Abstract
Neutrophilic granulocytes are the most abundant type of myeloid cells and form an essential part of the innate immune system. In vertebrates the first neutrophils are thought to originate during primitive hematopoiesis, which precedes hematopoietic stem cell formation. In zebrafish embryos, it has been suggested that primitive neutrophils may originate in two distinct sites, the anterior (ALPM) and posterior lateral plate mesoderm (PLPM). An ETS-family transcription factor Etsrp/Etv2/ER71 has been implicated in vasculogenesis and hematopoiesis in multiple vertebrates. However, its role during neutrophil development is not well understood. Here we demonstrate using zebrafish embryos that Etv2 has a specific cell-autonomous function during primitive neutropoiesis in the anterior lateral plate mesoderm (ALPM) but has little effect on erythropoiesis or the posterior lateral plate mesoderm (PLPM) expression of neutrophil marker myeloperoxidase mpo/mpx. Our results argue that ALPM-derived neutrophils originate from etv2-expressing cells which downregulate etv2 during neutropoiesis. We further show that Scl functions downstream of Etv2 in anterior neutropoiesis. Additionally, we demonstrate that mpx expression within the PLPM overlaps with gata1 expression, potentially marking the cells with a dual myelo-erythroid potential. Intriguingly, initiation of mpx expression in the PLPM is dependent on gata1 but not etv2 function. Our results demonstrate that mpx expression is controlled differently in the ALPM and PLPM regions and describe novel roles for etv2 and gata1 during primitive neutropoiesis.
Keywords: etv2, etsrp, er71, gata1, neutrophil, primitive hematopoiesis, zebrafish, granulocytes, myeloid
Introduction
Neutrophils are the most abundant type of leukocyte in the body, and are an important component of the rapid non-specific innate immune response. Defects in the formation and differentiation of myeloid cells can lead to different disorders such as leukemia and neutropenia (Hall and Crosier, 2010; Lakshman and Finn, 2001). During embryogenesis, the first myeloid cells in different vertebrates such as mouse and zebrafish are thought to originate during primitive hematopoiesis, prior to the emergence of hematopoietic stem cells (Galloway and Zon, 2003; Xu et al., 2012). However, the relationships between the factors that control different cell fate decisions during primitive hematopoiesis are not well understood.
Zebrafish have recently emerged as an advantageous model to study hematopoietic development. Their large progeny numbers, external development and early transparency allows for in vivo observation of early hematopoietic processes (Bradbury, 2004). Zebrafish possess all of the major hematopoietic lineages that exist in mammalian species, and the genetic pathways directing differentiation of these blood cell types are highly conserved (Burns et al., 2009; Davidson and Zon, 2004). Similar to mammalian embryos, hematopoiesis in the zebrafish occurs in two successive, distinct waves: primitive and definitive hematopoiesis. Primitive hematopoiesis occurs in two anatomically separate regions, the anterior lateral plate mesoderm (ALPM) and the posterior lateral plate mesoderm (PLPM) (Galloway and Zon, 2003). Cells of the ALPM and PLPM co-express hematopoietic and vascular markers, such as scl, lmo2, gata2, fli1 and etv2 (Liao et al., 1998; Sumanas et al., 2005; Thompson et al., 1998). These cells have the potential to give rise to both blood and vascular endothelial cells, and are termed “hemangioblasts” (Vogeli et al., 2006). The ALPM forms the rostral blood island (RBI) and gives rise to macrophages, neutrophils, vascular endothelial and endocardial cells. The PLPM produces the intermediate cell mass (ICM) and generates erythroid, vascular endothelial cells and potentially neutrophils (Davidson and Zon, 2004; Hsia and Zon, 2005; Jin et al., 2012; Warga et al., 2009).
There has been some controversy regarding the origin of the earliest primitive neutrophils in a zebrafish embryo. Fate mapping approaches in two independent studies have demonstrated that the ALPM region gives rise to both macrophages and neutrophils (Jin et al., 2012; Le Guyader et al., 2008). However, it was also argued that neutrophils come exclusively from the PLPM region (Warga et al., 2009). During later embryonic stages neutrophils originate during the process of definitive hematopoiesis from hematopoietic stem cells (HSCs) or a recently identified population of erythromyeloid progenitors (EMPs) located within the posterior blood island (PBI) (Bertrand et al., 2007; Le Guyader et al., 2008; Murayama et al., 2006).
One of the most critical factors that regulate differentiation of multiple hematopoietic cell types is stem cell leukemia (scl/tal1). scl has been shown to be required for both erythroid and myeloid development and is expressed in the ALPM and PLPM starting at the 2-somite stage where it initiates hematopoietic and vascular endothelial progenitor (angioblast) development (Dooley et al., 2005; Liao et al., 1998; Patterson et al., 2005). In zebrafish, scl knockdown embryos fail to form erythrocytes, macrophages and neutrophils (Dooley et al., 2005; Patterson et al., 2005). In mice, scl is expressed in the blood islands of the yolk sac, and knockout embryos are embryonic lethal due to defects in primitive erythropoiesis (Robb et al., 1995; Shivdasani et al., 1995). Subsets of scl-expressing cells in both the ALPM and PLPM acquire a myeloid fate by expressing pu.1, an ETS-family transcription factor essential for myeloid development (Bennett et al., 2001; Lieschke et al., 2002). pu.1 knockdown zebrafish embryos show absence of neutrophils and macrophages, which could be rescued by injection of murine pu.1 mRNA (Rhodes et al., 2005). In mice, pu.1 is expressed exclusively in hematopoietic cells, and knockout mice lack monocytes, T and B lymphocytes, macrophages and neutrophils, however still possess erythroid cells (McKercher et al., 1996; Scott et al., 1994).
In all vertebrates, one of the most specific markers for neutrophil precursors is myeloperoxidase (mpx / mpo). In zebrafish, mpx expression is first initiated within the PLPM by the 20-somite stage (Bennett et al., 2001; Le Guyader et al., 2008; Lieschke et al., 2001; Warga et al., 2009). In the ICM, a subset of cells transiently co-express pu.1 and mpx right before 24 hpf, although not all pu.1 positive cells express mpx, and vice versa (Bennett et al., 2001; Herbomel et al., 1999; Lieschke et al., 2001). Shortly thereafter, a separate pool of mpx expressing cells is observed in the ALPM region by 22-24 hpf. Fate-mapping studies have argued that both ALPM and PLPM-derived cells contribute to neutropoiesis suggesting that mpx expression in both regions labels neutrophil progenitors (Jin et al., 2012; Le Guyader et al., 2008; Warga et al., 2009).
Another important hematopoietic transcription factor, gata1, is first expressed at the 5-somite stage in the PLPM, downstream of scl (Detrich et al., 1995). gata1-expressing cells give rise to erythroid cells. Zebrafish gata1 knockdown embryos display a loss of erythroid cells and an increase in neutrophils and macrophages at the embryonic stages past 24 hpf, after blood circulation has been established (Galloway et al., 2005; Rhodes et al., 2005). Mice null for gata1 die during gestation due to failure of erythrocyte precursors to differentiation into red blood cells (Fujiwara et al., 1996). Studies in mouse and zebrafish demonstrate that gata1 and pu.1 both repress expression of each other (Rhodes et al., 2005; Zhang et al., 1999).
