Abstract
Neurotrophins, such as brain-derived neurotrophic factor (BDNF), are initially expressed in a precursor form (e.g., proBDNF) and cleaved to form mature BDNF (mBDNF). Following pilocarpine-induced status epilepticus (SE), increases in neurotrophins regulate a wide variety of cell signaling pathways including pro-survival and cell-death machinery in a receptor-specific manner. ProBDNF preferentially binds to the p75 neurotrophin receptor (p75NTR), while mBDNF is the major ligand of the tropomyosin related kinase receptor (TrkB). To elucidate a potential role of p75NTR in acute stages of epileptogenesis, rats were injected prior to and at onset of SE with LM11A-31, a small molecule ligand that binds to p75NTR to promote survival signaling and inhibit neuronal cell death. Modulation of early p75NTR signaling and its effects on (1) electrographic SE, (2) SE-induced neurodegeneration, and (3) subsequent spontaneous seizures were examined following LM11A-31 administration. Despite an established neuroprotective effect of LM11A-31 in several animal models of neurodegenerative disorders (e.g., Alzheimer’s disease, traumatic brain injury, and spinal cord injury), high-dose LM11A-31 administration prior to and at onset of SE did not reduce the intensity of electrographic SE, prevent SE-induced neuronal cell injury, nor inhibit the progression of epileptogenesis. Further studies are required to understand the role of p75NTR activation during epileptogenesis and in seizure-induced cell injury in the hippocampus among other potential cellular pathologies contributing to the onset of spontaneous seizures. Additional studies utilizing more prolonged treatment with LM11A-31 are required to reach a definite conclusion on its potential neuroprotective role in epilepsy.
Keywords: status epilepticus, pilocarpine, neurotrophin, seizure, LM11A-31, p57NTR
Introduction
The most common form of acquired epilepsy, temporal lobe epilepsy (TLE), afflicts 65 million people worldwide (England et al., 2012), and represents a therapeutic challenge as 30% of patients with TLE have seizures that are refractory to commonly used anticonvulsant drugs (Sillanpaa and Schmidt, 2006; Brodie and Kwan, 2012; Bauer and Burr, 2001). TLE can be progressive with seizures becoming more severe (or more frequent) over months to years after the onset of epilepsy in a subset of patients (Berg et al., 2003), and it is often associated with cognitive and psychological co-morbidities. All currently marketed anticonvulsants symptomatically treat seizures, and there are currently no approved medications that are capable of modifying the disease to lessen the severity of the underlying epilepsy and the associated co-morbidities.
Neurotrophins (e.g., nerve growth factor [NGF], brain-derived neurotrophic factor [BDNF], neurotrophin-3 [NT-3], and neurotrophin-4 [NT-4]) promote the survival and differentiation of neurons by binding and activating two receptor subtypes, the Trk receptor family of tyrosine kinases and p75NTR, a member of the TNF superfamily. P75NTR is primarily expressed during development, but can be re-expressed in adulthood during various pathological conditions, such as axotomy, neurodegeneration, and epilepsy (Dechant and Barde, 2002). P75NTR can interact with Trk receptors to activate survival signaling or other types of receptors, such as the sortillin receptor, to elicit apoptosis upon binding of active ligands. Both full-length neurotrophins and pro-neurotrophins are bioactive. Pro-neurotrophins (i.e., proNGF and proBDNF) preferentially bind and activate p75NTR once they are released (Chao and Bothwell, 2002) and are up-regulated in apoptotic neurons after seizures (Roux et al., 1999). Although neurotrophins are typically thought to play a trophic role after seizure activity (e.g., status epilepticus), recently it has been demonstrated that the p75NTR may have a pro-apoptotic role in neurons and that increases in neurotrophin expression after seizure activity could potentially promote cell death via p75NTR-dependent, apoptotic mechanisms (Unsain et al., 2008). Seizures induce necrotic and apoptotic cell death, and alterations in neurotrophin and TrkB receptor expression (Isackson et al., 1991; Humpel et al., 1993; Bengzon et al., 1993). Pilocarpine-induced SE also results in p75NTR protein and mRNA increases in the hippocampus, piriform cortex, and entorhinal cortex (Roux et al., 1999). Multiple studies have demonstrated neuronal cell loss in the hippocampus in human epileptic tissue (Arzimanoglou et al., 2002) and in experimental animal models of epilepsy (Represa et al., 1995; Turski et al., 1983). SE in the rat, specifically, results in damage in multiple brain regions including hippocampus, amygdala, thalamus, olfactory cortex, neocortex and substantia nigra (Turski et al., 1983; Olney et al., 1986; Turski et al., 1987). It is also well established that damage produced by SE precedes and may promote the development of the recurrent spontaneous seizures characteristic of epilepsy (Aicardi and Chevrie, 1983; Cavalheiro et al., 1991; Priel et al., 1996). The role, if any, of p75NTR activation following SE in subsequent neuronal loss and epilepsy development has not been well delineated.
LM11A-31 is a small molecule, non-peptide, p75NTR ligand originally developed by Massa et al. (2006) that inhibits degenerative signaling and promotes survival signaling (Yang et al, 2008; Longo and Massa, 2013). LM11A ligands, of which LM11A-31 emerged as a promising compound, block the ability of pro-neurotrophins to promote cell death. LM11A ligands (1) may induce different patterns of p75NTR adapter recruitment than neurotrophins, shifting the balance towards survival mechanisms, or (2) may prevent the association of p75NTR with other receptors influencing cell death (e.g., sortillin, Longo and Massa, 2008, 2013). More recently, LM11A-31 administration in vivo was shown to reduce cognitive deficits and neurite degeneration in a murine model of Alzheimer’s disease (the APPL/S mouse; Knowles et al., 2013) and to prevent binding of proNGF to p75NTR and associated oligodendrocyte death following spinal cord injury (Tep et al., 2013).
In an animal model of acquired epilepsy (specifically the pilocarpine model of TLE), continuous video-EEG monitoring was used to determine whether modulation of the p75NTR at the time of brain insult (SE) using a high-dose administration of LM11A-31 would alter the magnitude of SE, prevent the development of spontaneous seizures, or affect seizure-induced neurodegeneration.
