Abstract
Epidemiologic studies associate low serum vitamin D levels with an increased risk of colon cancer and inflammatory diseases such as inflammatory bowel disease (IBD). 129-Smad3tm1Par/J (Smad3−/−) mice are a model of bacterial-driven colitis and colon cancer when infected with Helicobacter bilis. Thus, we used this mouse model to determine whether increased dietary vitamin D would reduce inflammation and colon cancer. Smad3−/− mice were fed purified diet with either maintenance (1 IU vitamin D/g diet; maintenance) or increased concentrations of vitamin D (5 IU vitamin D/g diet; high vitamin D). One week after diet initiation, mice were inoculated with broth or H. bilis and were necropsied at several time points post-inoculation to assess inflammation, dysplasia, and neoplasia incidence. At 16 weeks post infection, 11% of mice fed high vitamin D diet had cancer compared to 41% of mice fed maintenance diet (p=0.0121). Evaluation at an early time point (1 week post-infection) showed that animals fed high vitamin D had decreased MAPK (p-p38 and p-JNK) activation in lamina propria leukocytes as well as decreased NFκB activation in colonic epithelial cells. Reduction in MAPK and NFκB activation correlated with decreased IBD scores (2.7 vs 15.5, p<0.0001) as well as decreased inflammatory cell infiltrates and reduced expression of proinflammatory cytokines in cecal tissue. These findings suggest that increased dietary vitamin D is beneficial in preventing inflammation-associated colon cancer through suppression of inflammatory responses during initiation of neoplasia or early stage carcinogenesis.
Keywords: Colon Cancer, Colitis, Mouse Model, Vitamin D, TGFβ
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in both men and women (1). Studies indicate that diet and lifestyle choices play a significant role in the development of and prognosis of colon cancer (2). Vitamin D status of an individual is also influenced by both diet and lifestyle (3). The link between vitamin D and colon cancer was first suggested by Garland et al. who observed that populations residing in the northeastern United States had an increased incidence of colon cancer-related mortality compared to those living in the southern United States (4). Since then, adequate serum vitamin D levels have been associated with decreased incidence of colon cancer and decreased mortality in patients diagnosed with colon cancer (5, 6). Adequate serum vitamin D levels also correlate with reduced risk for developing inflammatory diseases, such as Crohn’s disease, a risk factor for colon cancer (3, 7).
The risk of colon cancer is increased in patients diagnosed with IBD (Crohn's disease or ulcerative colitis) compared to the general population, supporting the notion that colonic inflammation impacts cancer development (8). The chronic inflammation seen in IBD, likely due in part to dysregulated mucosal immune responses to enteric antigens (8), is believed to progress to cancer through the promotion of angiogenesis, tumor-promoting cytokine production, tumor cell invasive behavior and cellular proliferation (9).
Vitamin D has a protective effect against colon cancer in various mouse models (10, 11). However, these cancer models, such as ApcMin/+ mice or mice given a chemical mutagen such as azoxymethane, are not driven by inflammation (12–15). Currently, the protective effect of vitamin D on inflammation-associated colon cancer is not known.
In order to investigate the potential chemopreventative effects of elevated dietary vitamin D on inflammation-associated colon cancer, we utilized Smad3−/− (Smad3tm1Par/J) mice which have defective TGFβ signaling due to the absence of the transcription factor, Smad3 (16). In humans, the TGFβ signaling pathway is commonly mutated in colon cancer including colitis-associated colorectal cancer (17). After being infected with an enteric microorganism, Helicobacter bilis (16), Smad3−/− mice develop transient colitis followed months later by colon cancer making them a useful model for studying inflammation-associated colon cancer. Using this model, we demonstrate that elevated dietary vitamin D increases serum vitamin D and protects H. bilis (Hb)-infected Smad3−/− mice from developing colon cancer. These protective effects are mediated through interactions between vitamin D and proinflammatory signaling pathways during the early stages of disease development.