Ets1-Related Protein (Etsrp/Etv2/ER71) was recently identified as a hemangioblast and vascular endothelial-cell-specific transcription factor being necessary and sufficient for vascular endothelial and myeloid cell development (Pham et al., 2007; Sumanas et al., 2008; Sumanas and Lin, 2006). etv2 is expressed within the putative vascular endothelial precursors in the zebrafish embryo starting at the 1-somite stage. Knockdown of etv2 causes a dramatic inhibition of vascular endothelial cell differentiation and loss of macrophages and neutrophils (Sumanas et al., 2008). Vascular endothelial and macrophage-specific scl expression is downregulated in the ALPM and PLPM region in etv2 morpholino (MO) knockdown embryos (morphants), while erythroid-specific scl expression in the PLPM is largely unaffected. Similar to zebrafish embryos, er71/etv2 knockout mouse embryos fail to undergo yolk sac vasculogenesis and exhibit defects in hematopoiesis (Ferdous et al., 2009; Lee et al., 2008). While previous studies have suggested that neutrophils in zebrafish are both ALPM and PLPM derived, it is currently not known if similar genetic pathways regulate formation of both pools of neutrophils and if Etv2 knockdown affects both populations of neutrophil progenitors. Most previous studies have focused on analysis of neutropoiesis after 24 hpf when the cells derived from both neutrophil populations are mixed (Dooley et al., 2005; Galloway et al., 2005; Jin et al., 2012; Sumanas et al., 2008)
In this study, we focused on the transcriptional regulation of neutropoiesis in the ALPM and PLPM regions. We demonstrate that etv2 has a critical cell-autonomous role during the primitive neutropoiesis in the ALPM region, but does not have a major role in initiating mpx expression in the PLPM. We show that scl functions downstream of etv2 in initiating neutropoiesis in the ALPM. We also demonstrate that gata1 initially has a supportive role during initiating mpx expression in the PLPM region, which later switches to an inhibitory role. Because genetic pathways that regulate early hematopoiesis are highly evolutionarily conserved, our findings will also promote understanding of neutropoiesis and hematopoiesis in mammalian embryos and may ultimately advance medical treatments for hematopoietic-related disorders.
Materials and Method
Zebrafish Strains
Embryos were raised and maintained at 28.5 oC under standard laboratory conditions (Westerfield, 2007). The following lines were used for experimental procedures: Tg(mpx:GFP)uwm1 (Mathias et al., 2006), Tg(etv2:GFP)ci1 (Proulx et al., 2010), etv2y11 (Pham et al., 2007) and Tg(etv2:Kaede)ci6 (Kohli et al., 2013). The etsrp/etv2y11 line was crossed into a Tg(fli1a:GFP)y1 (Lawson and Weinstein, 2002) background, and mutants were either identified prior to 24 hpf by lack of fli1a:GFP expression in the anterior head vasculature and in the endocardial cells fusing at the midline, or later at 24 hpf by lack of intersomitic vessels. etv2:Kaede embryos were exposed to UV light from a DAPI filter, photoconverting the Kaede protein from green to red, to allow for detection of mpx:GFP/etv2:Kaede positive cells.
Whole Mount In Situ Hybridization
In situ hybridization (ISH) was performed as previously described (Jowett, 1999). For the two-color in situ hybridization, the same protocol was followed except that embryos were incubated with 2 probes, a digoxigenin-UTP labeled probe, developed with NBT/BCIP and a fluorescein-UTP labeled probe, developed with Vector Red (Vector Laboratories). The following riboprobes were used: etsrp/etv2 (Sumanas et al., 2005),mpx (Bennett et al., 2001), scl (Liao et al., 1998), pu.1 (Lieschke et al., 2002), gata1 (Detrich et al., 1995), hbae3 (Brownlie et al., 2003), lyz (Liu and Wen, 2002), and irf8 (Li et al., 2011).
Immunohistochemistry following Whole Mount In Situ Hybridization
Embryos were fixed at the appropriate stage in 4% paraformaldehyde for 3-4 hours at 4°C, and then dehydrated and stored at −20°C until ISH was performed. On the last day of the ISH protocol, after development of probe, embryos were rinsed with PBT and then washed with acetone for 30 minutes at -20°C, followed by a 1 minute wash with water and 3-5 minute washes with 0.1% PBT. Embryos were blocked with MAB (100mM maleic acid, 150mM NaCl) and 2% blocking reagent (Roche Applied Science) for 1 hour at room temperature, and incubated overnight at 4°C in a 1:200 dilution of anti-GFP-Alexa488 (Invitrogen #A21311) in MAB and blocking buffer. Following six 15 minute washes in TNT (0.1M Tris-HCl, pH 7.5, 150mM NaCl, 0.1% Tween-20), a secondary antibody was used to amplify the GFP signal. Embryos were incubated in a 1:200 dilution of goat anti-rabbit-Alexa488 (Invitrogen #A11008) in MAB or TNT overnight at 4°C. Embryos were rinsed 3-4 times in TNT before imaging.
Real-Time RT-PCR
Embryos were injected at the one- to two-cell stage with 25–50 pg of circular etsrp-XeX as previously described (Sumanas et al., 2008) in addition to scl MO. Batches of 20 injected and control uninjected embryos were frozen on dry ice at the tail bud stage. Total RNA was purified using the RNAquous-4PCR kit (Ambion). cDNA was synthesized using Superscript III reverse transcriptase and oligo-dT primer (Invitrogen). Real-time PCR was performed using Chromo4 thermal cycler (Bio-Rad) and iQ SYBR Green Supermix (Bio-Rad). The following PCR profile was used: 95°C 5 min; 95°C 1 min, 58°C 1 min, 72°C 1 min, detection at 82°C for 10 sec; steps 2 through 5 repeated 44 times. Relative cDNA amounts for mpx were calculated using the Opticon 3 software (Bio-Rad) and normalized to the expression of elongation factor 1 α (EF1α). Primers for measuring mpx expression span an intron-exon boundary to detect cDNA instead of genomic DNA. The following primer sequences were used. mpx-Forward: CTG CGG GAC CTT ACT AAT GAT GG; mpx-Reverse: CCT GGA TAT GGT CCA AGG TGT C; EF1α-Forward: TCA CCC TGG GAG TGA AAC AGC; EF1α-Reverse: ACT TGC AGG CGA TGT GAG CAG
Morpholino Knockdown
Morpholinos (MO) were injected at the one- to two-cell stage against the following mRNA sequences: Translation blocking MOs against etv2 (7.5 ng MO1 + 7.5 ng MO2)(Sumanas and Lin, 2006), gata1 (10.8 ng)(Rhodes et al., 2005), and tnnt2a (4ng)(Sehnert et al., 2002), a splice-site blocking MO against scl (12.5 ng)(Dooley et al., 2005), and a combination of translation (11 ng) and splice site blocking (0.7 ng) MOs against pu.1 (Clay et al., 2007).