Methods
Pilocarpine-induced SE and LM11A-31 administration
Adult Sprague-Dawley rats (Charles-River Labs, Kingston, PA) pre-treated with 1 mg/kg i.p. scopolamine to block peripheral cholinergic effects were injected with 385 mg/kg i.p. pilocarpine to induce SE. A subconvulsive dose of pilocarpine (38.5 mg/kg i.p.) was administered to control rats. After 1 hour of sustained SE, diazepam (6 mg/kg i.p.) was administered. Supplemental doses of diazepam (3 mg/kg) were administered every 2 hours as needed to abolish any persistent seizures. Control rats received one tenth of the dose of diazepam (0.6 mg/kg). For all treatment studies, LM11A-31 (200 mg/kg in sterile saline) was administered by i.p. injection following the pilocarpine administration and a second dose of LM11A-31 (200 mg/kg) was injected at onset of SE (defined as the first Class 5 motor seizure). Motor seizures were scored by standard behavioral classes (Racine, 1972). Pilocarpine was purchased from Sigma (St. Louis, MO), diazepam was purchased from Hospira (Lake Forest, IL), and LM11A-31 was synthesized by Ricerca Biosciences (Concord, Ohio) under the direction of Dr. Frank Longo.. LM11A-31 levels in plasma and brain extract were measured using LC-MS/MS by Absorption Systems (Exton, PA) under the direction of Dr. Frank Longo as previously described (Knowles et al., 2013). For all experimental procedures, the animals’ care was in accordance with institutional guidelines.
EEG acquisition and analysis
To accurately analyze electrographic seizure frequency, two bilateral subdural stainless steel screws (4.0 mm posterior, 2.5 mm lateral relative to bregma) were placed over the temporolimbic cortices. Additional stainless steel screws (reference and ground electrodes) were placed bilaterally behind lambda. Animals recovered from surgery for at least 1 week prior to any further experimentation. Epileptic rats were video-EEG monitored 24 hours/day using Pinnacle digital video-EEG systems which utilizes a recording chamber, a commutator (i.e., electric swivel) system, and flexible cables that allow the animal to move freely. EEG signals were acquired at 1 kHz, amplified by 500×, and band-pass filtered between 0.3 Hz and 600 Hz.
Off-line data analysis was performed by a trained technician blinded to all experimental parameters. Manually detected electrographic seizures were differentiated from background noise by the appearance of large-amplitude (at least three times baseline), high frequency (minimum of 5 Hz) activity, with progression of the spike frequency that lasts for a minimum of 10 sec; and correlated with behavioral manifestations in continuous video recordings utilizing a modified Racine scale ([Racine, 1972]; described in Grabenstatter et al., 2013). Electrographic seizures class 3 (e.g., forelimb clonus) and above were classified as convulsive and seizures scored as class 2 (e.g., head bobbing) and below were classified as non-convulsive.
The power analysis was performed using a Fast Fourier Transform (FFT) algorithm written using subroutines in Visual Basic. For the initial analyses, a rectangular window was used to analyze continuous segments of data containing 8192 points (8.192 seconds) each. Maximum power, integrated power and time that elapsed from maximum power to 20%, 10%, and 5% of the baseline power were measured. Compressed spectral analyses (CSAs) were also generated for each rat using FFT demonstrating the magnitude of power in each frequency bin. For the short-term analyses, rectangular windows of 131072 points (131 seconds) were used. For both EEG data channels, the power in each frequency band (1-4 Hz, 4-8 Hz, 8-13 Hz, 13-30 Hz, 30-50 Hz, and 50-70 Hz) was averaged for 0.5 h, 1 h, 2 h, 4 h, and 6 h-long bins following onset of status and second administration of vehicle or drug.
Western Blots
Rats were anesthetized with inhaled isoflurane, decapitated, and whole brains were dissected in chilled 1× PBS medium (with 1:1000 phosphatase inhibitors) for dentate gyrus microdissection. Longitudinal hippocampal slices (500 μm thick) were cut and the dentate gyrus was identified and dissected under an anatomical microscope. Alternate slices from each hippocampus were used for protein extraction to control for any difference in the amount of damage and/or seizure activity in one hippocampus compared to the other. The dentate gyrus was chosen because it is relatively well preserved during epileptogenesis.
For western blots, protein (50 μg) extracted from microdissected DG was loaded into 8% SDS–polyacrylamide gels and run for ~1.5 h at 115 V. Blots were then transferred to nitrocellulose membranes and blocked in 5% milk/trisbuffered saline with Tween-20 (TBS-T) 1 h at room temperature. Membranes were incubated with rabbit polyclonal antibodies raised against p75NTR (p75NTR/NGF Receptor Antibody [EP1039Y], NB110-58000 Novus Biologicals; 1:250) overnight at 4°C in 5% bovine serum albumin/TBS-T. Membranes were then washed and incubated with anti-rabbit secondary antibody (GE health, 1:10,000 in 1% milk/TBST) conjugated to horseradish peroxidase for 1 h at room temperature.
Protein bands were detected with the use of chemiluminescent solution (Pierce). Membranes were stripped and probed with rabbit polyclonal antibody raised against β-actin (1:40,000, Sigma) in 1% milk/TBS-T overnight. P75NTR values were normalized to β-actin expression in the same samples to control for loading amount variability and then expressed as percent change with respect to mean control values in the same run (defined as 1). Densitometry was performed with NIH Image J version 1.42q. Statistical significance was defined as a P value of less than 0.05 and was calculated with Prism software using a one-way ANOVA with a Tukey’s test for multiple comparisons.
Fluoro-Jade B staining
To assess degenerating neurons in the hippocampus, rats were sacrificed 48 h following onset of SE and administration of either LM11A-31 (200 mg/kg with pilocarpine and 200 mg/kg at SE onset) or vehicle, perfused transcardially with cold phosphate buffered saline (0.01 M) and 4% paraformaldehyde (in 0.1 M), post-fixed overnight, then cryoprotected in 30% sucrose. Frozen, embedded (in Tissue-Tek OCT compound, Sakura Finetek, Torrance, CA) whole brains were serial sectioned (12 μm thickness, in the coronal plane), mounted, and processed for Fluoro-Jade B staining as previously published (Grabenstatter et al., 2013). A 1-in-20 series of sections (i.e., −2.3 mm to −4.8 mm from Bregma) from each brain (e.g., ~10 sections per animal, SE+LM11A-31, n=5; SE+vehicle, n=4) was processed. Stained sections were examined under a fluorescence microscope using fluorescein isothiocyanate (FITC) filter sets, and neurons were morphologically identified by size and cytological characteristics, and glial cells were excluded from counts. Fluoro-Jade B positive neuronal cell bodies were counted in the CA1, CA3, and hilar region of the hippocampus using NIS-Elements Analysis software. The selected areas of interest used to quantify Fluoro-Jade B cell density were the same for each section examined. Images were obtained using a 10× objective (Nikon Eclipse TE2000-U). The technician conducting cell counts was blind to treatment administered. Statistical differences between treatments were determined using a Mann-Whitney test.