Material and Methods
Mice and diets
Study mice were colony-bred 129-Smad3tm1Par/J (Smad3−/−) mice (age 6–14 weeks) housed in a specific pathogen-free facility. Mice were screened for rodent pathogens as previously described (18) except that sentinels were collected three times yearly rather than quarterly. In addition to referenced pathogens, annual screens were performed for Minute Virus of Mice, Lymphocytic Choriomeningitis Virus, and Ectromelia virus. Mice were maintained in a Helicobacter and Mouse Norovirus - free colony as previously described (19). The mice were group-housed in ventilated cages and fed a purified, irradiated diet with 1 IU vitamin D (5SRH, maintenance), 5 IU vitamin D (5AAA, high vitamin D) or 0 IU vitamin D per gram (5AV4, AIN93Null). All diets were manufactured by PMI Nutrition International (LabDiet/TestDiet, St.Louis, MO) based on AIN93M diet that is formulated for the maintenance of rodents’ health. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Experimental design
While no age-associated alterations in endpoint disease have been noted in our experience with Smad3−/− mice, care was taken to evenly distribute mice across treatment groups with regard to age and sex. Independent studies were performed to evaluate the effects of high and low dietary vitamin D levels on cancer. For high vitamin D studies, mice were started on either maintenance or high vitamin D diets one week prior to infection. For vitamin D deficient studies, maintenance or AIN93Null diets were initiated two weeks prior to infection (Supplemental Fig 1). For each diet, mice were infected with either ~2 × 107 CFU Hb in Brucella broth or Brucella broth alone (controls) by oral gavage as previously described (16) (Supplemental Fig 1). Helicobacter infection status was monitored by fecal polymerase chain reaction (PCR) using previously published primer sequences (18). Fecal samples were collected at 3, 6, and 14 days post infection for subjective fecal scoring and at 7 days post infection for fecal cytokine analysis. Mice were weighed weekly and monitored at least three times weekly for dehydration, diarrhea, lethargy, or weight loss. Animals were euthanized by CO2 asphyxiation at the designated end points.
Serum vitamin D and calcium determination and tissue collection
Following euthanasia, blood was obtained via cardiac puncture. Serum samples were submitted to Heartland Assays (Ames, IA) for quantification of 25-hydroxyvitamin D (radioimmunoassay) and calcium levels. Mesenteric lymph nodes, cecum, colon and rectum were fixed in 10% phosphate buffered formalin and processed for routine histologic examination. For study of the early inflammatory phase, cross-sections of proximal colon (5mm) were prepared for immunohistochemistry (IHC) and histology, and cross-sections of proximal colon (5mm) and cecum (3mm) were stored in RNA later (Qiagen, Valencia, CA) for cytokine analysis by qRT-PCR. For evaluation of Hb colonization, 3 cm sections of mid-jejunum, proximal colon, distal colon and whole cecum were harvested, rinsed gently in sterile PBS to remove fecal material and stored at −20°C until DNA extraction. For fecal cytokine analysis, individual fecal samples were collected, homogenized in 250 µl RNAlater stabilization solution (Qiagen) and stored at −80°C until RNA extraction. At the time of collection, a subjective fecal score was assigned to each animal, ranking presence of diarrhea and blood in the stool as a clinical measure of IBD (20).
Histopathology and immunohistochemistry
Whole colon and cecum were evaluated by a board certified veterinary pathologist (PT) blinded to experimental groups to assess the severity of colitis and incidence of neoplasia. An overall IBD score was determined as described (21) with the exceptions that scores were summed from cecum, proximal, mid and distal colons and none were weighted. Analysis of the rectum was included with the distal colon. IBD scores incorporate the severity of mucosal epithelial changes, degree of inflammation, and extent of lesions. A dysplasia score was also generated by determining the degree of dysplasia present in each of four segments as described (22): cecum, proximal colon, mid colon, and distal colon. For each segment, a score ranging from 0–4 was assigned: 0= none, 1= indefinite, 2= low grade, 3=high grade, and 4= high grade with frank invasion beyond tunica muscularis and distinguished from mucosal herniation. Cancers were classified as adenocarcinomas and mucinous adenocarcinomas (16, 22). The four individual segmental scores were summed to generate the overall dysplasia score for each animal.
For study of the early inflammatory phase, colonic expression of CD3, F4/80, cleaved caspase 3, and MHCII were evaluated on transverse cross-sections of proximal colon in animals euthanized 1week post-infection. IHC staining within the mucosa, excluding any gastrointestinal lymphoid tissue, was scored by a pathologist blinded to groups (PT), using a range of 0–4: 0= no positive cells, 1= few single positive cells, 2= few (3 or less) scattered small clusters of positive cells, 3= many (>3) small clusters of positive cells or larger clusters of positive cells, 4= large or coalescing clusters of positive cells. For early time points, in addition to IHC staining, cecum and serial transverse cross-sections of proximal colon were evaluated histologically for evidence of IBD.