DNA/RNA Injections
To overexpress Etv2, a DNA construct driven under the Xenopus EF1α promoter (referred to as the XeX-etv2 construct) was injected at a concentration of 25 pg/embryo (Sumanas et al., 2008). To overexpress scl, the sense RNA for scl (Liao et al., 1998)was obtained by digesting scl-pCS2+ with NotI and transcribing with SP6 RNA polymerase mMessage Machine Kit (Ambion) and injected 3nl at a concentration of 133 pg/nl.
Cell Transplantation
Donor embryos (mpx:GFP) were injected with a mixture of etv2 DNA (55 pg) and Tetramethylrhodamine isothiocyanate (TRITC) dextran at a concentration of 1 mg/ml (Invitrogen, Mw=2MDa) into the blastomere at the 1-cell stage. Donor and recipient embryos (mpx:GFP) were dechorionated with 2% Pronase (20 mg/ml) and then transferred to embryo media. Twenty to 50 cells were transplanted at the sphere to 30% epiboly stages using a CellTram Air (Eppendorf). Embryos were observed at 24-27 hpf for presence of TRITC-labeled donor cells and mpx:GFP fluorescence.
Imaging
Embryos were whole-mounted in 2% methylcellulose or 1% low melt agarose on glass slides. Images were captured using 20x/0.5 NA objective or 10x/0.3 NA objective on an AxioImager Z1 (Zeiss) compound microscope with Axiocam ICC3 color camera (Zeiss) and Axiocam MMR black and white camera (Zeiss). Images in different focal planes were combined using Extended Focus module within Axiovision software (Zeiss). Image levels were adjusted using Adobe Photoshop CS2 and CS6 to increase the contrast. For fluorescent in situ hybridization imaging, embryos were mounted in 0.6% low melting point agar with an overlay of embryo media. Confocal images were acquired using a Nikon D-Eclipse C1 (Nikon Instruments Inc., USA) equipped with a Plan Aprochromat 20X/0.75 NA microscope objective (Nikon Instruments Inc., USA) or an Apo LWD 40X/1.15 NA microscope objective (Nikon Instruments Inc., USA). NBT/BCIP fluorescence was imaged as previously described,(Trinh le et al., 2007) with an excitation wavelength of 647 nm and an emission wavelength of 745 nm. Vector Red fluorescence was imaged with an excitation wavelength of 561 nm and an emission wavelength of 685 nm. Images in different focal planes were combined and brightness and contrast levels were adjusted using Imaris software (Bitplane).
Results
Etv2 is required for primitive neutropoiesis in the ALPM
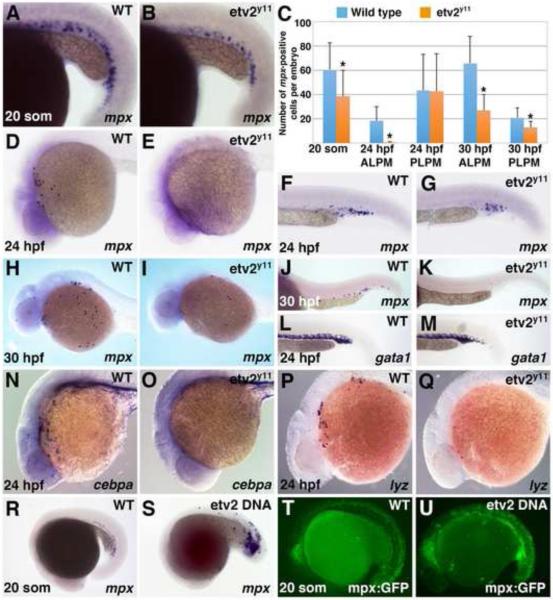
Previous studies have implicated a role for Etv2 during neutropoiesis, as etv2-MO injected embryos show a reduction in the neutrophil marker mpx expression at 24 hpf (Sumanas et al., 2008). However, it was not clear if both ALPM and PLPM-derived neutrophils were affected in etv2 morphants. To investigate the role for Etv2 during neutropoiesis in greater detail, we analyzed mpx expression in the previously identified etv2y11 mutants (Pham et al., 2007). At the 20-somite stage, when mpx expression is first evident in the PLPM, etv2 morphants displayed slight reduction in both mpx expression level and the number of mpx-expressing cells (Fig. 1A-C). At 24 hpf, two separate ALPM and PLPM -derived mpx-positive cell populations were apparent in wild-type (wt) embryos (Fig. 1D,F). In contrast, the anterior mpx-positive cell population was absent in etv2y11 mutant embryos while mpx-positive cells in the trunk and tail region were not significantly affected (Fig. 1C-G). After 24 hpf, circulation is initiated in zebrafish embryos and both populations are likely to undergo some mixing. At 30 hpf, the level of mpx expression and the number of mpx expressing cells were greatly reduced in both the anterior and posterior regions of etv2y11 mutant embryos as compared with their wild-type siblings (Fig. 1C,H-K). Additional markers for the anterior myeloid population cebpa and a neutrophil-specific marker lysozyme C (lyz) (Liu and Wen, 2002; Lyons et al., 2001; Meijer et al., 2008; Pase et al., 2012) were nearly completely absent in etv2y11 mutant embryos at 24 hpf (Fig. 1N-Q). In contrast, and consistent with our previous etv2 MO knockdown studies (Sumanas and Lin, 2006), erythroid specific gata1 expression remained largely unaffected in the ICM region of etv2y11 mutants at 24 hpf (Fig. 1L,M). Thus, Etv2 is required for myelopoiesis in the ALPM and but has little effect in initiating erythropoiesis and mpx expression in the ICM prior to the initiation of circulation.
Figure 1. Etv2 function is necessary for neutropoiesis in the ALPM and is sufficient to initiate mpx expression.
(A-K) Anterior but not posterior mpx expression is greatly reduced or absent in etv2y11mutants, as analyzed by in situ hybridization. (A,B) mpx expression is slightly reduced in the ICM region of etv2y11 mutants (B) as compared to their wild-type siblings (A) at the 20 somite stage. (C) Number of mpx-expressing cells at various stages in the anterior and posterior lateral plate mesoderm of wild type and etv2y11 mutant embryos. The cells were counted in 30 wt and 23 etv2y11 mutant embryos at the 20-somite stage, 16 wt and 16 mutant embryos at 24 hpf and 10 wt and 9 mutant embryos at 30 hpf. Error bars correspond to ± standard deviation. Asterisks denote significant differences (p<0.05) calculated by Student's T-Test. (D, E) mpx expression is strongly reduced at 24 hpf in the ALPM of etv2y11 mutants (E) compared to wild type siblings (D). (F, G) mpx expression has only a minor reduction in the ICM region of etv2y11 mutants (G) compared to their wild-type siblings (F). (H-K) mpx expression is reduced at 30 hpf in the anterior (I) and posterior (K) of etv2y11 mutants compared to wild type siblings (H, J). (L, M) gata1 expression is not significantly affected in etv2y11 mutants (M) as compared to wild-type siblings at 24 hpf (L). (N, O) cebpa expression at 24 hpf is strongly reduced in 100% of etv2y11 mutants (O, n=11) while it is strongly expressed in 100% of wild-type siblings (N, n=11). (P, Q) lyz expression is strongly reduced in 100% of etv2y11 mutants (Q n=14) compared to 100% of wild-type siblings with normal lyz expression (P, n=15). (R-U) Etv2 DNA overexpression results in ectopic mpx expression as analyzed by in situ hybridization (R,S) and mpx:GFP live fluorescence (T,U) at the 20-somite stage (65% of etv2-injected embryos with increased or ectopic mpx:GFP, n=115 as opposed to 0% controls, n=39). Note that mpx:GFP exhibits non-specific GFP fluorescence in the neural tube. All embryos are positioned with the anterior to the left and dorsal side up.