Results
P75NTR receptor expression increases in a time-dependent manner following SE
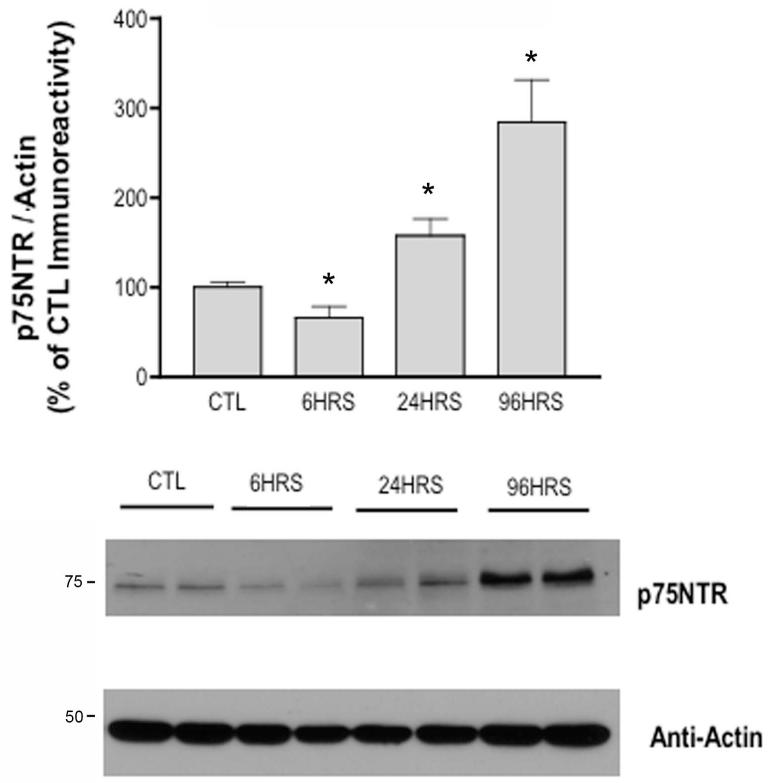
Previous studies have demonstrated increases in p75NTR protein expression in apoptotic neurons of the hippocampus following pilocarpine-induced SE (Roux et al., 1999; Troy et al., 2002). Specifically, a slight increase in p75NTR protein expression at 12 and 24 h after SE, and a significant increase in p75NTR expression at 72 h after SE has been reported (Unsain et al., 2008). Evaluating an expanded time course specifically in the dentate gyrus, our results demonstrate p75NTR protein levels first decrease 6 hours after SE onset, slightly increase relative to controls 24 hours after SE onset, and demonstrate significantly increased p75NTR protein 96 hours (284% higher than control levels) after SE onset (Figure 1).
Fig 1. Time-dependent alterations in p75NTR protein levels following pilocarpine-induced SE.
Western blot of protein homogenates from dentate gyrus of rats after SE probed with anti-p75NTR and anti-Actin antibodies. Although p75NTR protein levels decreased 6 hours after SE onset, Western Blot analyses demonstrate p75NTR levels increase relative to controls (i.e., rats treated with subconvulsive doses of pilocarpine) 24 hours after SE onset and significantly increase 96 hours (*, p=0.0002) after SE onset (CTL, n=5; SE at 6 hours, n=4; SE at 24 hours, n=4; SE at 96 hours, n=4). The ratio of p75NTR / Actin was expressed as percentage relative to mean values of control (CTL) group. Error bars, mean ± S.E.M. Statistically significant differences (p<0.05) were determined via a one-way ANOVA and Tukey’s post-hoc test for multiple comparisons.
LM11A-31 is blood-brain permeable
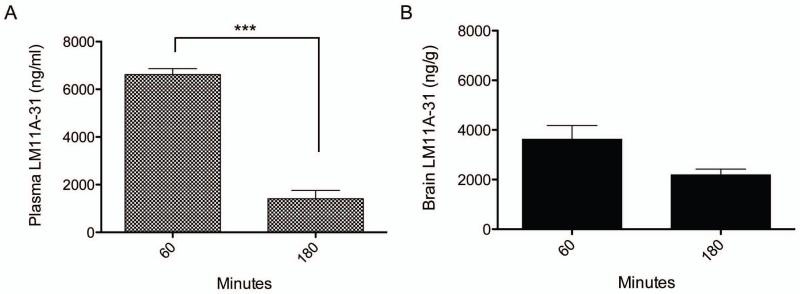
The goal of this study was to determine if p75NTR modulation by LM11A-31 administered prior to and at onset of SE would prevent SE-induced neurodegeneration and later seizures. An essential first step was to determine if LM11A-31 crossed the blood brain barrier following intraperitoneal injection in animals undergoing SE. Two doses of 200 mg/kg LM11A-31 were administered: the first with pilocarpine and prior to onset of SE, and the second immediately after the onset of SE. To assess the brain and plasma concentrations, rats were sacrificed 60 minutes and 3 h after the second dose. The peak brain concentration observed at 60 minutes was 3625.00 ± 958.63 ng/g, greater than 10 times the peak concentrations in mice receiving 50 mg/kg orally (~262.2 ng/g), which has been reported to be neuroprotective in an Alzheimer’s mouse model (Knowles et al., 2013). There was significantly less LM11A-31 present in the plasma 3 h after SE and also a lower brain concentration of LM11A-31 at 3 h (2191.67 ± 401.29 ng/g) when compared to levels 1 h after SE (Figure 2), consistent with the ~1 hour plasma half-life of the compound found previously in mice (Knowles et al., 2013). The brain-to-plasma ratio for the 1 h time point is 0.55 ± 0.16, and improves to >1 or 1.78 ± 0.82 at 3h (similar to brain-to-plasma ratios described in studies of normal mice [Knowles et al., 2013] and a favorable profile for CNS uptake). High doses of LM11A-31 were used to overcome the rapid metabolism occurring during recurrent seizures of pilocarpine-induced SE, and this may account for the initial high plasma and brain concentrations using this treatment paradigm. Within the first 24 h of SE, a 35% mortality incidence was measured in LM11A-31-treated rats relative to 31.25% mortality in vehicle-treated rats (across all experiments), expected attrition rates for the pilocarpine-induced model of temporal lobe epilepsy and not statistically different between groups.
Fig 2. LM11A-31 concentrations peak early in Plasma (A) and Cortex (B) of rats undergoing status epilepticus and are maintained for at least 3 h.
LM11A-31 concentrations were measured 1 h and 3 h after SE onset. LM11A-31 levels were lower in both plasma (1 h, n=3; 3 h, n=3; p<0.001) and cortex (1 h, n=3; 3 h, n=3) 3 hours after SE when compared to levels 1 h after SE. Statistically significant differences (p<0.05) were determined using a Student’s t-test. Error bars, mean ± S.E.M. Cortex and plasma was harvested from animals receiving 200 mg/kg of LM11A-31 with pilocarpine administration and a second 200 mg/kg LM11A-31 at onset of SE. Rats were sacrificed 1 h and 3 h after SE onset (i.e., 1 h and 3 h after second LM11A-31 dose) and results demonstrate blood-brain permeability, and brain and plasma concentrations that have been previously demonstrated as therapeutic in both in vitro and in vivo models of neurodegenerative diseases (Knowles et al., 2013). Error bars, mean ± SEM.