All IHC staining was performed by Experimental Histopathology Services at Fred Hutchinson Cancer Research Center (Seattle, WA). Rat monoclonal antibodies were used to detect MHC class II (I-A/I-E, BD Pharmingen), CD3 (MCA1477, Serotec) and F4/80 (MCA497, Serotec). Signals were detected using biotinylated goat anti-rat (Jackson ImmunoResearch) followed by streptavidin HRP (Jackson ImmunoResearch). Cleaved caspase-3 antibody (Biocare Medical CP229B) was followed by Mach 2 anti-rabbit HRP-labeled polymer (Biocare Medical RHRP520L). Staining was visualized with 3,3’-diaminobenzidine (DAB, Dako) and counter-stained with hematoxylin (Dako). Concentration-matched isotype-control slides were run for each tissue sample (Jackson ImmunoResearch).
Cytokine analysis and Helicobacter quantification by quantitative real-time PCR analysis
For cytokine analysis, RNA was extracted from cecum, colon, and feces using the RNeasy kit (Qiagen). Fecal RNA samples were further concentrated using the Qiagen mini-elution/cleanup kit. RNA was converted to cDNA using SuperScript First Strand Synthesis System (Invitrogen, Grand Island, NY) followed by qRT-PCR using Power Sybr Green Master Mix (Applied Biosystems, Carlsbad, CA) and a Stratagene Mx3005P analyzer (Agilent Technologies, Santa Clara, CA). Samples were run in duplicate. Cytokine levels were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) and expressed relative to a control sample (RNA from cecum or proximal colon) that was run on every plate as a calibrator. For Helicobacter quantification, DNA was extracted from mid-jejunal, cecal, proximal colon and distal colon samples using a previously published protocol (23). Primer sequences used are outlined in Supplemental Table 1. Data were analyzed using Stratagene's MxPro v4.10 software (Agilent Technologies).
Epithelial and lamina propria cell preparations
Proximal colon (4cm sections) samples were rinsed in PBS to remove fecal material, and epithelial and lamina propria leukocyte (LPL) populations were isolated using the Lamina Propria Dissociation Kit and gentleMACS Dissociator according to manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). Cells were pelleted, snap-frozen in liquid nitrogen, and stored at −80°C.
Western blotting
Protein was extracted by homogenizing cell pellets in RIPA buffer (Thermo Fisher Scientific, Rockford, IL) containing protease and phosphatase inhibitors (Thermo Fisher Scientific). Western blot analysis was performed according to a previously published protocol (19) with the following modifications. Proteins (40 µg/lane) were run on gradient (4–15%) Tris-HCl polyacrylamide gels (Biorad, Hercules, CA) and transferred to polyvinylidene fluoride membranes using 100 mM N-cyclohexyl-3-aminopropanesulfonic acid buffer with 10% methanol. Primary antibodies used were vitamin D receptor (VDR, SC-1008, Santa Cruz, Santa Cruz, CA), NFκB signaling (Phospho-NF-κB p65 (Ser536), and IκBα, L35A5, Cell Signaling, Danvers, MA), MAPK signaling (Phospho-MAPK Family, 9910, and MAPK Family, 9926, Antibody sampler Kits Cell Signaling), Bcl-xl (54H6, Cell Signaling), and proliferating cell nuclear antigen (PCNA, PC10, Cell Signaling). Beta actin (A5441, Sigma-Aldrich, St. Louis, MO) was used as a loading control.
Statistical analysis
Prior to statistical analysis, distribution of data was assessed for normality. If data were not normally distributed, transformation was attempted; if transformation did not normalize the distribution, non-parametric tests were performed. Serum vitamin D, serum calcium, histologic scoring, fecal scoring, and densitometries were analyzed using either Unpaired or Mann-Whitney t test. QRT-PCR data were analyzed using the Kruskal-Wallis non-parametric test followed by Dunn’s post-hoc test to adjust for multiple comparisons. Cancer and dysplasia incidence significance was determined by Fisher’s exact test. All data are presented as mean ± SEM. Differences with a p-value of 0.05 or less were considered significant. All statistical analyses were performed using GraphPad Prism software (Version 5.04, GraphPad Software Inc, La Jolla, CA).
