Abstract
Previous examination in a small number of individuals with Williams syndrome (also referred to as Williams-Beuren syndrome) has shown subtly softer skin and reduced deposition of elastin, an elastic matrix protein important in tissue recoil. No quantitative information about skin elasticity in individuals with Williams syndrome is available; nor has there been a complete report of dermatologic findings in this population. To fill this knowledge gap, 94 patients with Williams syndrome aged 7-50 years were recruited as part of the Skin and Vascular Elasticity (WS-SAVE) study. They underwent either a clinical dermatologic assessment by trained dermatologists (2010 WSA family meeting) or measurement of biomechanical properties of the skin with the DermaLab™ suction cup (2012 WSA family meeting). Clinical assessment confirmed that soft skin is common in this population (83%), as is premature graying of the hair (80% of those 20 years or older), while wrinkles (92%) and abnormal scarring (33%) were detected in larger than expected proportions. Biomechanical studies detected statistically significant differences in dP (the pressure required to lift the skin), dT (the time required to raise the skin through a prescribed gradient), VE (viscoelasticity) and E (Young’s modulus) relative to matched controls. The RT (retraction time) also trended longer but was not significant. The biomechanical differences noted in these patients did not correlate with the presence of vascular defects also attributable to elastin insufficiency (vascular stiffness, hypertension, and arterial stenosis) suggesting the presence of tissue specific modifiers that modulate the impact of elastin insufficiency in each tissue.
Keywords: Williams Syndrome, elastin, skin wrinkling, graying of hair, skin, biomechanics
INTRODUCTION
To provide both a competent barrier to external exposures and to allow movement of the body, the skin must be both strong and elastic. Tensile strength resists tearing of a tissue in response to a force while elasticity allows a tissue to return to its original shape once an applied force is removed. Two proteins, collagen and elastin, imbue human skin with these qualities. Collagen constitutes 77% of the fat-free dry weight of the skin and is responsible for its tensile strength, while elastin, makes up 4%, and provides elasticity to the skin [Hussain et al., 2013]. Collagen defects that decrease the functional ratio of collagen to elastin cause hyperelastic skin and poor wound healing in a family of conditions called Ehlers Danlos syndrome (EDS). Scars in individuals with classic EDS are widened and atrophic. Additionally, patients with EDS exhibit pathology associated with tissue instability, including lax joints, hernias, organ prolapse and rupture. Abnormal elastic fiber proteins, however, result in another family of conditions called cutis laxa. At least eleven subtypes of cutis laxa have been identified [Mohamed et al., 2011; Uitto et al., 2013], all of which are associated with loose, redundant skin that lacks recoil. Nondermal features such as emphysema, heart valve dysfunction and hernias have also been described [Mohamed et al., 2011; Roussin et al., 2011; Urban et al., 2005].
The most common cutis laxa subtype is autosomal dominant cutis laxa caused by mutations in the elastin (ELN) gene (ELN-ADCL, MIM #123700) [Callewaert et al., 2011; Rodriguez-Revenga et al., 2004; Tassabehji et al., 1998; Zhang et al., 1999]. Mutations (most commonly frameshift mutations) in the 3′ end of the ELN gene (exon 28 to the C-terminus) yield an abnormal protein with an aberrant C-terminus that multimerizes poorly, producing ultrastructurally abnormal fibers and decreased crosslinked elastin. Skin in these patients appears prematurely aged with poor recoil and excessive skin folds. Unlike individuals with EDS, patients with ADCL caused by ELN mutations are felt to have normal wound healing.
In contrast to the proposed dominant negative and gain of function effects in ADCL, ELN mutations (or deletions) that result in haploinsufficiency for the elastin protein lead to familial supravalvular aortic stenosis (SVAS, OMIM #185500)[Curran et al., 1993; Ewart et al., 1994; Li et al., 1997] a disorder in which affected individuals predominantly display vascular features with relatively few findings reported to date in other elastic tissues [Metcalfe et al., 2000; Urban et al., 2000a].
Individuals with a related disorder, Williams syndrome (Williams-Beuren syndrome, WS, OMIM #194050) have vascular abnormalities that phenotypically overlap with those in SVAS syndrome. WS, however, is a contiguous gene deletion syndrome caused by loss of 25-27 genes on chromosome 7q11.23, in addition to the loss of one copy of the elastin gene [Meng et al., 1998]. Given the loss of additional genes in WS, many non-vascular features are present, including characteristic facial dysmorphisms, a distinctive neurocognitive profile, and polyendocrinopathy [Pober, 2010]. Because of the prevalent morbidity and mortality associated with the vascular disease in this condition, there has been less analysis of the effects of elastin haploinsufficiency on other elastic tissues. Case reports have noted emphysema, bladder, and colonic diverticuli, [Ignacio et al., 2012; Partsch et al., 2005; Sammour et al., 2006; Schulman et al., 1996; Wan et al., 2010] features potentially attributable to elastin insufficiency, and two studies have commented on the skin findings in the condition. Urban et.al. [2000b] showed that individuals with WS (n=4) have subtly softer skin with mild changes in elastin deposition as demonstrated by electron microscopy, while Dridi et al., [1999] used light microscopy and immunofluorescence (n=10) to demonstrate abnormal volume and organization of skin elastic fibers. In addition, individual clinical reports have made note of early graying and soft skin patients with WS [Morris et al., 1988]. To date, however, neither quantitative assessment of cutaneous elasticity nor comprehensive clinical assessment of skin and integumentary findings in a large series of individuals with WS has been reported.