Early treatment with LM11A-31 does not alter electrographic SE
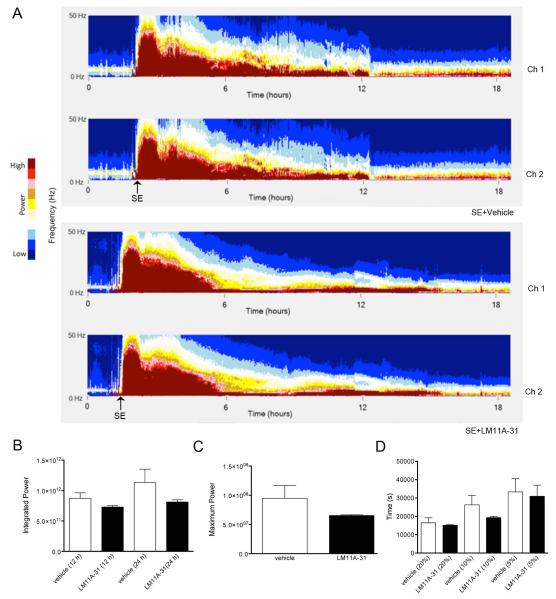
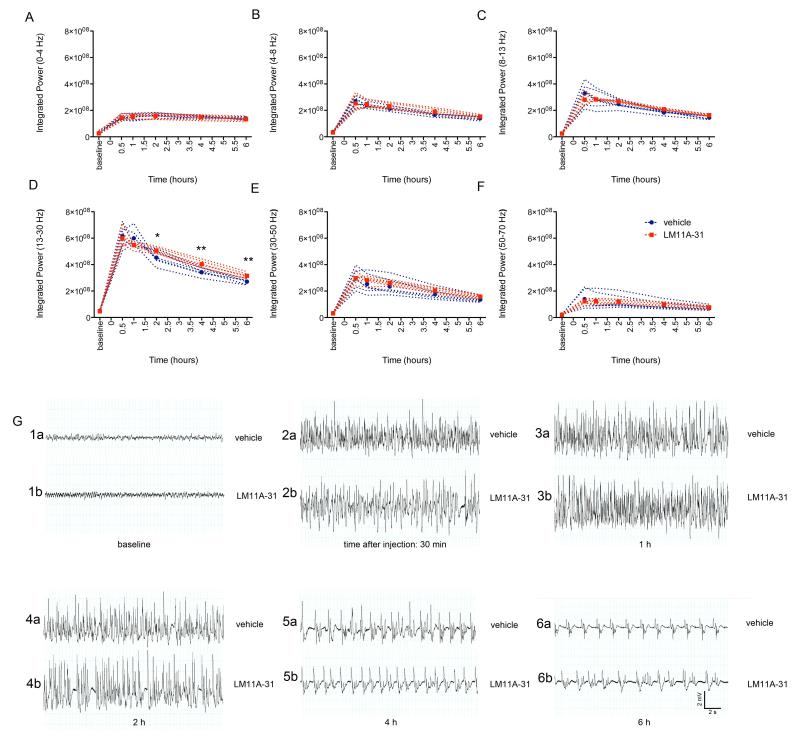
As the administration paradigm involved treatment with LM11A-31 prior to and at onset of SE, it was essential to analyze for any effects of LM11A-31 administration on the severity of SE that might impact subsequent cell loss or spontaneous seizure development. An injection of 200 mg/kg of LM11A-31 was given i.p. prior to and at onset of SE as described above. LM11A-31- and vehicle-treated rats were continuously video-EEG monitored during and after pilocarpine-induced SE for 48 hours. LM11A-31-treated rats did not demonstrate a significant difference in mean power (0 - 50 Hz, Figure 3A), integrated power 12 or 24 hours after SE onset (Figure 3B), nor maximum power (Figure 3C, all measures of the severity of SE) relative to vehicle-treated rats. No significant change was observed in the time required for power to degrade to 20%, 10%, and 5% of maximum power levels (a measure of SE duration) following LM11A-31 treatment (Figure 3D). These data suggest that LM11A-31 administered prior to and at onset of SE does not alter the severity, magnitude, nor duration of subsequent SE utilizing a simple, quantitative analysis of SE. However, 83.3% (5/6) of rats pre-treated with LM11A-31 required a second administration of pilocarpine to induce SE compared to only 42.9% (3/7) rats pre-treated with vehicle, and a trend towards a longer latency to onset of SE was observed in animals treated with LM11A-31 (92.8 ± 41.8 min.) relative to those treated with vehicle (56.0 ± 31.4 min.), but these differences were not significant. Discrete effects of the drug on specific bandwidths or at acute time points, however, could potentially be masked utilizing the analysis of power within the broad 0-50 Hz bandwidth for the first 48 h following SE. Additionally, reduced p75NTR function has been associated with impaired long-term depression, increased long-term potentiation and increased spatial memory (Rosch et al., 2005; Barret et al., 2010), thus, a more detailed analysis of multiple time points (i.e., 30 min, 1 h, 2 h, 4 h, and 6 h) and power in multiple bandwidths (0-4 Hz, 4-8 Hz, 8-13 Hz, 13-30 Hz, 30-50 Hz, and 50-70 Hz) following injection of vehicle or high-dose LM11A-31 were also analyzed to determine if early p75NTR modulation altered electrographic indices of SE (Figure 4A-G). No significant differences were observed in integrated power demonstrated in the delta (0-4 Hz), theta (4-8 Hz), alpha (8-13 Hz), 30-50 Hz, or 50-70 Hz bandwidths within 30 min, 1 h, 2 h, 4 h, or 6 h epochs after LM11A-31 or vehicle administration. However, an increase in integrated power within the beta-gamma (13-30 Hz) bandwidth was observed 2 h (p<0.05), 4 h (p<0.01), and 6 h (p<0.01) after the second dose of LM11A-31 and the onset of SE, suggesting early p75NTR modulation may increase power in discrete EEG bandwidths, specifically 13-30 Hz, during SE, but does not affect overall induction of SE or severity of ongoing SE. Raw traces (0.3 Hz - 600 Hz) from LM11A-31-treated rats compared to those from vehicle-treated rats (Fig 4 G1a-6a and G1b-6b) at specific time points following administration do not demonstrate striking treatment-induced differences in the progression of early electrographic SE (i.e., at 30 min, 1 h, 2 h, 4 h, or 6 h).
Fig. 3. Effect of LM11A-31 on electrographic status epilepticus.
Rats were continuously video-EEG monitored during and after pilocarpine-induced SE to conduct quantitative analyses of electrographic SE. LM11A-31 (200 mg/kg) or vehicle was injected i.p. after pilocarpine administration and again at the onset of SE. (A) Representative compressed spectral analyses (CSAs) are shown for the first ~19 hours following start of recording (including the time of pilocarpine administration). CSAs demonstrate magnitude power in low frequencies (0 - 50 Hz) over time following the onset of SE in animals implanted with 2 cortical EEG-recording electrodes treated with either vehicle (top 2 spectrographs) or LM11A-31 (bottom 2 spectrographs). Color map represents intensity of power graded from dark blue (lower magnitude) to dark red (higher magnitude). Each pixel in the x direction represents approximately 32 seconds.