Results
Increased dietary vitamin D significantly increases serum 25-hydroxyvitamin D without altering serum calcium levels
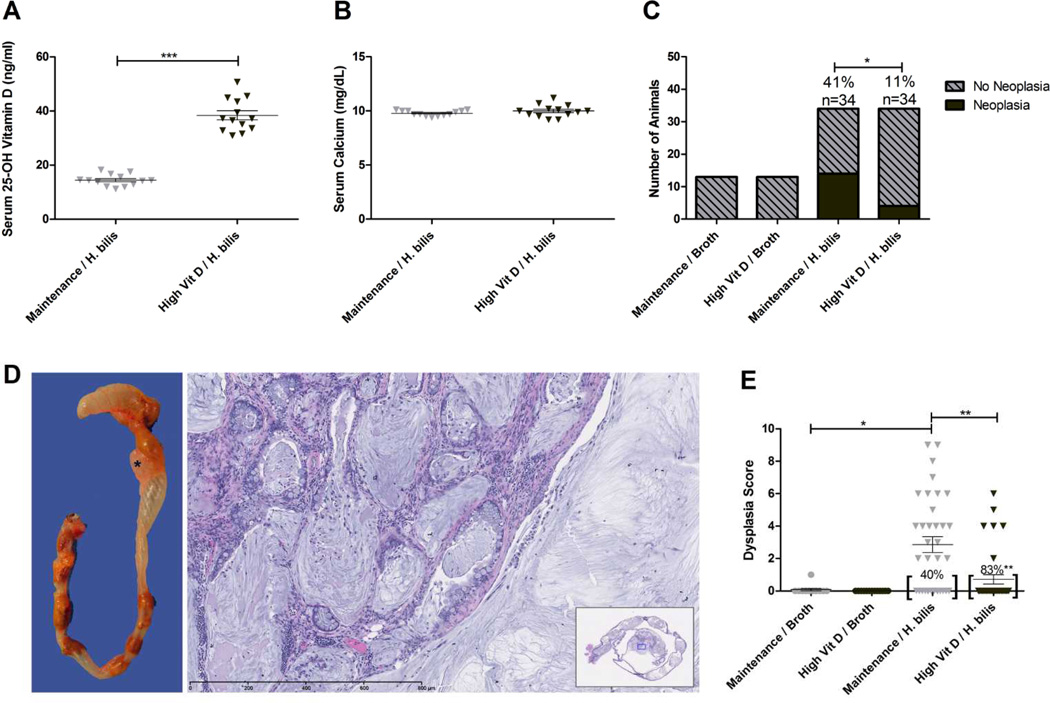
To determine if high dietary vitamin D increases serum vitamin D status without causing toxicity in Smad3−/− mice, we measured serum 25-hydroxyvitamin D and serum calcium in mice fed high vitamin D diet or maintenance diet (control) for one week. The high vitamin D diet significantly increased serum 25-hydroxyvitamin D levels without altering serum calcium (25–hydroxyvitamin D mean: 37.6 vs. 17.6 ng/ml, p=0.016; calcium mean: 9.6 vs. 10.6 mg/dl, p=0.1), demonstrating that the dietary regimen rapidly elevated serum vitamin D levels without causing hypercalcemia. Similarly, after 16 weeks on diet, Smad3−/− mice fed high vitamin D diet had serum vitamin D levels that were roughly double that of mice fed maintenance diet (mean: 38.4 vs. 14.4 ng/ml, p<0.0001) (Fig 1A) while serum calcium levels remained unchanged (Fig 1B).
Figure 1. Increased dietary vitamin D increases serum vitamin D and decreases dysplasia and cancer in Hb-infected Smad3−/− mice.