This study aims to define more fully the dermatologic phenotype in individuals with WS. Toward this end, we recruited individuals with a diagnosis of WS, aged 7 to 50, to participate in the Williams Syndrome-Skin and Vessel Elasticity (WS-SAVE) study. Qualitative assessment of the skin by trained dermatologists, and/or quantitative analysis of skin elasticity using a DermaLab™ suction cup device, were performed. The study identified biomechanical differences in skin of individuals with WS compared to matched controls and also identified a subpopulation of the participants with WS who have significantly less stiff skin than either the rest of the WS cohort or controls. In addition, we describe previously under-appreciated dermatologic features of this syndrome, at least some of which appear unrelated to the elastin insufficiency. We discuss the other WS deletion genes that may be responsible for these findings.
MATERIALS AND METHODS
Human subjects
Human studies were conducted in accordance with the Institutional Review Board protocols at Washington University School of Medicine (WUSM) and Massachusetts General Hospital. Subjects with WS were recruited either through the Williams Syndrome Patient & Clinical Research Registry (www.registry.williams-syndrome.org) or as walk-ins during successive Williams Syndrome Association Family Conventions (St. Louis, MO, July 2010 or Boston, MA, July 2012). All parents/guardians consented, while all cognitively able subjects assented. Parents completed a surgical and medical history questionnaire and signed a release for medical records including laboratory confirmation of the WS diagnosis. Those for whom molecular diagnosis was not obtainable from medical records had research genetic testing to confirm the WS diagnosis (according to methods previously reported for this cohort [Kozel et al., 2014]).
During the 2010 Family Convention, 50 participants with WS underwent a complete dermatologic evaluation by one of two trained dermatologists (Drs. Bayliss or Berk). Race was reported as follows: 39 white, one black/African American, and one white /Japanese ancestry. Review of photographs in the nine patients who did not answer this question showed white race in seven of nine and uncertain race in the remaining two. In terms of smoking, 0/25 reported “no” to current or past smoking; the remainder declined to answer, although based on the authors’ experiences the smoking prevalence is expected to be extremely low. During the 2012 Family Convention, 61 participants with WS underwent a series of biomechanical skin measurements using a DermaLab™ device. Study participation was open to any person with WS ≥ 7 yrs of age, attending either (or both) the 2010 and 2012 Conventions. Accordingly, 33 persons participated solely in the 2010 (dermatologic) portion of the study, 44 in only the 2012 (DermaLab) portion of the study, and 17 in both.
Controls: 111 adult and pediatric control subjects were recruited and consented solely for skin biomechanical (DermaLab) measurements from WUSM clinical and research populations. Controls (or parents if the participant was a minor) also answered a directed set of medical history questions. Controls were matched to patients with WS by age, gender and body mass index (BMI).
Dermatologic exam
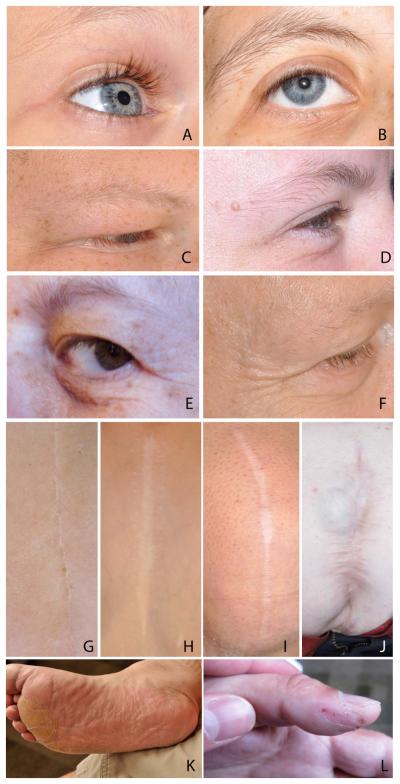
Participants’ hair, skin and nails were evaluated by trained dermatologists using a specifically designed examination checklist. With consent, photos were taken of representative findings. To assess for wrinkles and scars, photos were taken of the face and of any scars detected on exam. Photos were rated by three observers who trained together on sample images but were blinded to the other’s ratings on the WS set. Scars were rated as normal=0, mild widening=1, or severe widening and/or hernia=2 (See Fig 2,G-J for examples). Facial photos were assessed for wrinkles in three locations: forehead, peri-ocular region, and peri-oral region. Photos were scored as no wrinkling=0, mild wrinkling/creasing=1, or moderate or greater wrinkling/creasing=2 (See Fig 2, A-F for examples). Scores were averaged among the three raters and scores of 0.66-1.33 (at least two scorers rating the finding as mild) were used to denote mild findings and a score of 1.66 or higher (at least two scorers rating the finding as moderate/severe) was used to denote more severe features. In no case did the scores for a feature vary by more than one point among examiners.
Figure 2.