(B) Progression of SE shown by integrated power quantified at 12 h and 24 h after the onset of SE. No significant differences were noted between DMSO-treated and LM11A-31-treated rats during the progression of SE. Maximum power (C) and the degradation of maximum power to 20%, 10%, and 5% of the level measured as maximum power (D) were quantified to evaluate the intensity and duration of SE. No significant differences were detected between treatment and vehicle groups. (SE+LM11A-31, n=3; SE+vehicle, n=4). Error bars, ±SEM.
Fig 4. Acute effects of LM11A-31 on discrete EEG bandwidths during SE.
Integrated power was measured for baseline periods prior to induction of SE, and periods 30 min, 1 h, 2 h, 4 h, and 6 h after onset of SE and the second administration of LM11A-31 (200 mg/kg with pilocarpine administration and a second dose of 200 mg/kg at SE onset) or vehicle for (A) delta (0-4 Hz), (B) theta (4-8 Hz), (C) alpha (8-13 Hz), (D) 13-30 Hz, (E) 30-50 Hz, and (F) 50-70 Hz bandwidths. Blue symbols and dotted lines, individual vehicle-treated rats, n=3. Red symbols and dotted lines, individual LM11A-31-treated rats, n=4. Significant increases in power were observed in the 13-30 Hz bandwidth 2 h (p<0.05), 4 h (p<0.001), and 6h (P<0.001) following LM11A-31 drug administration relative to vehicle treatment. Potential significant differences were evaluated using a one-way ANOVA with Tukey’s test for multiple comparisons. Representative traces sampled from a vehicle-treated rat (G1a-6a) and LM11A-31-treated rat (G1b-6b) at baseline (1 h prior to scopolamine administration), and at 30 min, 1 h, 2 h, 4 h, and 6 h following drug or vehicle injection demonstrate no alteration in the progression of pilocarpine-induced electrographic SE by treatment (SE+vehicle, n=3 rats; SE+LM11A-31, n=4 rats). Error bars, ±SEM.
Early LM11A-31 administration does not prevent or lessen the severity of pilocarpine-induced epilepsy
A number of studies have examined changes in p75NTR and neurotrophin expression following recurrent seizures and the contribution of these changes to neuronal cell death (Roux et al., 1999; Volosin et al., 2008; Troy et al., 2002). While p75NTR-mediated apoptosis has been suggested to be a mediator of epileptogenesis, there have been no studies that investigate the role of p75NTR, its co-receptors, and associated ligands in the development of spontaneous seizures. P75NTR double knockout mice exhibit an increased susceptibility to intrahippocampal kainate-induced SE compared to wild-type mice. In the absence of p75 receptors, both ictal and interictal spiking (during SE) were increased suggesting enhanced neuronal excitability (Balosso et al., 2005). Early administration of high-dose LM11A-31, a p75NTR ligand, was used here to modulate neurotrophin binding to p75NTR during SE, and multiple measures of epileptogenesis were assessed using long-term, video-EEG monitoring (Figure 5). High-dose LM11A-31 did not affect the overall severity of eventual pilocarpine-induced epilepsy (Figure 5A), nor did it slow the progression of epileptogenesis (i.e., the daily seizure frequency over time; Figure 5B). One month after SE, LM11A-31-treated rats had a cumulative seizure frequency similar to vehicle-treated rats, and measures of seizure severity, including seizure duration (Figure 5C) and the proportion of convulsive vs. nonconvulsive seizures (Figure 5D) did not differ in LM11A-31-treated rats relative to vehicle-treated rats. Modulating p75NTR activation with early LM11A-31 administration also did not slow the onset of spontaneous seizures (Figure 5E) and no difference was detected in the percentage of seizure-free days over the course of the first two weeks following SE in LM11A-31-treated rats relative to vehicle-treated rats (Figure 5F). Collectively, these results suggest that high-dose LM11A-31 administered prior to and at onset of SE is not an effective anti-epileptogenic strategy. However, alternative treatment regimes incorporating chronic, multi-day administration with different timing, potentially initializing LM11A-31 treatment later in the progression of epileptogenesis, when P75NTR expression is higher (i.e., 72 – 96 h after SE when p75NTR is most abundant), need to be explored to better assess its utility in epilepsy and the role of P75NTR receptors in the generation of seizures.
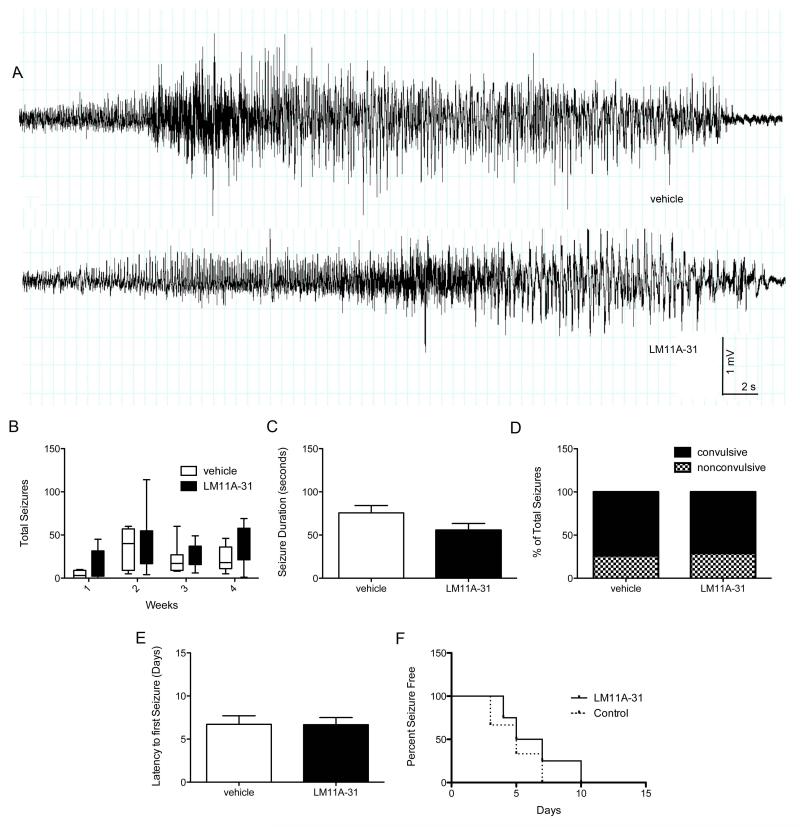
Fig 5. LM11A-31 does not prevent the onset of spontaneous seizures, slow the progression of epileptogenesis or reduce the severity of eventual epilepsy.