Serum 25-OH D (A) and serum calcium (B) were measured 16 weeks post Hb-infection in Hb-infected/ maintenance (n=13) vs. high vitamin D (n=13) diet. ***p<0.0001, Mann-Whitney t test. Colon and cecum were analyzed at 16 weeks post Hb-infection for histopathologic evidence of invasive adenocarcinoma and dysplasia (C–E). (C) Cancer incidence is reduced in mice fed high vitamin D (*p=0.0121, Fisher’s exact test). (D) Hb-infected mice typically develop grossly visible tumors in the cecum or proximal colon as represented by the pale, multilobulated mass in the proximal colon (*).Note the mucin lakes and neoplastic epithelial cells penetrating the colonic wall and proliferating within the muscularis and serosa. H&E staining. Original magnification 20X. Inset: Subgross of the whole colon section; blue box indicates magnified region. E, Hb-infected animals fed high vitamin D diet had decreased mean dysplasia scores and an increased number of animals with no evidence of dysplasia *p<0.01, **p<0.001
Treatment with increased dietary vitamin D significantly reduces cancer incidence in Hb-infected Smad3−/− mice
Hb-infected mice fed increased dietary vitamin D had a significantly reduced incidence of invasive colon cancer compared to Hb-infected mice fed maintenance diet (11% vs. 41%, p=0.0121) (Fig 1C). No neoplastic lesions developed in uninfected mice on either diet (Fig 1C). Mucinous adenocarcinomas located in the proximal colon were the primary neoplasm diagnosed, as previously noted in this model (16) (Fig 1D). Well-differentiated mucinous adenocarcinomas were characterized by expansile mucin-filled, epithelial-lined cysts that disrupt the muscular tunics, serosa and expand into mesentery (Fig 1D). Consistent with the decreased incidence of invasive adenocarcinoma, Hb-infected mice fed high vitamin D diet had an average four-fold decrease in dysplasia scores compared to mice fed maintenance diet (mean: 0.71 vs. 2.85, p<0.001, score range 0–16) (Fig 1E) with a significantly higher percentage of animals with no evidence of dysplasia (83% vs. 40%, p=0.0005). Dysplasia was primarily observed in the cecum and proximal colon. Minimal dysplasia was observed in the mid colon of mice fed maintenance diet but not in high vitamin D fed mice. No dysplasia was observed in the distal colon regardless of diet.
Clinical disease and colonic inflammatory cell infiltrates are reduced during the inflammatory phase in Hb-infected Smad3−/− mice fed increased dietary vitamin D
Smad3−/− develop acute inflammation approximately 3–7 days following Hb infection which is characterized by diarrhea, frank blood in the stool, dehydration, lethargy and loss of body condition. Clinical signs typically resolve within 7–14 days until the time that cancers develop ((16), unpublished observations). In order to determine the effects of elevated dietary vitamin D on early disease stages we assessed clinical disease parameters and alterations in inflammatory infiltrates in the colons of Hb-infected mice fed high vitamin D diet compared to Hb-infected mice fed maintenance diet. During the initial inflammatory period, mice were assigned