Skin findings in patients with WS. Photos showing progression of facial wrinkles with age. Participants aged 7 (A), 15 (B), 20 (C), 27 (D), 32 (E), and 50 years (F). Peri-ocular areas evaluated in this study included the crow’s feet area lateral to the eye and the under-eye area. Surgical scars ranging from normal spinal scar (G), to widened chest scar (H), widened knee scar (I) and widened abdominal scar with hernia (J). Exfoliative dermatitis of the foot (K) and skin picking on the thumb (L).
Biomechanical skin measurements
Quantitative skin measurements were obtained using the elasticity module for the Dermalab suction cup (Cortex Technology, Hadsund, Denmark). The suction cup was placed on the volar surface of each forearm midway between the wrist and the elbow using adhesive stickers, and measurements were taken from each side independently. Pressure was applied by the coupled vacuum in increasing increments to the small segment of skin under the suction cup, causing the skin to be lifted into the apparatus. When the skin crosses a light beam emitted at the base of the suction cup, P1 is recorded. The second pressure, P2, is recorded after the skin has moved 1.5 mm further into the cup, as detected by interference with a second light beam. This cycle is repeated two subsequent times. Data directly collected by this device include 1) dP, (the pressure required to lift the skin 1.5 mm into the device (P2-P1) measured in mBar), 2) dT, (the amount of time needed to raise the skin 1.5 mm using a prescribed pressure gradient, in milliseconds (ms)) and 3) RT, (retraction time, the amount of time required for the raised skin to return to flat, in ms). Calculations were then made for Young’s modulus (E: Δx = Ψ * dP1 * r4 / (E * s3), where Δx is skin displacement (0.0015 m (1.5 mm) for this probe), Ψ is a constant, r is the radius of the skin patch displaced and s is the thickness of the skin, estimated at 0.001 m (1 mm) and dP and viscoelasticity (VE= E/RTn where RTn is a normalized retraction time obtained by dividing RT with 260 ms, the average control underarm retraction time, also in MPa) using the assumption of constant skin thickness. Thus, E is proportional to dP and VE is proportional to dP/RT. For the findings presented here, data from the first pull were analyzed. Subsequent cycles showed similar trends but different magnitude. Measurements were excluded from analyses when right versus left arm measurements were significantly discrepant (e.g., when the left vs. right differed by a value greater than two standard deviations) as determined in the patients with WS or control population.
Statistical analysis
The WS vs control comparison for skin biomechanical properties: RT, dP, dT, E and VE were compared between matched WS and control participants using paired t-tests.
Correlation of skin biomechanical properties with vascular features of WS: To look for associations between biomechanical properties of the skin and vascular features of WS (stenosis, hypertension, elevated pulse wave velocity (a proxy for vascular stiffness)), t-tests were performed to evaluate for differences in RT, dP, dT, VE and Y in those with and without the feature. Presence or absence of vascular features (stenosis, hypertension, and pulse wave velocity) were determined as previously described [Kozel et al., 2014]. Individuals were classified as having elevated PWV when their measurement was >0.5 m/s faster than age-gender matched peers, and were excluded from analysis if taking anti-hypertensive medication due to the protective effect of these medications on PWV.
RESULTS
Demographic information for participants in WS cohort 1 (2010), WS cohort 2 (2012), and the control cohort are summarized in Table I.
Table I.
WS-SAVE Demographics in WS Subjects and Controls*
| WS subjects Cohort 1 (Family Convention, 2010) |
WS subjects Cohort 2 (Family Convention, 2012) |
Control subjects* |
|
|---|---|---|---|
| # Individuals participating |
50 | 61 | 111 |
| Molecular confirmation of WS diagnosis |
50 | 61 | N/A |
| # examined by study Dermatologist |
50 | 0 | 0 |
| # with quality Dermalab™ data |
N/A | 53 | 100 |
| Number successfully matched |
N/A | 50 | 50 |
| Age of studied participants Average± SD, range |
24.7 ± 12.1, 7-54 |
23.7 ± 11.1, 7-49 |
24.0 ± 11.5, 7-50 |
| Gender of studied/matched participants M:F |
22:28 | 21:29 | 21:29 |
| BMI of studied or matched participants |
24.9 ± 7.8 | 24.2 ± 6.9 | 24.0 ± 6.0 |
Only quantitative measure biomechanical skin properties performed on controls.
A. Family Convention, St Louis, MO., 2010 (Cohort-2010)
Fifty subjects aged 7-50 with WS underwent a complete examination of their hair, skin and scalp by a board-certified dermatologist. The overall prevalence of common scalp, skin, and hair findings in subjects participating in this portion of the study are noted in Table IIA and B, and are further described below.
Table IIa.
Hair and scalp findings in WS
| # with Feature /#Assessed |
Percentage | Comment | |
|---|---|---|---|
| Hair graying (>20 yrs old) |
26/32 | 81% | Oldest subject w/o gray hair =33 |
| Hair graying (<20 yrs old) |
3/18 | 17% | Youngest subject with gray hair =11 |
| Low frontal and/or posterior hairline |
32/50 | 64% | Slightly low in most cases |
| Scalp flakiness/dandruff/ seborrheic dermatitis |
32/50 | 64% | 30 current; + 2 with history. Most cases mild. |
| Soft hair texture | 11/50 | 22% |
Table IIb.