To test if Etv2 was sufficient to initiate mpx expression, an Etv2 DNA overexpression construct was injected and embryos were collected for in situ hybridization. mpx expression was ectopically induced at the 20-somite stage after etv2 injection (Fig. 1R,S). Similar induction of GFP expression was also observed in the mpx:GFP reporter line (Fig. 1T,U). These findings demonstrate that Etv2 is both required and sufficient to induce mpx expression during primitive neutropoiesis.
Neutrophils in the ALPM are derived from etv2-positive progenitors
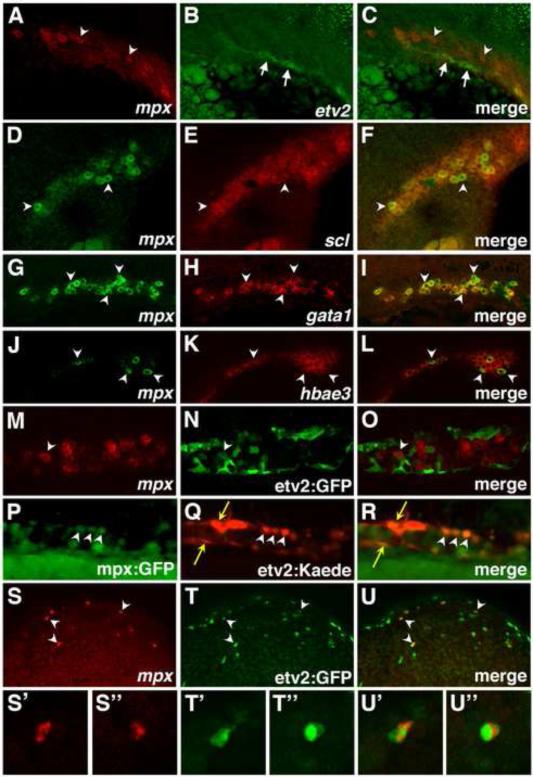
Previous work has demonstrated etv2 expression in vascular endothelial progenitors (Sumanas and Lin, 2006). However, it is not clear if etv2 is expressed in any hematopoietic progenitors, including neutrophil precursors. To test if mpx and etv2 expression overlapped during primitive hematopoiesis, double in situ hybridization was performed. It was apparent that mpx expression largely did not overlap with etv2 expression in the ICM region at the 20-somite stage (Fig. 2A-C). In contrast, posterior mpx expression overlapped with the hematopoietic master regulator scl (Fig. 2D-F), the erythroid precursor marker gata1 (Fig. 2G-I), and the erythroid cell marker alpha embryonic hemoglobin hbae3 (Fig. 2J-L). These results suggest that mpx may not specifically mark neutrophils in the ICM, and instead mpx may be transiently expressed in the erythroid population (see Discussion).
Figure 2. Comparison of mpx, etv2, scl, gata1, hbae3, etv2, GFP and etv2:Kaede expression.
(A-L) mpx expression overlaps with scl, gata1 and hbae3, but not etv2, in the ICM of wild-type embryos at the 22-somite stage, as analyzed by two color in situ hybridization. (A-C) etv2 (arrows), and mpx (arrowheads) expression domains largely do not overlap. Maximum intensity projections of confocal images are shown . Autofluorescent yolk granules are apparent in the lower left corner in (B,C). (D-F) mpx expression partially overlaps with scl expression (selected overlapping cells, arrowheads). Single confocal slices are shown. (G-I) mpx and gata1 expression largely overlap (arrowheads, selected cells). Single confocal slices are shown. (J-L) mpx and hbae3 expression largely overlap (arrowheads, selected cells). Note that not all hbae3 cells are positive for mpx expression. Single confocal slices are shown. (M-O) Only a fraction (10.8%) of mpx-expressing cells in the ICM overlap with etv2:GFP expression at the 24 somite stage, as observed by combined ISH for mpx expression and immunohistochemistry for GFP. (P-R) etv2:Kaede and mpx:GFP expression overlaps in neutrophils (white arrowheads) in the trunk ICM region of embryos at 24hpf. Yellow arrows denote vascular endothelial cells. (S-U) Multiple (64%) ALPM-derived mpx-expressing cells, positioned over the yolk, overlap with etv2:GFP expression at 24 hpf (white arrowheads) as observed by combined ISH for mpx expression (red) and immunohistochemistry for GFP (green). (S’-U’’) Magnified views of individual cells from S-U. Images show anterior to the left and dorsal up in all panels.
Because etv2 expression initiates significantly earlier than that of mpx, it is possible that neutrophils are derived from etv2-positive precursors, which may downregulate etv2 expression upon differentiation. In order to determine if mpx-expressing neutrophils had at one time expressed etv2, we utilized an etv2:GFP transgenic line (Proulx et al., 2010). GFP has a long half-life, therefore its expression can be used as a lineage tracer to mark the progeny of etv2-expressing cells. As observed by a combined GFP immunofluorescence and mpx in situ hybridization assay, only 10.8% of mpx-expressing cells in the ICM region also showed etv2:GFP-fluorescence (out of the total of 417 mpx cells counted in 13 embryos) (Fig. 2M-O). Similar results were obtained when using the etv2:Kaede transgenic line (Kohli et al., 2013) to detect co-expression of mpx:GFP and etv2:Kaede in neutrophils. etv2:Kaede was photoconverted prior to imaging to completely convert the Kaede protein from green to red. Only rarely was overlap of mpx:GFP and etv2:Kaede observed in the posterior region (Fig. 2P-R). These results argue that the majority of mpx-positive cells in the ICM region are not derived from etv2-positive cells. etv2 expression in the ALPM region as observed by ISH at 24 hpf is restricted to vascular endothelial cells and is not apparent in myeloid cells or neutrophils (Sumanas et al., 2008; Sumanas and Lin, 2006). However, in contrast to the ICM, 64% of mpx-positive cells located over the yolk (out of 89 total cells analyzed in 5 embryos), were also positive for etv2:GFP expression (Fig. 2S-U). As shown in the earlier studies, these cells originate in the ALPM region (Le Guyader et al., 2008). These data suggest that the majority of anterior mpx cells originate from etv2-expressing cells.