a subjective fecal score in order to assess clinical evidence of IBD. Animals fed high vitamin D diet had significantly decreased fecal scores at both 3 and 6 days post infection (mean 0.1 vs. 0.8, p=0.0015 and 0.2 vs. 1.0 p=0.0003 respectively, Supplemental Fig 2A–B) compared to animals fed maintenance diet. Broth-treated animals showed no evidence of diarrhea, as expected, and by 14 days post-infection all animals had minimal evidence of diarrhea regardless of diet (Supplemental Fig 2C). There was no significant difference in body weight change associated with diet following Hb infection (data not shown).
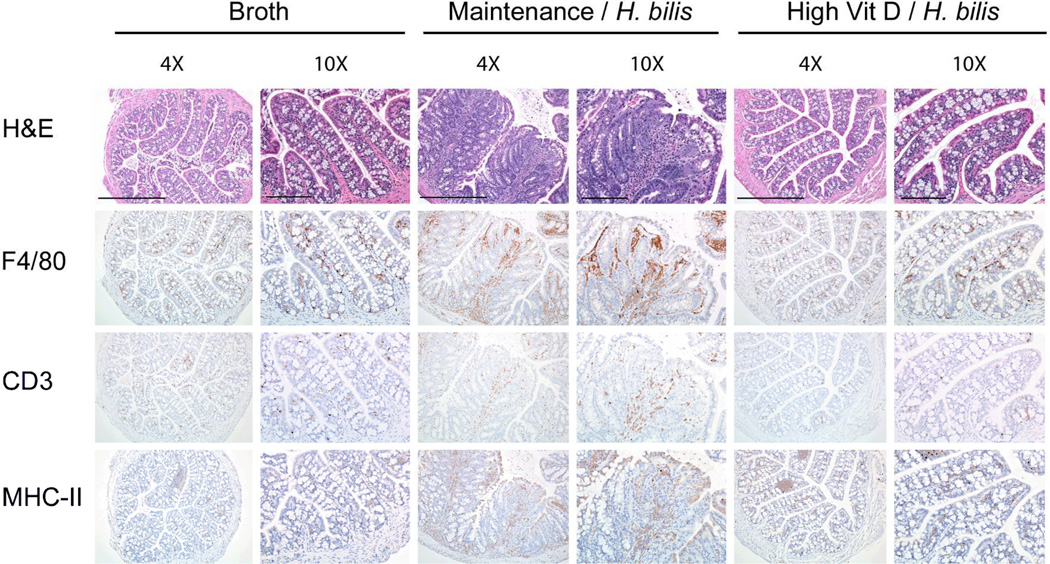
To determine if improved clinical signs associated with increased dietary vitamin D correlate with decreased colonic inflammation during early disease, proximal colon and cecum of mice fed high vitamin D or maintenance diet were analyzed for inflammation one week post Hb infection. Hb-infected animals fed high vitamin D diet had significantly reduced colitis compared to infected animals fed maintenance diet (mean 2.7 vs. 15.5, p<0.0001, Fig 2 and Supplemental Fig 3A). Immunohistochemistry studies further demonstrated that infected mice fed high vitamin D diet had decreased inflammatory infiltrates compared to those fed maintenance diet (F4/80+ cells {mean ± SEM: 2.7 ± 0.19 vs. 3.7± 0.11, p=0.0003}, CD3+ T cells {mean ± SEM: 2.1 ± 0.15 vs. 2.6 ± 0.17, p=0.04}, and MHC II+ cells {mean ± SEM: 2.05 ± 0.20 vs. 2.85 ± 0.13, p=0.001}) (Fig 2 and Supplemental Fig 3B, 3C, and 3D). Broth-treated controls had minimal inflammation regardless of diet.
Figure 2. Increased dietary vitamin D decreases proinflammatory infiltrates 1 week post Hb-infection.
Serial sections of paraffin-embedded proximal colon were stained with H&E for orientation and immunohistochemically for F4/80, CD3, and MHCII antigen. Images from representative samples were captured at the same low (4X – scale bars indicated on top row are 500µm) and high (10X – scale bars indicated on top row are 200µm, all lower rows same magnification) original magnifications. Broth-treated controls fed maintenance diet are shown. No significant differences were noted between maintenance and high vitamin D diet groups treated with broth.
Because inflammation is often associated with increased cell proliferation, we evaluated if increased dietary vitamin D was associated with changes in cell proliferation in either lamina propria or epithelial cell populations within the colon one week post Hb-treatment. Proliferating Cell Nuclear Antigen (PCNA) was used as a marker of cellular proliferation by Western blot. Analysis of PCNA showed no significant differences associated with diet in either cell population (LPL mean 0.73 vs. 0.67, epithelial cell mean 0.99 vs. 0.85, high vitamin D diet vs. maintenance diet respectively, data not shown).
High dietary vitamin D decreases cecal, proximal colon and fecal proinflammatory cytokines 1 week post Hb-infection
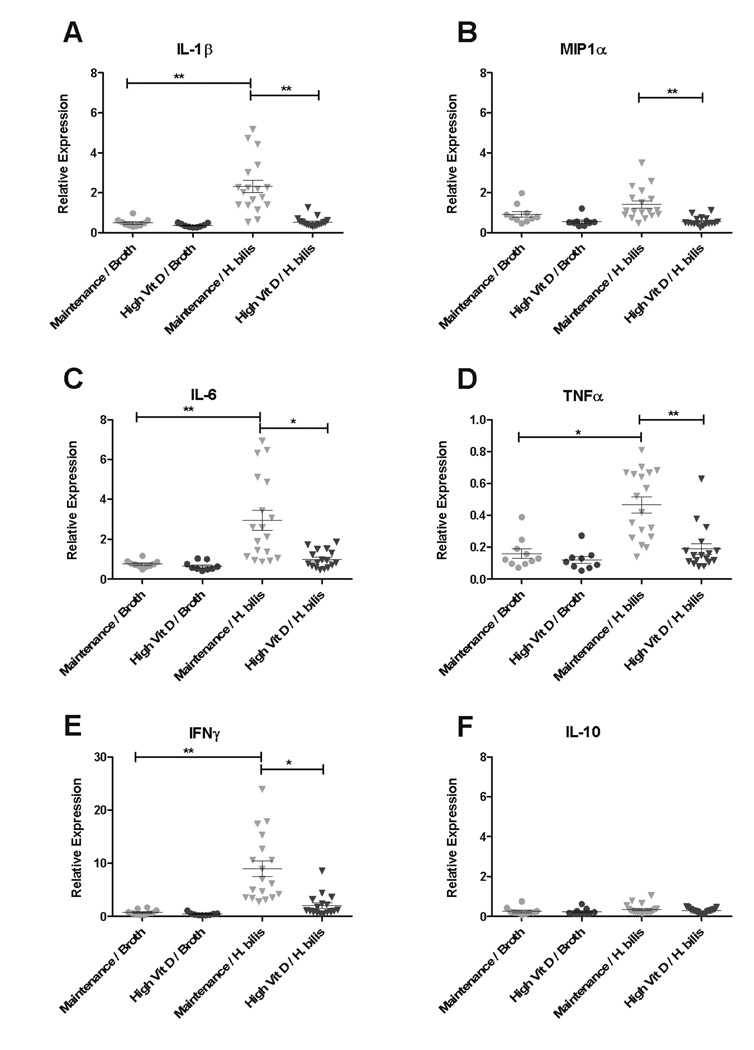