Skin and Nail Findings in WS
| # With feature/ # assessed |
Percentage | Comment | |
|---|---|---|---|
| Soft skin | 40/48 | 83% | |
| Presence of a Scar | 30/50 | 60% | 18/30 are surgical |
| Mildly abnormal scar if scar present |
15/30 | 50% | |
| Markedly abnormal scar if present |
10/30 | 33% | |
| Forehead wrinkling | 20/47 | 43% | |
| Peri-ocular wrinkling | 39/48 | 81% | |
| Peri-oral wrinkling | 30/49 | 61% | |
| Nail biting and/or skin picking |
21/50 | 42% | 19 current; + 2 with history. |
| Keratosis pilaris | 20/50 | 40% | Most cases mild |
| Atrophic areas of skin | 14/50 | 34% | |
| Stretch Marks | 10/50 | 20% | 4/10 with normal/low BMI |
| Dry skin / Hyperkeratosis | 10/50 | 20% | Most cases mild |
Hair and Scalp (Table IIA): Premature graying (Fig 1) was observed (or reported to be present in those who dyed their hair) in 58% of the total cohort. The prevalence increased with advancing age: 18% of those under 20 (3/18) had some amount of gray hair; the youngest person in our cohort with currently graying hair was 11 years old. Eighty-one percent of individuals 20 years of age or older (26/32) showed or reported some gray hair. The oldest individual without gray hair was 33 years old. Hair texture was abnormally soft in 22%. Hair distribution revealed a low posterior hairline in 58% while the hairline was anteriorly advanced in five individuals (10%). Abnormalities of the scalp, mild in most cases, were reported or observed in 64%; these included sebhorreic dermatitis, scalp flakiness, and/or dandruff.
Figure 1.
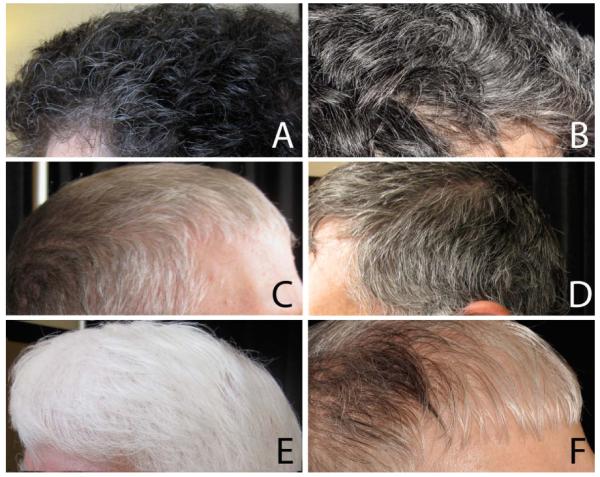
Graying hair in patients with WS. Photographs showing hair graying in WS individuals in their 30s (A and B), 40s (C and D), and 50s (E and F).
Skin and Nails (Table IIB): Eighty-three percent (40/48) of individuals were deemed to have soft skin (described as “doughy” or “babylike”) when clinically assessed. Facial wrinkling (Fig 2A-D) was noted in 46/50 participants on at least one portion of the face (forehead, periocular, or peri-oral) with occurrence of mild wrinkling as young as 7 years of age. However, the age of onset and severity of wrinkling varied based on location. When stratified by decade (Fig S1 in supporting information online), peri-ocular wrinkles were noted earliest, with mild wrinkling/creasing already present in 9/11 of 11-20 year olds. Mild peri-oral wrinkles were first seen in the 21-30 year age group, with 12/16 receiving a “mild’ score. These were noted to qualify as more moderate/severe by age 41-50 (i.e., all individuals in this age group received a “moderate/severe” score). Finally, forehead wrinkles were the last to appear, being initially detectable, on average, in the 31-40 year category (with seven of nine being classified as “mild”).
Ten of 50 patients (20%, aged 10-37 years) had stretch marks, mainly on the abdomen, hips and posterior arms. The BMI for these individuals ranged from 15-40; four of these ten had normal or low BMI. Atrophic lesions on the extremities were present in 34% and scars were noted in 30/50 (60%) of individuals, owing in large part to a history of surgery (19/30). Scars varied in appearance, ranging from normal (Fig 2E) to moderately splayed and disfigured (Fig 2F-H and Table IIb). Of note, scarring showed greater inter-individual than intra-individual variability (Fig S3 in supporting information).
Dry skin and/or hyperkeratosis were relatively common, present in 10/50 (20%). It was generally mild and patchy, though a few individuals displayed severe retention hyperkeratosis (Fig 2I). This finding was noted predominantly on the extremities, especially on the feet, but other skin regions such as the trunk and neck were occasionally affected. Keratosis pilaris was noted in 40% of WS participants, most commonly mild and affecting the extremities.
Nail structure was normal in the majority of individuals (only one showed hypoplastic nails and two had nail ridging). However, 42% of individuals displayed evidence of, or gave a history of, nail biting or periungual skin-picking (Fig 2J). Onychodystrophy was noted in 22%.