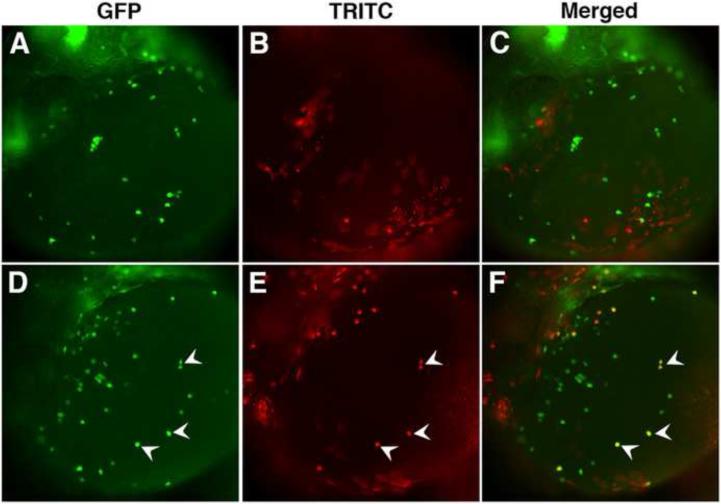
To demonstrate further that etv2-expressing cells give rise to mpx-positive precursors, we utilized cell transplantation to generate mosaic embryos. In a control experiment, 20-50 cells were transplanted at the sphere stage from mpx:GFP donor embryos into mpx:GFP recipient embryos close to the animal pole. This region normally contributes to ectodermal tissues and is not expected to give rise to myeloid progenitors (Kimmel et al., 1990). Indeed, none of the donor cells showed mpx:GFP expression in more than 20 embryos analyzed at 26 hpf (Fig. 3A-C). In contrast, when mpx:GFP donor embryos were injected with etv2 DNA, 83% of the recipient embryos (20 out of 24) had mpx:GFP positive donor cells (Fig. 3D-F). This argues that etv2-overexpressing cells can autonomously induce mpx expression.
Figure 3. etv2 functions cell autonomously in neutropoiesis.
Donor mpx:GFP embryos were injected with either TRITC-dextran tracer or TRITC-dextran and etv2 DNA, and the cells were transplanted at the sphere stage (4 hpf) into mpx:GFP recipient embryos. (A-C) Wild type cells transplanted into a wild type host do not induce mpx expression. (A) mpx:GFP, (B) TRITC and (C) merged fluorescence in the recipient embryos at 26 hpf stage. (D-F) A subset of etv2-overexpressing cells transplanted into a wild type host induce mpx expression autonomously. (D) mpx:GFP, (E) TRITC and (F) merged fluorescence in the recipient embryos at 26 hpf stage. White arrowheads point to overlapping cells, indicating that they are derived from the donor etv2-overexpressing embryos.
Scl functions downstream of Etv2 in the anterior neutropoiesis
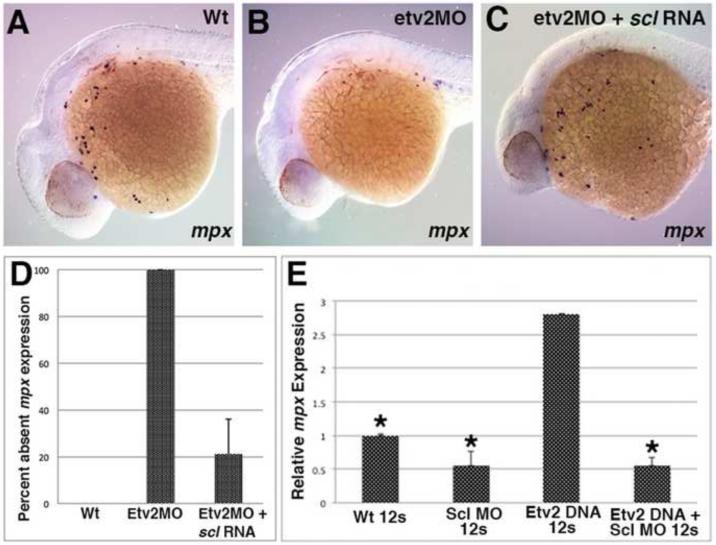
Scl has been previously implicated in the formation of all hematopoietic lineages, including myelopoiesis in the ALPM (Dooley et al., 2005; Patterson et al., 2005). Our previous work has shown that etv2 is required for scl expression in the ALPM (Sumanas et al., 2008). To determine if scl functions downstream of etv2 in initiating anterior mpx expression, we tested if scl overexpression would rescue mpx expression in etv2 knockdown embryos. Indeed, injection of scl RNA effectively restored anterior mpx expression in etv2 MO-injected embryos (Fig. 4A-D).
Figure 4. scl functions downstream of etv2 during neutropoiesis in the ALPM.
(A-D) scl RNA can rescue anterior mpx expression in etv2 knockdown embryos, as analyzed at 24 hpf. mpx expression in etv2 morphants (B, n=19/19) is decreased as compared to the wild type siblings (A, n=26/26). Injection of scl RNA rescues mpx expression in etv2 morphants (C, n=23/29). All embryos are positioned with anterior to the left and dorsal side up. (E) Morpholino knockdown of scl reduces the induction of mpx by etv2 DNA, as analyzed by real-time RT-PCR at the 12-somite stage. mpx expression is increased in etv2 DNA injected embryos, relative to uninjected controls but is not changed significantly when etv2 DNA is co-injected with scl MO. Relative expression levels have been normalized to EF1α expression. Asterisk denotes statistical significance vs. etv2 DNA over-expression (p<0.01) as analyzed by one-way ANOVA test with pair-wise comparison. Error bars correspond to ± standard deviation.. (n=3).
To determine if scl was required for precocious induction of mpx expression by etv2, qPCR analysis was performed at the 12-somite stage, when endogenous mpx expression is not yet observed. While etv2 DNA overexpression resulted in strong precocious induction of mpx expression, this induction was absent in the embryos coinjected with etv2 DNA and a previously characterized scl MO (Dooley et al., 2005) (Fig. 4E). These results demonstrate that scl functions downstream of etv2 during neutropoiesis in the ALPM.