(A) Representative traces of a spontaneous, electrographic seizures in rats with pilocarpine-induced epilepsy. (B) The total seizures per week over the course of four weeks after SE demonstrates no difference in spontaneous seizure frequency over time after SE in vehicle-treated rats (n=7) relative to rats treated with 200 mg/kg LM11A-31 prior to SE and 200 mg/kg LM11A-31 at onset of SE (n=6). Potential statistically significant differences were determined using a one-way ANOVA with Tukey’s test for multiple comparisons. Box-and-whiskers plots. Upper and lower extremes of box are 75th and 25th percentiles. Whiskers extend from maximum value to minimum value. Line in box is plotted at median. (C) LM11A-31-treated rats do not demonstrate significantly different seizure durations or a (D) difference in the proportion of convulsive seizures (71.2%) relative to vehicle-treated rats (73.9%). Potential statistically significant differences in mean seizure duration were determined using a Student’s t-test and differences in the proportion of convulsive to non-convulsive seizures following LM11A-31 relative to vehicle treatment were determined using a Fischer’s exact test. (E) There is no difference in latency time to first spontaneous seizure after pilocarpine-induced SE in LM11A-31-treated rates relative to vehicle-treated rats. (F) LM11A-31 failed to reduce the “loss of seizure control” rate (i.e., the number of animals developing spontaneous seizures over time) when compared to vehicle-treated rats (LM, n=6; vehicle, n=6). Error bars, ±SEM for all graphs except (B and F).
Early LM11A-31 does not prevent SE-induced neuronal degeneration in the hippocampus
Following pilocarpine-induced SE, increased expression of p75NTR receptor has been shown to be co-expressed with TUNEL labeling demonstrating a potential link of the receptor and cell death (Roux et al., 1999). Further, in p75−/− mice, caspase-3 was not detected and a significantly lower number of dying neurons were observed (as evaluated by Fluoro-Jade B) relative to wild-type mice following SE suggesting that p75NTR-mediated cell death plays a role in neuronal loss following brain injury (Troy et al., 2002). Thus, histological experiments were conducted to determine whether early LM11A-31 administration prevents SE-induced neuronal cell injury in the hippocampus. Neuronal degeneration was evaluated using Fluoro-Jade B staining at 48 h after SE in the dentate hilus, CA1, and CA3 layers of the hippocampus. No difference in Fluoro-Jade B positive cell densities in any of the cell layers was found in rats treated with LM11A-31 compared to vehicle-treated rats (Figure 6). These results suggest that LM11A-31, at least administered using this particular protocol and dose, neither reduces the symptoms (i.e., the seizures) of epilepsy nor does it protect from seizure-induced neuronal cell loss.
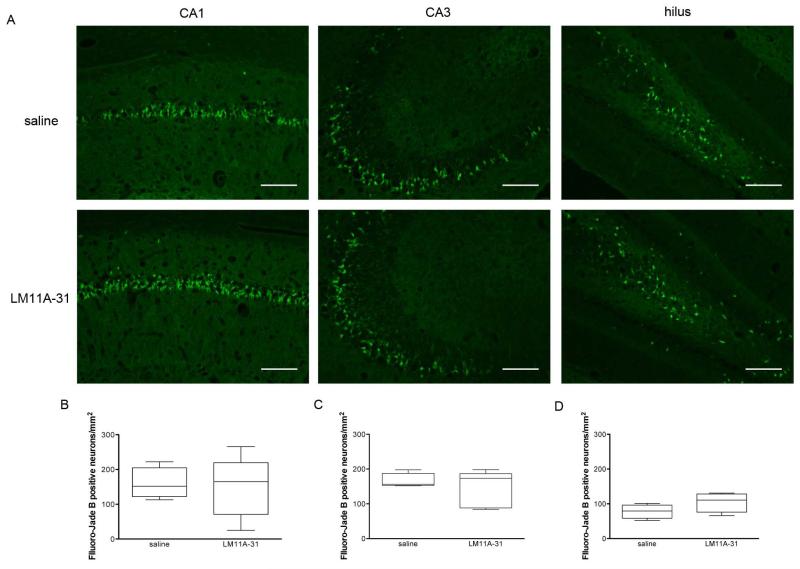
Fig 6. LM11A-31 does not reduce SE-induced neuronal cell death in hippocampus.
Representative images of Fluoro-Jade B (A-D) stained CA1, CA3, and dentate hilus subregions from a saline-treated (n=4) or LM11A-31-treated (n=5) rat 48 hours after SE and administration of either two doses of 200 mg/kg LM11A-31 or two doses of vehicle. Acquired using 10× objective (for A-D) and 40× objective (for E-F) on Nikon Eclipse TE2000-U fluorescent microscope. Calibration bars, 200 μm. Mean Fluoro-Jade B-positive neuronal cell densities (i.e., positively stained neuronal cells per mm2) averaged across a 1-in-20 series of serially sectioned and stained coronal sections (~ten 12-μm sections per rat) did not differ between LM11A-31-treated rats and saline-treated rats in (B) CA1, (C) CA3, or (D) dentate hilus. Potential statistically significant differences were determined using Mann-Whitney test. Box-and-whiskers plots, upper and lower extremes of box are 75th and 25th percentiles. Whiskers extend from maximum value to minimum value. Line in box is plotted at median.
Discussion
LM11A-31, administered as one 200 mg/kg i.p. dose prior to and a second 200 mg/kg dose at the onset of SE, is blood-brain permeable and reaches brain concentrations compatible with receptor modulation (Yang et al., 2008; Knowles et al., 2013), but does not reduce the magnitude of SE, nor the severity of subsequent epilepsy in an animal model of TLE. LM11A-31 was designed to specifically target p75NTR and prevents pro-neurotrophin-induced cell death (Massa et al., 2006; Tep et al., 2013), however it did not prevent acute pilocarpine-induced neuronal cell loss in the hippocampus when administered early relative to SE onset. These data do not support a role for acute phase administration of p75NTR ligands, like LM11A-31, in neuroprotection acutely following SE, nor for prevention of epilepsy development after prolonged seizures. However, it remains unknown if p75NTR modulators may have utility if they are administered at additional time points following SE or following other forms of brain injury such as TBI and hypoxia that are also associated with p75NTR receptor increases, and can lead to epilepsy with associated cognitive deficits.