B. Family Convention, Boston, MA, 2012 (Cohort-2012)
Skin Elasticity: Sixty-one individuals with WS, aged 7-49, were consented for skin elasticity testing as part of the WS-SAVE study. Eleven WS subjects were excluded from analyses for the following reasons: five were unable to tolerate the noise generated by Dermalab™ elasticity suction cup evaluation; three had significantly discrepant readings between their left and right arms; and three had BMIs >42 which precluded successful matching to a member of our control cohort. Among our control cohort of 111 individuals, 11 were excluded due to significantly discrepant left vs. right arm measurements.
Final analyses were therefore performed on 50 subjects with WS (21 male and 29 female), each matched to one control. After matching, there were no statistically significant differences in age or BMI between patients with WS and controls (data not shown), and all were gender-matched.
Individuals with WS demonstrate altered skin biomechanical characteristics
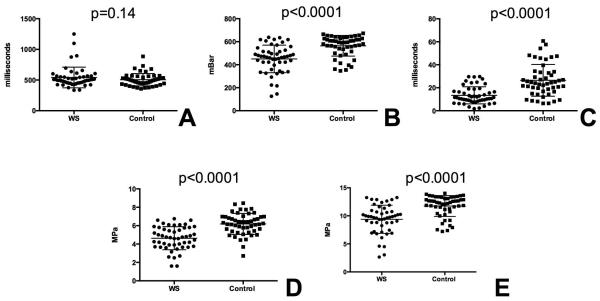
Subjects with WS differed significantly from matched controls in requiring less pressure to lift the skin, (dP, 450.4 ± 120.2 (WS) vs. 564.5 ± 89.6 (control), p<0.0001, Fig 3B) and decreased time to lift the skin through the prescribed pressure gradient (dT, 13.4 ± 7.4 (WS) vs 26.5± 13.8 (control), p<0.0001, Fig 3C). Though skin retraction time (RT) trended longer in participants with WS, the results were non-significant (541.4 ± 168.8 (WS) vs. 505.7 ± 100.5, p=0.14, Fig 3A). The calculated statistics VE and E were also altered, with with WS showing reduced viscoelasticity (VE, 4.6 ±1.3 vs. 6.2± 1.1, p<0.0001, Fig 3D) and a reduced Young’s modulus (E, 9.4±2.5 vs. 11.8± 1.9, p<0.0001, Fig 3E).
Figure 3.
Biomechanical characteristics of skin in patients with WS and matched controls. Measurements are shown for retraction time (RT, panel A), lift pressure (dP, panel B), lift time (dT, panel C), viscoelasticity (VE, panel D), and Young’s modulus (E, panel E). WS participants are noted as circles and controls are squares. The mean (long line) and SD (shorter line) are also noted. P values are recorded above each comparison.
For each biomechanical property, a subpopulation of participants with WS had skin mechanical parameters that were more extreme than the rest of the WS cohort and controls (Fig 3). For example, five patients with WS required markedly decreased force to lift their skin, while four additional subjects had markedly longer retraction time. These participants could not be distinguished from the other WS participants on the basis of gender, BMI, or age, suggesting the possibility of other genetic modifiers contributing to this variation.
Skin biomechanical properties do not predict severity of vascular disease in WS
We next compared biomechanical skin measurements between WS individuals who did and did not have cardiovascular features of the disease (hypertension, stenosis, and vascular stiffness (pulse wave velocity > 0.5 m/s faster than age and gender control norms)). No association between skin biomechanical properties and vascular features was detectable nor did p values trend in the direction of significance to suggest that a larger sample size would reveal detectable differences (see Figure S2 in supporting information online). Similarly, no correlation was identified between outlier status on any of the skin biomechanical measurements and the vascular features of WS (data not shown); however, this analysis is underpowered.
DISCUSSION
Elastic fibers contribute to the recoil capability of multiple tissues including the skin, blood vessels, and lung. Much is known about the effect of elastin haploinsufficiency on the vascular system in individuals with WS. Far less information is available about the skin in these individuals. Previous investigation on a small number of patients by Urban et al.,.[2000b] showed that patients with WS have subtly softer skin and some minor ultrastructural abnormalities of elastic fiber deposition. There has been no quantitative assessment of the biomechanical properties of skin in this population, nor has there been a complete description of the dermatologic features.
Our study confirmed the clinically reported findings of early graying of hair and soft skin. Hair graying is quite early, with all participants 34 or older reporting some gray hair. In fact, onset of graying occurred as early as 11 years of age. By contrast, only 40% of Caucasians without WS report some graying by age 40, with the average age of onset 34.2 ± 9.6 years [Keogh and Walsh, 1965].
Our dermatologists clinically appreciated “soft skin” in 83% of study subjects, independent of age. This finding corroborates previous mention of soft skin in the literature [Urban et al., 2000b]. It is unknown why elastin insufficiency would cause softer skin but it is possible that the decreased elastin leads to a functional increase, either as a result of frank up-regulation or simply due to relatively increased ratios of other matrix molecules, including glycosaminoglycans, that retain water in the tissue giving it a softer texture.
Nail biting and skin picking were common and are likely physical manifestations of the anxiety disorder that commonly complicates WS [Woodruff-Borden et al., 2010]. Other common dermatological conditions in this population included keratosis pilaris (20%) and excessive scalp flakiness and/or dandruff (64%). Increased sloughing of skin would not be predicted as a result of elastin insufficiency as the elastic fibers are present lower in the dermis. Similar skin and scalp changes have also been described in individuals with Down syndrome [Weijerman and de Winter, 2010] and may be a non-specific finding in individuals with cognitive impairment. However, the hyperkeratosis and scalp flakiness was severe in several individuals suggesting the possibility of a common underlying pathology.