scl and gata1, but not pu.1 are required for posterior mpx expression
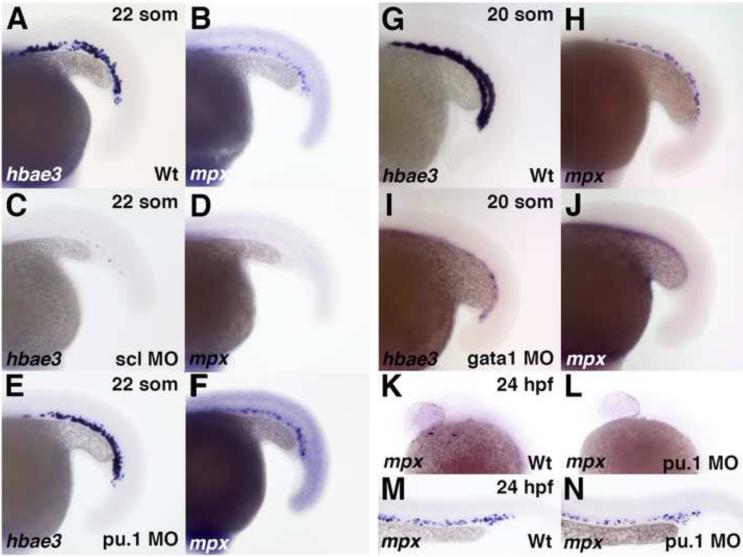
Our data argue that Etv2 has only a minor effect on mpx expression in the ICM. Therefore we examined the role of other transcription factors that have been previously implicated in hematopoiesis. Previous studies have demonstrated a requirement for scl and pu.1 in neutropoiesis (Dooley et al., 2005; Rhodes et al., 2005). However, the analysis focused largely on embryonic stages past 24 hpf after neutrophils have already entered circulation. To determine if scl and pu.1 have similar requirements in the initiation of mpx expression within the ICM region, we performed functional knockdown using previously validated scl and pu.1 MOs (Clay et al., 2007; Dooley et al., 2005). Expression of mpx was absent in scl knockdown embryos and was largely unaffected in pu.1 knockdown embryos at the 22-somite stage (Fig. 5B,D, F). As expected, expression of erythroid specific marker hemoglobin alpha embryonic 3 (hbae3) was absent only in scl morphants and not affected in pu.1 morphants (Fig. 5A,C, E). To confirm the efficacy of the pu.1 MO, we examined anterior mpx expression at 24 hpf. Anterior mpx expression was strongly reduced or absent in pu.1 knockdown embryos as previously shown (Fig. 5K,L)(Rhodes et al., 2005), whereas the posterior domain was largely unaffected (Fig. 5M,N). Additionally, the expression of the myeloid marker lplastin was strongly reduced in pu.1 morphant embryos (data not shown).
Figure 5. Knockdown of scl and gata1, but not pu.1, results in the loss of PLPM-specific mpx expression at the 22- and 20-somite stage.
(A, B) Wild type expression of embryonic globin hbae3 (A), and mpx (B) is normal in 100% (n=23) and 61% (n=28) of control uninjected embryos, respectively, in the intermediate cell mass region at the 22-somite stage. (C, D) scl morpholino knockdown results in the loss of hbae3 (C) and mpx (D) expression in 100% (n=24) and 97% (n=32) of embryos, respectively. (E, F) Knockdown of pu.1 does not affect hbae3 expression (E, 100% of embryos, n=54), or mpx expression (F, 70% of embryos, n=37). (G, H) Wild type expression of embryonic globin hbae3 (G), and mpx (H) in the intermediate cell mass region at the 20-somite stage is normal in 100% (n=20) and 78% (n=60) of control uninjected embryos, respectively. (I, J) Knockdown of gata1 results in the loss of expression of both hbae3 (I) and mpx in 100% of embryos, n=28 and n=100, respectively (J). (K, L) mpx expression is strongly reduced or absent in the anterior of mpx morpholino knockdown embryos (L, 92% of embryos, n=25) compared to uninjected controls (K, 13% of embryos, n=8). (M, N) mpx expression is normal in the posterior of mpx morpholino knockdown embryos (N, 88% of embryos, n=25) compared to uninjected controls (M, 75% of embryos, n=8).
gata1, which is an important transcription factor for erythroid development, has previously been shown to repress pu.1 expression during hematopoiesis (Rhodes et al., 2005). Previous studies have demonstrated that mpx expression is greatly increased at 32 hpf in gata1 knockdown embryos, arguing that erythroid precursors assume myeloid fates in the absence of gata1 (Rhodes et al., 2005). Interestingly, reduced mpx expression was observed in gata1 morphants at 22 hpf (Galloway et al., 2005). We observed a similar loss of mpx expression in gata1 morphants at the 20 somite stage (Fig. 5H, J). As expected, hbae3 expression was strongly reduced by gata1 knockdown (Fig. 5G, I). This result suggested an early requirement for gata1 in initiating mpx expression in the ICM, which is different from its later role in suppressing myelopoiesis.
gata1 is required for early initiation of mpx expression in the ICM, however later acts to repress the neutrophil fate
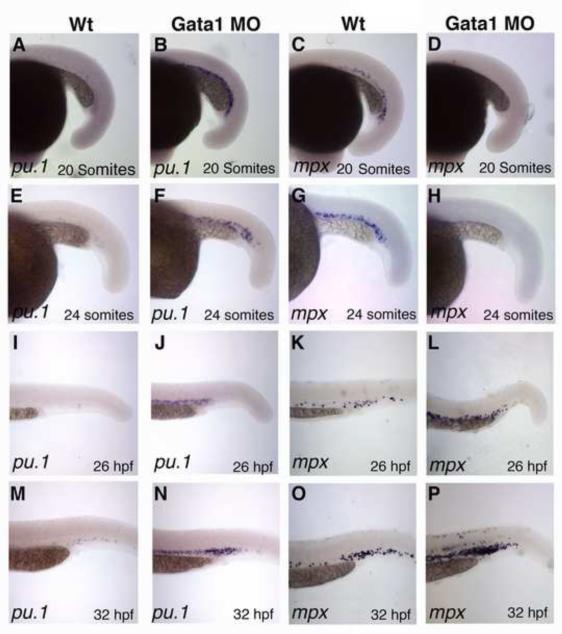
To better understand how mpx expression in the ICM region is affected in gata1 morphants, we used a time-course approach to assay for mpx and pu.1 expression after gata1 MO knockdown. Although pu.1 is initially expressed in the PLPM region, by the 20-somite stage its expression is downregulated and is no longer apparent in wild-type embryos (Fig. 6A) (Lieschke et al., 2002). In agreement with the previous studies (Galloway et al., 2005; Rhodes et al., 2005), in gata1 morphants pu.1 was dramatically increased at the 20-somite stage in the ICM region (Fig. 6A,B). In contrast, mpx expression was absent at the 20-somite stage in gata1 morphants, as compared to uninjected control embryos (Fig. 6C,D). pu.1 expression was also increased in the ICM region at the 24-somite, 26 hpf and 32 hpf stages, when it is not observed in control uninjected embryos (Fig. 6E,F,I,J,M,N). mpx expression was also strongly decreased in gata1 morphants at the 24-somite stage (Fig. 6G,H). Other myeloid markers such as neutrophil-specific lyz and macrophage-specific interferon regulatory factor 8 (irf8) were not expressed at the 24-somite stage in control uninjected embyros (Suppl. Fig. S1A,C) but were expressed in a small number of cells at the 24-somite stage in gata1 morphants (Suppl. Fig. S1B,D). At the 26 hpf and 32 hpf stages, mpx expression was increased in the gata1 morphants as compared to control uninjected embryos (Fig. 6K,L,O,P), which is in accordance with the previous reports (Galloway et al., 2005; Rhodes et al., 2005). Consistent with this result, lyz and irf8 expression was increased in gata1 morphants at 32 hpf (Suppl. Fig. S1E-H).