Potential role of increased p75NTR and neuronal cell death in epileptogenesis
P75NTR-positive labeled neurons exhibit caspase-9, -6, and -3 activation and chromatin condensation following pilocarpine-induced SE (Troy et al., 2002), evidence of seizure-induced apoptotic signaling. An antibody to proNGF infused unilaterally for 3 days into the hippocampus after SE reduced expression of cleaved caspase-3 and Fluoro-Jade B-positive neurons in the hippocampus compared to control antibody infused into the contralateral side (Volosin et al. 2008). The lack of cleaved caspase-3 in hippocampal neurons of p75NTR−/− mice following pilocarpine-induced SE and reduced Fluoro-Jade B-positive neuronal cell counts compared to wild-type mice suggests p75NTR activity plays a critical role in neuronal cell death following seizures (Troy et al., 2002). However, p75NTR−/− mice are also reported to have more seizures during SE than wild-type mice suggesting that, at least, seizure susceptibility increases in the constitutive absence of p75NTR receptor (Balosso et al., 2005). Thus, p75NTR receptor activation by pro-neurotrophins may be integral to cell death mechanisms following prolonged seizures, but may not be necessary for the development of spontaneous seizures. The potential role of p75NTR receptor activation in chronic seizure-induced neuronal loss in the hippocampus and elsewhere in the brain that may contribute to cognitive and psychological co-morbidities associated with TLE remains to be defined. Pro-neurotrophins are cleaved by serine proteases and matrix metalloproteases once outside of the cell, and the activity of many of these cleavage enzymes are altered after SE ([e.g., PC1, furin], Meyer et al., 1996), making it difficult to predict the relative effects of mature BDNF vs proBDNF, and their associated pro-survival vs pro-apoptotic signaling, in the progression of epileptogenesis following SE.
P75NTR activation: a balance between cell death and pro-survival signaling
Because p75NTR is associated with a number of pro-survival, cell death, and synaptic plasticity machinery, competitive binding of the LM11A-31 ligand may inhibit or activate pro-epileptogenic mechanisms. Whether the p75NTR receptor promotes degenerative or survival signaling is dependent on the type and concentration of ligand present, and the presence or absence of co-receptors (Barker, 2004; Dechant and Barde, 2002; Ibanez and Simi, 2012; Underwood and Couldon, 2008). Unsain and co-authors (2008) suggested that the time-dependent increase in p75NTR protein expression (see Figure 1) occurs in parallel with a decrease in TrkB protein expression in hippocampus and precedes Fluoro-Jade B detection of neuronal injury in the dentate hilus or CA1 cell body layers, potentially promoting the onset of spontaneous seizures. When ligands for both receptors are present (as they are following status), it has been suggested that p75NTR-mediated apoptotic signaling will suppress the ability of BDNF to activate trkB-dependent survival mechanisms, but the inverse is not true. Treatment with BDNF does not suppress the ability of pro-neurotrophins to elicit p75NTR-mediated apoptosis (Volosin et al., 2006). The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), mitogen activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (Erk) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathways, have each been shown to contribute to pro-epileptogenic mechanisms, are key components of p75NTR-induced, pro-survival signaling (Massa et al., 2006; Volosin, 2006). P75NTR may be up-regulated in both neurons and glia after CNS injury (e.g., cortical axotomy and stroke [Harrington et al., 2004; Andsberg et al., 2001]), thus, modulation of the receptor using LM11A-31 may have different functional consequences depending on the injury incurred and potential pathological processes involved. Intranasal LM11A-31 administration following controlled cortical impact (10 minutes after injury and once daily for up to 14 days with 6 μl of 33.3 μM LM11A-31 in water) reduced microglial activation, improved progenitor cell survival and proliferation, promoted neurogenesis, and reduced neuronal cell loss (in total, preserving hippocampal structure), and thus, improved injury-induced deficits in spatial memory (Shi et al., 2013). Specifically, Fluoro-Jade B-positive neuronal staining was reduced in the injured cortex and in hippocampal pyramidal cells 24 h after injury. Oral administration of LM11A-31 (50 mg/kg/day for 2.5 - 3 months) to a mouse model of Alzheimer’s disease (the APPL/S mouse) prevented novel object recognition and Y-maze deficits, and reduced hippocampal and cortical neurite degeneration (Knowles et al., 2013). In the current study, systemic, high-dose, administration of two doses of LM11A-31 prior to and at onset of pilocarpine-induced SE did not reduce acute neuronal loss in the hippocampus (as measured by Fluoro-Jade B) nor did it significantly alter the severity of electrographic SE nor subsequent development of spontaneous seizures. This suggests that the manner in which the ligand is administered, including dosage, timing, duration of treatment and administration route combined with the nature of the brain injury influences the efficacy of LM11A-31. Considering the demonstrated beneficial effects of chronic administration of LM11A-31 in animal models of traumatic brain injury and Alzheimer’s disease, future studies of the utility of LM11A-31 in epilepsy should likely include longer periods of administration after the inciting injury and/or examination of the effects of chronic administration on progression of epilepsy after it develops.
P75NTR as a target to treat co-morbidities of epilepsy
The p75NTR receptor has been implicated in activity-dependent synaptic plasticity and in learning and memory deficits suggesting that modulation of the neurotrophin signaling via LM11A-31 could be a beneficial adjunctive treatment to ameliorate adverse cognitive symptoms associated with TLE. In two different strains of mice carrying mutations of the p75NTR gene, hippocampal long-term depression was reduced (Rosch, 2005). The p75NTR knockout mice had enhanced long-term potentiation in the Schaffer collateral fiber synapses of the hippocampus, displayed markedly superior learning in the Barnes maze, and exhibited increased stress (e.g., freezing) in the open-field test relative to control animals (Barrett, 2010). Bath application of LM11A-31 rescues amyloid-beta-induced CA1 long-term potentiation impairment in hippocampal slice experiments (Yang et al., 2008). Based on the gradual increase in p75NTR receptor protein expression after SE (figure 1), a delayed and/or more prolonged period of treatment with LM11A-31 after SE than the one used in the current study may be more appropriate to prevent SE-induced, P75NTR-dependent neuronal loss and potential cognitive deficits. For example, a study utilizing chronic dosing of LM11A-31 to chronically epileptic rats with spontaneous seizures and cognitive deficits to determine the long-term effects on learning, memory and seizure frequency may provide more promising results than those found here after only acute administration at the time of SE. The dosage and timing of the drug administration needs to be carefully designed based on seizure-induced changes in neurotrophins, p75NTR receptor expression and expression of its co-receptors so as to balance the downstream pro-survival and pro-apoptotic mechanisms and potential adverse consequences on disease pathogenesis. Future studies using chronic administration of LM11A-31 after SE and throughout epileptogenesis and after the onset of spontaneous seizures are thus required to fully evaluate its potential efficacy for the prevention of long-term neurodegeneration and associated memory deficits in epilepsy.
Acknowledgements
These studies were funded by NIH, National Institute of Neurological Disorders and Stroke R01NS051710 (to ABK and SJR), Citizens United for Research in Epilepsy [(CURE) to ABK and SJR], Epilepsy Foundation (to HLG), and Jean Perkins Foundation (FML). COI to disclose: Dr. Longo is a founder of PharmatrophiX, a company focused on the development of neurotrophin receptor ligands. The authors would like to thank the University of Colorado Neurophysiology Core for assistance related to EEG monitoring.