Because these and other findings, such as premature graying of the hair, have not been described in individuals with isolated SVAS, we believe they are more likely to be associated with deletion of other genes within the WS critical region. In the general population, hair graying is thought to be caused by loss of melanocytes in the hair follicles [Nishimura et al., 2005]. The mechanism for graying, and early graying in particular, is unknown in WS but intriguingly, the gene BAZ1b (WSTF) has been implicated. BAZ1b is a chromatin remodeling factor expressed by multiple cell types. When knocked out in neural crest derivatives (such as melanocytes), the mice exhibit premature graying of their fur in addition to developmental and behavioral abnormalities [Liu et al., 2012]. Excessive skin turnover, on the other hand, may be related to changes in maturation of epidermal cells or abnormal connections between them. Other than elastin, WS-deletion genes expressed in the skin include the claudins (CLDN3 and CDN4), GTF2iRD1, LIMK1 and NCF1. The claudins, in particular, are interesting candidates for this phenotype. CLDN3 and CLDN4 are expressed by the keritinocytes of the epidermis and are components of tight junctions. In the skin, tight junctions form a barrier for electrolyte and water loss. In mouse models, knockdown Cldn4 changed skin permeability for certain ions and molecules and human studies have shown altered CLDN3 expression in psoriatic plaques [Kirschner et al., 2013; Watson et al., 2007]. No studies have investigated cell turnover in chronic CLDN3 or CLDN4 haploinsufficiency. Additional work will be needed to solidify a role for these proteins in the skin changes that we observed in WS.
Our report is the first to document variable skin scarring in WS, ranging from normal (5/30) or minimally abnormal scars (15/30) to markedly widened and irregular lesions (10/30). In the study participants who had undergone multiple surgical procedures, scars healed comparably (i.e., all scars were normal, or all were abnormal). These observations, plus identification of atrophic areas of skin in moderate numbers (17/50) of participants with WS, suggest subtle and variable abnormalities in wound healing in this population. The presence of facial wrinkling was noted in most participants (46/50), often starting in the second or third decade, but generally remained mild until the fifth decade (age 41-50 years). In contrast, studies evaluating the onset of wrinkling in Caucasian populations reported that mild peri-ocular wrinkling presented, on average, in the fifth decade [Daniell, 1971], three decades later than when it was noted in our WS cohort. Similarly, moderate to severe wrinkling is felt to be relatively rare in non-smoking individuals younger than 40 [Ernster et al., 1995]. However, our cohort showed moderate to severe wrinkling in at least one facial region in 7/11 of the 30-39 year olds. Though the craniofacial differences seen in patients with WS, such as the periocular fullness and micrognathia, may contribute to earlier onset wrinkling or drooping, its occurrence in WS also may reflect a mild end of the spectrum for elastic fiber-related skin disease.
Biomechanical testing showed that participants with WS required less pressure to lift the skin (dP) and that it took less time for the skin to be raised through a prescribed pressure gradient (dT). This suggests that skin in patients with WS stretches more readily when force is applied. Retraction times in patients with WS trend longer, with a few individuals with significantly longer measurements. Similarly, the calculated factors VE (viscoelasticity) and E (Young’s modulus) were both decreased in participants with WS. Viscoelasticity is defined as the tendency for a material to deform only briefly when a stress is applied and then removed quickly (elastic) but for the deformation to remain if the stress is prolonged (viscous)[Hussain et al., 2013]. Typically the skin is described as having viscoelastic properties, but our measurements show reduced viscoelasticity in WS skin relative to control skin. VE is proportional to the ratio of dP to RT. Consequently, the change seen in WS occurs predominantly due to decreased dP. The elastic modulus (E) reflects a deformed material’s ability to return to its original shape once the deforming force is removed [Hussain et al., 2013]. A lower elastic modulus is associated with lower stiffness in a material. Taken together, these features suggest decreased stiffness in the skin in WS compared to control skin. The biomechanical properties of the skin, however, do not correlate well with the vascular disease present in individual WS patients. This lack of correlation could be due to variations in tissue specific architecture including differences in elastic fiber organization, structure, and quantity as well as the composition of remaining extracellular matrix. Tissue specific alternative splicing of the elastin gene also likely plays a role [Sugitani et al., 2012].
Two previous studies have identified an association between age of onset for hair graying and risk for earlier onset cardiovascular disease in non-WS individuals [Erdogan et al., 2013; Kocaman et al., 2012]. Though it would be interesting to examine the presence or absence of this correlation in WS, the cross-sectional design of our study precluded this. Specifically, we do not have data on age of onset for features such as hair graying or skin wrinkling. Similarly, over-representation of surgical chest scars precluded unbiased analysis of scar healing and vascular disease. We recommend that future studies evaluate for an association between age of onset for these features and vascular pathology of WS.