Figure 6. gata1 is required to initiate mpx expression in the ICM region, but later represses it.
(A-H) pu.1 expression is greatly increased (n=16/16 and 31/31) while mpx expression is greatly reduced (n=66/66 and 26/26) as analyzed by ISH in gata1 MO knockdown embryos at the 20-somite (A-D) and 24-somite (E-H) stages, respectively. (I-P) Both pu.1 and mpx expression are greatly increased in gata1 morphant embryos at 26 hpf and 32 hpf (n=35/35 and 30/33 for pu.1 expression; n=27/31 and 21/24 for mpx expression at 26 hpf and 32 hpf, respectively). Dorsal is up and anterior is to the left in all panels.
To confirm that the increased mpx expression at 32 hpf represents posteriorly-derived cells only, we used the troponin T2a (tnnt2a) morpholino to stop blood circulation (Sehnert et al., 2002). Posterior mpx expression was normal in embryos injected with tnnt2a MO only (Suppl. Fig. S2A, B). The average number of anterior and posterior mpx-expressing cells in control uninjected (75 ± 22.7 in anterior, 14 ± 6.7 in posterior, n=19) and in tnnt2a morphants (65 ± 20.4 in anterior, 17 ± 11.7 in posterior, n=15) was not significantly different (p>0.1). Injection of gata1 MO and tnnt2a in combination caused a similar increase in posterior mpx expression as injection of gata1 MO alone, indicating that the additional posterior cells are derived from the initial posterior ICM expression domain (Suppl. Fig. S2C,D). These results argue that gata1 has a specific requirement in initiating mpx expression in the ICM, while at later stages its role switches to the repression of myelopoiesis.
Discussion
In this study, we demonstrate that etv2 has a critical cell autonomous requirement during neutropoiesis in the anterior lateral plate mesoderm. We further show that scl functions downstream of etv2 in ALPM-derived neutropoiesis. In contrast, posterior mpx expression largely does not depend on etv2 or pu.1 function, but is regulated by scl and, surprisingly, gata1 expression. Similar to its zebrafish homolog, mouse etv2 function has been previously implicated in both vasculogenesis and hematopoiesis (Ferdous et al., 2009; Kataoka et al., 2011; Lee et al., 2008). Interestingly, HSC-specific knockout of mouse er71/etv2 displays defects in myelopoiesis, similar to the zebrafish knockdown phenotype (Lee et al., 2011). This argues for a high degree of functional conservation between mammalian and zebrafish Etv2 function.
Our results show that mpx expression in the zebrafish ALPM and PLPM are regulated differently. mpx expression in the ALPM depends on etv2 function while its expression in the PLPM is largely etv2-independent. Because etv2 expression is not observed in ALPM-derived neutrophil progenitors, while etv2:GFP is, this supports the hypothesis that neutrophil progenitors in the ALPM are derived from etv2-expressing cells that downregulate etv2 expression upon myeloid differentiation. Although not all ALPM-derived mpx cells displayed etv2:GFP expression, it is quite possible that some cells downregulated etv2:GFP beyond the detectable level. Cell transplantation data provide further support for a cell autonomous function of etv2 during myelopoiesis. However, our experimental data do not exclude the possibility that etv2 also has a non-autonomous function during neutropoiesis in the ALPM.
As evident from this and previous studies, mpx expression is initiated in the ALPM and PLPM regions at distinct times and under different genetic control. Two independent studies have demonstrated using fate mapping that ALPM region gives rise to neutrophil progenitors (Jin et al., 2012; Le Guyader et al., 2008). On the other hand, the fate of posterior mpx-expressing cells is currently less clear. Our data show that all mpx-positive cells in the ICM are also positive for the erythroid marker gata1 and embryonic globin hbae3 expression. This argues for two possibilities. First, gata1+mpx+ cells in the ICM represent erythro-myeloid progenitors and may contribute to both erythroid and myeloid lineages. A second possibility is that all gata1+mpx+ cells in the ICM express mpx only transiently, and lose mpx expression as they undergo erythroid differentiation. This scenario supports the model where all primitive myeloid lineages (macrophages and neutrophils) originate within the ALPM while all PLPM gives rise only to erythroid progenitors (as well as endothelial cells). The coexpression of mpx and hbae3 in the ICM supports this model. However, hematopoietic progenitors in the ICM do have myeloid potential and can differentiate into neutrophils and macrophages if gata1 function is inhibited.
In a previous study, single cell labeling in the ICM region at 24-26 hpf produced neutrophils and erythrocytes among its progeny (Warga et al., 2009). This result would support bipotent fates of gata1+mpx+ progenitors. However, it is possible that some of the ICM derived cells contribute to the erythro-myeloid progenitors (EMPs) that have been previously observed in the posterior blood island (PBI) at slightly later stages of 30-42 hpf (Bertrand et al., 2007). In fact, cell labeling has shown that these definitive EMPs originate in the ICM region (Bertrand et al., 2007). It remains to be determined what percentage of gata1+mpx+ cells in the ICM contribute to myeloid and erythroid lineages.
It is intriguing that mpx expression is regulated differently in the ALPM and PLPM. Our results argue that the majority of ALPM-derived neutrophil precursors originate from etv2-expressing cells, while the majority of mpx-positive cells in the PLPM are not derived from etv2-expressing cells. scl is required for mpx expression in both anterior and posterior regions, while only the anterior scl domain is regulated by Etv2. We have previously demonstrated that scl expression in the PLPM is induced independently of Etv2 (Sumanas et al., 2008). It is likely that there are other yet unidentified ETS factors that regulate posterior scl and mpx expression.
In situ hybridization analysis revealed that mpx expression overlaps with both scl and gata1 in the ICM of the early embryo. Previous studies have also demonstrated overlap between mpx and pu.1-expressing cells (Bennett et al., 2001). Our results show that scl is required for the initiation of mpx expression in the ICM, consistent with the previous studies that analyzed mpx expression at later stages. Surprisingly, we and others (Galloway et al., 2005) have revealed an early requirement for the erythroid transcription factor gata1 to initiate mpx expression in the ICM region. This is a transient loss in mpx expression since mpx becomes upregulated at 26 hpf in gata1 morphants. As expected, when gata1 is knocked down, expression of pu.1 increases dramatically. In the previous studies an opposite effect of increased mpx expression was observed, as pu.1 expression has been shown to drive mpx expression and pu.1 and gata1 have been demonstrated to have an antagonistic relationship (Bennett et al., 2001; Rhodes et al., 2005). However, this analysis was performed after 24 hpf, and our results indicate an early role for gata1 but not pu.1 in initiating mpx expression in the ICM region. Both pu.1 and gata1 are known to function in a transcriptional complex with multiple other co-factors. Presence or absence of one of these gata1 co-factors may account for the switch in permissive and inhibitory roles of gata1.
Our results describe the transcriptional regulatory interactions during the earliest steps of primitive neutropoiesis, which have been poorly understood. Because the factors that regulate hematopoiesis are highly conserved between different vertebrates, our findings will promote further understanding of the molecular mechanisms that control hematopoiesis in mammalian embryos and may ultimately contribute to the development of novel therapeutic strategies to treat hematopoietic disorders.
