.1515/bchm3.1986.367.2.695. [DOI] [PubMed] [Google Scholar]
- Richter D. Molecular events in expression of vasopressin and oxytocin and their cognate receptors. Am J Physiol. 1988 Aug;255(2 Pt 2):F207–F219. doi: 10.1152/ajprenal.1988.255.2.F207. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983 Sep;113(3):939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- Robinson B. G., Frim D. M., Schwartz W. J., Majzoub J. A. Vasopressin mRNA in the suprachiasmatic nuclei: daily regulation of polyadenylate tail length. Science. 1988 Jul 15;241(4863):342–344. doi: 10.1126/science.3388044. [DOI] [PubMed] [Google Scholar]
- Russo A. F., Crenshaw E. B., 3rd, Lira S. A., Simmons D. M., Swanson L. W., Rosenfeld M. G. Neuronal expression of chimeric genes in transgenic mice. Neuron. 1988 Jun;1(4):311–320. doi: 10.1016/0896-6273(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Schmale H., Heinsohn S., Richter D. Structural organization of the rat gene for the arginine vasopressin-neurophysin precursor. EMBO J. 1983;2(5):763–767. doi: 10.1002/j.1460-2075.1983.tb01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman T. G., Day R., Civelli O., Douglass J., Herbert E., Akil H., Watson S. J. Regulation of hypothalamic magnocellular neuropeptides and their mRNAs in the Brattleboro rat: coordinate responses to further osmotic challenge. J Neurosci. 1988 Oct;8(10):3785–3796. doi: 10.1523/JNEUROSCI.08-10-03785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaneanu L., Rindi G., Horvath E., Murphy D., Polak J. M., Kovacs K. Morphology of adenohypophysial tumors in mice transgenic for vasopressin-SV40 hybrid oncogene. Endocrinology. 1992 Apr;130(4):1789–1795. doi: 10.1210/endo.130.4.1312426. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Södersten P., De Vries G. J., Buijs R. M., Melin P. A daily rhythm in behavioral vasopressin sensitivity and brain vasopressin concentrations. Neurosci Lett. 1985 Jul 4;58(1):37–41. doi: 10.1016/0304-3940(85)90325-8. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Reppert S. M. Suprachiasmatic nucleus vasopressin messenger RNA: circadian variation in normal and Brattleboro rats. Science. 1986 Apr 18;232(4748):390–393. doi: 10.1126/science.3961487. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Watts A. G., Swanson L. W. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987 Apr 8;258(2):230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]
- Wuenschell C. W., Mori N., Anderson D. J. Analysis of SCG10 gene expression in transgenic mice reveals that neural specificity is achieved through selective derepression. Neuron. 1990 Apr;4(4):595–602. doi: 10.1016/0896-6273(90)90117-x. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res. 1986 Dec;387(3):231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Zoeller R. T. Neuroendocrine gene expression in the hypothalamus: in situ hybridization histochemical studies. Cell Mol Neurobiol. 1987 Dec;7(4):353–366. doi: 10.1007/BF00733788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E. A., Silverman A. J. Vasopressin and adrenal cortical interactions. Prog Brain Res. 1983;60:493–504. doi: 10.1016/S0079-6123(08)64415-5. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D. L., Almazan G. Regulation of poly(A) tail size of vasopressin mRNA. J Biol Chem. 1988 Aug 15;263(23):11041–11043. [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D., Almazan G. Regulation of vasopressin gene expression in rat hypothalamic neurons. Response to osmotic stimulation. J Biol Chem. 1986 Oct 5;261(28):12956–12959. [PubMed] [Google Scholar]