Funded by: NIH, National Institute of Neurological Disorders and Stroke R01NS051710 (to ABK and SJR), Citizens United for Research in Epilepsy [(CURE) to ABK and SJR], Epilepsy Foundation (to HLG), and Jean Perkins Foundation (FML).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- proBDNF
proneurotrophin, precursor form of BDNF
- SE
status epilepticus
- p75NTR
p75 neurotrophin receptor
- TrkB
tropomyosin related kinase receptor
- NGF
nerve growth factor
- proNGF
proneurotrophin, precursor form of BDNF
- TLE
temporal lobe epilepsy
References
- Aicardi J, Chevrie JJ. Consequences of status epilepticus in infants and children. Adv Neurol. 1983;34:115–125. [PubMed] [Google Scholar]
- Andsberg G, Kokaia Z, Lindvall O. Upregulation of p75 neurotrophin receptor after stroke in mice does not contribute to differential vulnerability of striatal neurons. Exp Neurol. 2001;169(2):351–363. doi: 10.1006/exnr.2001.7646. [DOI] [PubMed] [Google Scholar]
- Arzimanoglou A, Hirsch E, Nehlig A, Castelnau P, Gressens P, Pereira de Vasconcelos A. Epilepsy and neuroprotection: an illustrated review. Epileptic Disord. 2002;4(3):173–182. [PubMed] [Google Scholar]
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57(6):804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42(4):529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Barrett GL, Reid CA, Tsafoulis C, Zhu W, Williams DA, Paolini AG, Trieu J, Murphy M. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 20(1):145–152. doi: 10.1002/hipo.20598. [DOI] [PubMed] [Google Scholar]
- Bauer J, Burr W. Course of chronic focal epilepsy resistant to anticonvulsant treatment. Seizure. 2001;10(4):239–246. doi: 10.1053/seiz.2000.0499. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Repressa A, Tremblay E, Nitecka L. Selective and non-selective seizure related brain damage produced by kainic acid. Adv Exp Med Biol. 1986;203:647–657. doi: 10.1007/978-1-4684-7971-3_49. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Ernfors P, Kokaia M, Leanza G, Nilsson OG, Persson H, Lindvall O. Regulation of neurotrophin and trkA, trkB and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 1993;53(2):433–446. doi: 10.1016/0306-4522(93)90207-v. [DOI] [PubMed] [Google Scholar]
- Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, Bazil C, Pacia SV, Spencer SS. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60(2):186–190. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32(6):778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33(1):9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5(11):1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 2012;25(2):266–276. doi: 10.1016/j.yebeh.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstatter HL, Del Angel YC, Carlsen J, Wempe MF, White AM, Cogswell M, Russek SJ, Brooks-Kayal AR. The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis. 2013;62C:73–85. doi: 10.1016/j.nbd.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greferath U, Trieu J, Barrett GL. The p75 neurotrophin receptor has nonapoptotic antineurotrophic actions in the basal forebrain. J Neurosci Res. 90(1):278–287. doi: 10.1002/jnr.22735. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101(16):6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C, Wetmore C, Olson L. Regulation of brain-derived neurotrophic factor messenger RNA and protein at the cellular level in pentylenetetrazol-induced epileptic seizures. Neuroscience. 1993;53(4):909–918. doi: 10.1016/0306-4522(93)90476-v. [DOI] [PubMed] [Google Scholar]
- Ibanez CF, Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 35(7):431–440. doi: 10.1016/j.tins.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6(6):937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Knowles JK, Simmons DA, Nguyen TV, Vander Griend L, Xie Y, Zhang H, Yang T, Pollak J, Chang T, Arancio O, Buckwalter MS, Wyss-Coray T, Massa SM, Longo FM. A small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol Aging. 2013;34(8):2052–2063. doi: 10.1016/j.neurobiolaging.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo FM, Massa SM. Small molecule modulation of p75 neurotrophin receptor functions. CNS Neurol Disord Drug Targets. 2008;7(1):63–70. doi: 10.2174/187152708783885093. [DOI] [PubMed] [Google Scholar]
- Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov. 2013;12(7):507–525. doi: 10.1038/nrd4024. [DOI] [PubMed] [Google Scholar]
- Massa SM, Xie Y, Yang T, Harrington AW, Kim ML, Yoon SO, Kraemer R, Moore LA, Hempstead BL, Longo FM. Small, nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. J Neurosci. 2006;26(20):5288–5300. doi: 10.1523/JNEUROSCI.3547-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Chretien P, Massicotte G, Sargent C, Chretien M, Marcinkiewicz M. Kainic acid increases the expression of the prohormone convertases furin and PC1 in the mouse hippocampus. Brain Res. 1996;732(1-2):121–132. doi: 10.1016/0006-8993(96)00502-1. [DOI] [PubMed] [Google Scholar]
- Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanisms of epileptic brain damage. Adv Neurol. 1986;44:857–877. [PubMed] [Google Scholar]
- Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26(1):115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Pollard H, Ben-Ari Y. Cell death, gliosis, and synaptic remodeling in the hippocampus of epileptic rats. J Neurobiol. 1995;26(3):413–425. doi: 10.1002/neu.480260313. [DOI] [PubMed] [Google Scholar]
- Rosch H, Schweigreiter R, Bonhoeffer T, Barde YA, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci U S A. 2005;102(20):7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19(16):6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpaa M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain. 2006;129(Pt 3):617–624. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- Tep C, Lim TH, Ko PO, Getahun S, Ryu JC, Goettl VM, Massa SM, Basso M, Longo FM, Yoon SO. Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J Neurosci. 2013;33(2):397–410. doi: 10.1523/JNEUROSCI.0399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem. 2002;277(37):34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Turski L, Cavalheiro EA, Czuczwar SJ, Turski WA, Kleinrok Z. The seizures induced by pilocarpine: behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm. 1987;39(5):545–555. [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9(3):315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Underwood CK, Coulson EJ. The p75 neurotrophin receptor. Int J Biochem Cell Biol. 2008;40(9):1664–1668. doi: 10.1016/j.biocel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Unsain N, Nunez N, Anastasia A, Masco DH. Status epilepticus induces a TrkB to p75 neurotrophin receptor switch and increases brain-derived neurotrophic factor interaction with p75 neurotrophin receptor: an initial event in neuronal injury induction. Neuroscience. 2008;154(3):978–993. doi: 10.1016/j.neuroscience.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26(29):7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Knowles JK, Lu Q, Zhang H, Arancio O, Moore LA, Chang T, Wang Q, Andreasson K, Rajadas J, Fuller GG, Xie Y, Massa SM, Longo FM. Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS One. 2008;3(11):e3604. doi: 10.1371/journal.pone.0003604. [DOI] [PMC free article] [PubMed] [Google Scholar]