Supplementary Material
Highlights.
Zebrafish Etv2 regulates anterior but not posterior myeloperoxidase mpx expression.
Anterior neutrophils originate from Etv2-expressing cells.
Scl functions downstream of Etv2 during anterior neutropoiesis.
Gata1 is required to initiate posterior mpx expression.
Acknowledgments
This research was supported by Cincinnati Children's Research Foundation and NIH R01 HL107369 award to S.S. J.A. Schumacher was supported by NIH T32HL00738 fellowship and E.J. Zhao was supported by AHA-Great Rivers Affiliate Summer Undergraduate Research Fellowship 12UFEL9990000. We thank M. Kofron for his help with the confocal imaging and B. Weinstein for providing etv2y11 mutants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N.O.G., J.A.S., H.J.K., E.J.Z. and J.S. performed experiments and analyzed the results, N.O.G., J.A.S, and S.S. wrote the paper, S.S. designed the study and analyzed the results.
The authors have no conflict of interest to declare.
References
- Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, Look AT. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J. Small fish, big science. PLoS biology. 2004;2:E148. doi: 10.1371/journal.pbio.0020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie A, Hersey C, Oates AC, Paw BH, Falick AM, Witkowska HE, Flint J, Higgs D, Jessen J, Bahary N, Zhu H, Lin S, Zon L. Characterization of embryonic globin genes of the zebrafish. Developmental biology. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Burns CE, Galloway JL, Smith AC, Keefe MD, Cashman TJ, Paik EJ, Mayhall EA, Amsterdam AH, Zon LI. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood. 2009;113:5776–5782. doi: 10.1182/blood-2008-12-193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell host & microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley KA, Davidson AJ, Zon LI. Zebrafish scl functions independently in hematopoietic and endothelial development. Developmental biology. 2005;277:522–536. doi: 10.1016/j.ydbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Developmental cell. 2005;8:109–116. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Galloway JL, Zon LI. Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Current topics in developmental biology. 2003;53:139–158. doi: 10.1016/s0070-2153(03)53004-6. [DOI] [PubMed] [Google Scholar]
- Hall C, Crosier P. Editorial: Maintaining the balance--fishing for drugs to treat persistent neutrophilic inflammation. Journal of leukocyte biology. 2010;87:189–191. doi: 10.1189/jlb.0909647. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- Hsia N, Zon LI. Transcriptional regulation of hematopoietic stem cell development in zebrafish. Experimental hematology. 2005;33:1007–1014. doi: 10.1016/j.exphem.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Jin H, Li L, Xu J, Zhen F, Zhu L, Liu PP, Zhang M, Zhang W, Wen Z. Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative feedback loop inhibiting Pu.1 expression. Blood. 2012;119:5239–5249. doi: 10.1182/blood-2011-12-398362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett T. Analysis of protein and gene expression. Methods in cell biology. 1999;59:63–85. doi: 10.1016/s0091-679x(08)61821-x. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H, Nishikawa S. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRalpha+ primitive mesoderm. Blood. 2011;118:6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- Kohli V, Schumacher JA, Desai SP, Rehn K, Sumanas S. Arterial and venous progenitors of the major axial vessels originate at distinct locations. Developmental cell. 2013;25:196–206. doi: 10.1016/j.devcel.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman R, Finn A. Neutrophil disorders and their management. Journal of clinical pathology. 2001;54:7–19. doi: 10.1136/jcp.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, Mordelet E, Zapata A, Shinomiya H, Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- Lee D, Kim T, Lim DS. The Er71 is an important regulator of hematopoietic stem cells in adult mice. Stem Cells. 2011;29:539–548. doi: 10.1002/stem.597. [DOI] [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell stem cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jin H, Xu J, Shi Y, Wen Z. Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood. 2011;117:1359–1369. doi: 10.1182/blood-2010-06-290700. [DOI] [PubMed] [Google Scholar]
- Liao EC, Paw BH, Oates AC, Pratt SJ, Postlethwait JH, Zon LI. SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes & development. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001;98:3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Oates AC, Paw BH, Thompson MA, Hall NE, Ward AC, Ho RK, Zon LI, Layton JE. Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Developmental biology. 2002;246:274–295. doi: 10.1006/dbio.2002.0657. [DOI] [PubMed] [Google Scholar]
- Liu F, Wen Z. Cloning and expression pattern of the lysozyme C gene in zebrafish. Mechanisms of development. 2002;113:69–72. doi: 10.1016/s0925-4773(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Lyons SE, Shue BC, Lei L, Oates AC, Zon LI, Liu PP. Molecular cloning, genetic mapping, and expression analysis of four zebrafish c/ebp genes. Gene. 2001;281:43–51. doi: 10.1016/s0378-1119(01)00774-0. [DOI] [PubMed] [Google Scholar]
- Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of leukocyte biology. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. The EMBO journal. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Meijer AH, van der Sar AM, Cunha C, Lamers GE, Laplante MA, Kikuta H, Bitter W, Becker TS, Spaink HP. Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Developmental and comparative immunology. 2008;32:36–49. doi: 10.1016/j.dci.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Pase L, Layton JE, Wittmann C, Ellett F, Nowell CJ, Reyes-Aldasoro CC, Varma S, Rogers KL, Hall CJ, Keightley MC, Crosier PS, Grabher C, Heath JK, Renshaw SA, Lieschke GJ. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Current biology : CB. 2012;22:1818–1824. doi: 10.1016/j.cub.2012.07.060. [DOI] [PubMed] [Google Scholar]
- Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Developmental biology. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K, Lu A, Sumanas S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Developmental biology. 2010;348:34–46. doi: 10.1016/j.ydbio.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Rhodes J, Hagen A, Hsu K, Deng M, Liu TX, Look AT, Kanki JP. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Developmental cell. 2005;8:97–108. doi: 10.1016/j.devcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey RP, Metcalf D, Begley CG. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nature genetics. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood. 2005;106:534–541. doi: 10.1182/blood-2004-12-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS biology. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Developmental biology. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Trinh le A, McCutchen MD, Bonner-Fraser M, Fraser SE, Bumm LA, McCauley DW. Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic stain. BioTechniques. 2007;42:756–759. doi: 10.2144/000112476. [DOI] [PubMed] [Google Scholar]
- Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- Warga RM, Kane DA, Ho RK. Fate mapping embryonic blood in zebrafish: multi- and unipotential lineages are segregated at gastrulation. Developmental cell. 2009;16:744–755. doi: 10.1016/j.devcel.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. 5th ed. Monte Westerfield; Eugene, OR: 2007. The Zebrafish Book. [Google Scholar]
- Xu J, Du L, Wen Z. Myelopoiesis during zebrafish early development. Journal of genetics and genomics = Yi chuan xue bao. 2012;39:435–442. doi: 10.1016/j.jgg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang P, Behre G, Pan J, Iwama A, Wara-Aswapati N, Radomska HS, Auron PE, Tenen DG, Sun Z. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.