In summary, this study broadens our understanding of the dermatologic features of Williams syndrome. We have quantified previously reported findings in WS such as soft skin and gray hair in a large cohort of individuals with WS. In addition, we have noted new findings related to altered appearance of wound healing and tissue aging in this condition. The biomechanical findings show reproducible differences between WS and control skin, highlighting the impact of elastin insufficiency on the mechanical properties of the skin. However, because the biomechanical properties of the skin are not well correlated with the vascular features of the condition, they cannot be presently be used as a proxy to predict or to monitor vascular disease severity individuals with elastin insufficiency.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Williams Syndrome Association and the families who participated in the WS-SAVE study.
GRANT SUPPORT Funding was provided to Dr. Kozel by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital. In addition, Dr. Kozel received funding for the study through her appointment as a scholar of the Child Health Research Center in Developmental Biology (NIH K12-HD01487) and the Genetic Basis of Inflammatory Airway Disease (NIH K12-HL089968).
Footnotes
CONFLICT OF INTEREST The authors have no conflicts of interest to report.
REFERENCES
- Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham RP, Loeys B, Coucke PJ, De Paepe A, Urban Z. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat. 2011;32:445–455. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- Daniell HW. Smoker’s wrinkles. A study in the epidemiology of “crow’s feet”. Annals of internal medicine. 1971;75:873–880. doi: 10.7326/0003-4819-75-6-873. [DOI] [PubMed] [Google Scholar]
- Dridi SM, Ghomrasseni S, Bonnet D, Aggoun Y, Vabres P, Bodemer C, Lyonnet S, de Prost Y, Fraitag S, Pellat B, Sidi D, Godeau G. Skin elastic fibers in Williams syndrome. Am J Med Genet. 1999;87:134–138. [PubMed] [Google Scholar]
- Erdogan T, Kocaman SA, Cetin M, Durakoglugil ME, Ugurlu Y, Sahin I, Canga A. Premature hair whitening is an independent predictor of carotid intima-media thickness in young and middle-aged men. Intern Med. 2013;52:29–36. doi: 10.2169/internalmedicine.52.7842. [DOI] [PubMed] [Google Scholar]
- Ernster VL, Grady D, Miike R, Black D, Selby J, Kerlikowske K. Facial wrinkling in men and women, by smoking status. American journal of public health. 1995;85:78–82. doi: 10.2105/ajph.85.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart AK, Jin W, Atkinson D, Morris CA, Keating MT. Supravalvular aortic stenosis associated with a deletion disrupting the elastin gene. J Clin Invest. 1994;93:1071–1077. doi: 10.1172/JCI117057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SH, Limthongkul B, Humphreys TR. The biomechanical properties of the skin. Dermatologic surgery : official publication for American Society for Dermatologic Surgery. 2013;39:193–203. doi: 10.1111/dsu.12095. [et al.,] [DOI] [PubMed] [Google Scholar]
- Ignacio RC, Jr., Klapheke WP, Stephen T, Bond S. Diverticulitis in a child with Williams syndrome: a case report and review of the literature. Journal of pediatric surgery. 2012;47:E33–35. doi: 10.1016/j.jpedsurg.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Keogh EV, Walsh RJ. Rate of greying of human hair. Nature. 1965;207(999):877–878. doi: 10.1038/207877a0. [DOI] [PubMed] [Google Scholar]
- Kirschner N, Rosenthal R, Furuse M, Moll I, Fromm M, Brandner JM. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol. 2013;133:1161–1169. doi: 10.1038/jid.2012.507. [DOI] [PubMed] [Google Scholar]
- Kocaman SA, Cetin M, Durakoglugil ME, Erdogan T, Canga A, Cicek Y, Dogan S, Sahin I, Satiroglu O, Bostan M. The degree of premature hair graying as an independent risk marker for coronary artery disease: a predictor of biological age rather than chronological age. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2012;12:457–463. doi: 10.5152/akd.2012.150. [DOI] [PubMed] [Google Scholar]
- Kozel BA, Danback JR, Waxler JL, Knutsen RH, De Las Fuentes L, Reusz GS, Kis E, Bhatt AB, Pober BR. Williams syndrome predisposes to increased vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension. 2014;63:74–79. doi: 10.1161/HYPERTENSIONAHA.113.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu T, Tahmasian M, Tseng Z, Kim J, Xiao A. Epigenetic Mechanisms in Neural Crest Cells Play a Critical Role in Preventing Williams Syndrome Development. Williams Syndrome Scientific Meeting; Boston, MA. 2012. [Google Scholar]
- Meng X, Lu X, Li Z, Green ED, Massa H, Trask BJ, Morris CA, Keating MT. Complete physical map of the common deletion region in Williams syndrome and identification and characterization of three novel genes. Hum Genet. 1998;103:590–599. doi: 10.1007/s004390050874. [DOI] [PubMed] [Google Scholar]
- Metcalfe K, Rucka AK, Smoot L, Hofstadler G, Tuzler G, McKeown P, Siu V, Rauch A, Dean J, Dennis N, Ellis I, Reardon W, Cytrynbaum C, Osborne L, Yates JR, Read AP, Donnai D, Tassabehji M. Elastin: mutational spectrum in supravalvular aortic stenosis. Eur J Hum Genet. 2000;8:955–963. doi: 10.1038/sj.ejhg.5200564. [DOI] [PubMed] [Google Scholar]
- Mohamed M, Kouwenberg D, Gardeitchik T, Kornak U, Wevers RA, Morava E. Metabolic cutis laxa syndromes. Journal of inherited metabolic disease. 2011;34:907–916. doi: 10.1007/s10545-011-9305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Demsey SA, Leonard CO, Dilts C, Blackburn BL. Natural history of Williams syndrome: physical characteristics. J Pediatr. 1988;113:318–326. doi: 10.1016/s0022-3476(88)80272-5. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307(5710):720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Partsch CJ, Siebert R, Caliebe A, Gosch A, Wessel A, Pankau R. Sigmoid diverticulitis in patients with Williams-Beuren syndrome: relatively high prevalence and high complication rate in young adults with the syndrome. Am J Med Genet A. 2005;137:52–54. doi: 10.1002/ajmg.a.30865. [DOI] [PubMed] [Google Scholar]
- Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Iranzo P, Badenas C, Puig S, Carrio A, Mila M. A novel elastin gene mutation resulting in an autosomal dominant form of cutis laxa. Arch Dermatol. 2004;140:1135–1139. doi: 10.1001/archderm.140.9.1135. [DOI] [PubMed] [Google Scholar]
- Roussin I, Sheppard MN, Rubens M, Kaddoura S, Pepper J, Mohiaddin RH. Cardiovascular complications of cutis laxa syndrome: successful diagnosis and surgical management. Circulation. 2011;124:100–102. doi: 10.1161/CIRCULATIONAHA.111.025056. [DOI] [PubMed] [Google Scholar]
- Sammour ZM, Gomes CM, Duarte RJ, Trigo-Rocha FE, Srougi M. Voiding dysfunction and the Williams-Beuren syndrome: a clinical and urodynamic investigation. J Urol. 2006;175:1472–1476. doi: 10.1016/S0022-5347(05)00666-X. [DOI] [PubMed] [Google Scholar]
- Schulman SL, Zderic S, Kaplan P. Increased prevalence of urinary symptoms and voiding dysfunction in Williams syndrome. J Pediatr. 1996;129:466–469. doi: 10.1016/s0022-3476(96)70086-0. [DOI] [PubMed] [Google Scholar]
- Sugitani H, Hirano E, Knutsen RH, Shifren A, Wagenseil JE, Ciliberto C, Kozel BA, Urban Z, Davis EC, Broekelmann TJ, Mecham RP. Alternative splicing and tissue-specific elastin misassembly act as biological modifiers of human elastin gene frame shift mutations associated with dominant cutis laxa. J Biol Chem. 2012 doi: 10.1074/jbc.M111.327940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Hurst J, Ashcroft GS, Kielty C, Wilmot C, Donnai D, Read AP, Jones CJ. An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa. Hum Mol Genet. 1998;7:1021–1028. doi: 10.1093/hmg/7.6.1021. [DOI] [PubMed] [Google Scholar]
- Uitto J, Li Q, Urban Z. The complexity of elastic fibre biogenesis in the skin--a perspective to the clinical heterogeneity of cutis laxa. Experimental dermatology. 2013;22:88–92. doi: 10.1111/exd.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban Z, Gao J, Pope FM, Davis EC. Autosomal dominant cutis laxa with severe lung disease: synthesis and matrix deposition of mutant tropoelastin. J Invest Dermatol. 2005;124:1193–1199. doi: 10.1111/j.0022-202X.2005.23758.x. [DOI] [PubMed] [Google Scholar]
- Urban Z, Michels VV, Thibodeau SN, Davis EC, Bonnefont JP, Munnich A, Eyskens B, Gewillig M, Devriendt K, Boyd CD. Isolated supravalvular aortic stenosis: functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum Genet. 2000a;106:577–588. doi: 10.1007/s004390000285. [DOI] [PubMed] [Google Scholar]
- Urban Z, Peyrol S, Plauchu H, Zabot MT, Lebwohl M, Schilling K, Green M, Boyd CD, Csiszar K. Elastin gene deletions in Williams syndrome patients result in altered deposition of elastic fibers in skin and a subclinical dermal phenotype. Pediatr Dermatol. 2000b;17:12–20. doi: 10.1046/j.1525-1470.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- Wan ES, Pober BR, Washko GR, Raby BA, Silverman EK. Pulmonary function and emphysema in Williams-Beuren syndrome. Am J Med Genet A. 2010;152A:653–656. doi: 10.1002/ajmg.a.33300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Poddar R, Walker JM, McGuill I, Hoare LM, Griffiths CE, O’Neill CA. Altered claudin expression is a feature of chronic plaque psoriasis. The Journal of pathology. 2007;212:450–458. doi: 10.1002/path.2200. [DOI] [PubMed] [Google Scholar]
- Weijerman ME, de Winter JP. Clinical practice. The care of children with Down syndrome. Eur J Pediatr. 2010;169:1445–1452. doi: 10.1007/s00431-010-1253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Borden J, Kistler DJ, Henderson DR, Crawford NA, Mervis CB. Longitudinal course of anxiety in children and adolescents with Williams syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:277–290. doi: 10.1002/ajmg.c.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MC, He L, Giro M, Yong SL, Tiller GE, Davidson JM. Cutis laxa arising from frameshift mutations in exon 30 of the elastin gene (ELN) J Biol Chem. 1999;274:981–986. doi: 10.1074/jbc.274.2.981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.