Because we previously observed elevated inflammatory cytokines in this model associated with Hb-infection (16), proinflammatory cytokine expression in cecal and proximal colon tissues one-week post Hb-infection were evaluated to determine whether increased vitamin D would dampen the inflammatory response induced by infection. Increased expression of IL1β, macrophage chemotaxis factor 1α (MIP1α), IL6, TNFα, and IFNγ were noted in cecal tissue from Hb-infected animals fed maintenance diet compared to broth-treated controls (Fig 3) as seen previously with this model (16). However, there was a significant reduction in expression of those same cytokines in Hb-infected mice fed increased dietary vitamin D compared with those fed maintenance diet (Fig 3A–E). Interestingly, there were no changes in expression of the anti-inflammatory cytokine, IL-10 in response to diet or Hb-infection (Fig 3F). Expression patterns of proinflammatory cytokines in the proximal colon were similar to those observed in cecal tissue (Supplemental Fig 4A–F).
Figure 3. High dietary vitamin D decreases cecal tissue expression of proinflammatory and chemotactic cytokines 1 week post Hb-infection.
Expression of IL-1β (A), MIP-1α (B), IL-6 (C), TNFα (D), IFNγ (E), and IL-10 (F) in cecal tissue from Smad3−/− mice fed high vitamin D or maintenance diet with or without Hb-infection was determined by real-time PCR. Hb-infected/ maintenance diet (n=20), Hb-infected/ high vitamin D diet (n=20), broth-treated/ maintenance diet (n=10), and broth-treated/ high vitamin D diet (n=10). Note differences in scale in (D) and (E). Kruskal-Wallis non-parametric test and Dunn’s post-hoc test to control for multiple comparisons. *p<0.05, **p<0.001
Fecal cytokines have been used in the Hb-infected Smad3−/− mouse model to characterize the inflammatory response and predict development of cancers (24). To determine if fecal cytokine expression correlated with tissue cytokine expression, expression of IL1β and MIP1α was evaluated in fecal pellets collected from animals 1, 2, and 3 weeks post Hb-infection. Similar to the expression pattern of IL1β and MIP1α observed in cecal and proximal colon tissues at 1 week post-infection (Fig 3A and B, Supplemental Fig 4A and B), expression of IL1β and MIP1α in feces were significantly increased in Hb-infected animals fed maintenance diet compared with broth-treated animals (Supplemental Fig 4G–H). Accordingly, Hb-infected mice fed high vitamin D diet had an average 3-fold decrease in fecal IL1β and 1.5-fold decrease in fecal MIP1α expression compared with mice fed the maintenance diet. However, these fecal cytokine changes were transient as there were no significant differences in IL1β or MIP1α expression between any treatment group at 2 or 3 weeks post-infection (data not shown).
Increased dietary vitamin D decreases p-P38 MAPK in the colon
Helicobacter species have been shown to elicit proinflammatory cytokine production through TLR-4 dependent activation of the MAPK and NFκB pathways (25). Thus, we determined whether decreased colonic inflammation in Smad3−/− mice in response to increased dietary vitamin D was associated with altered MAPK and NFkB signaling pathways during early disease (1 week post Hb infection).
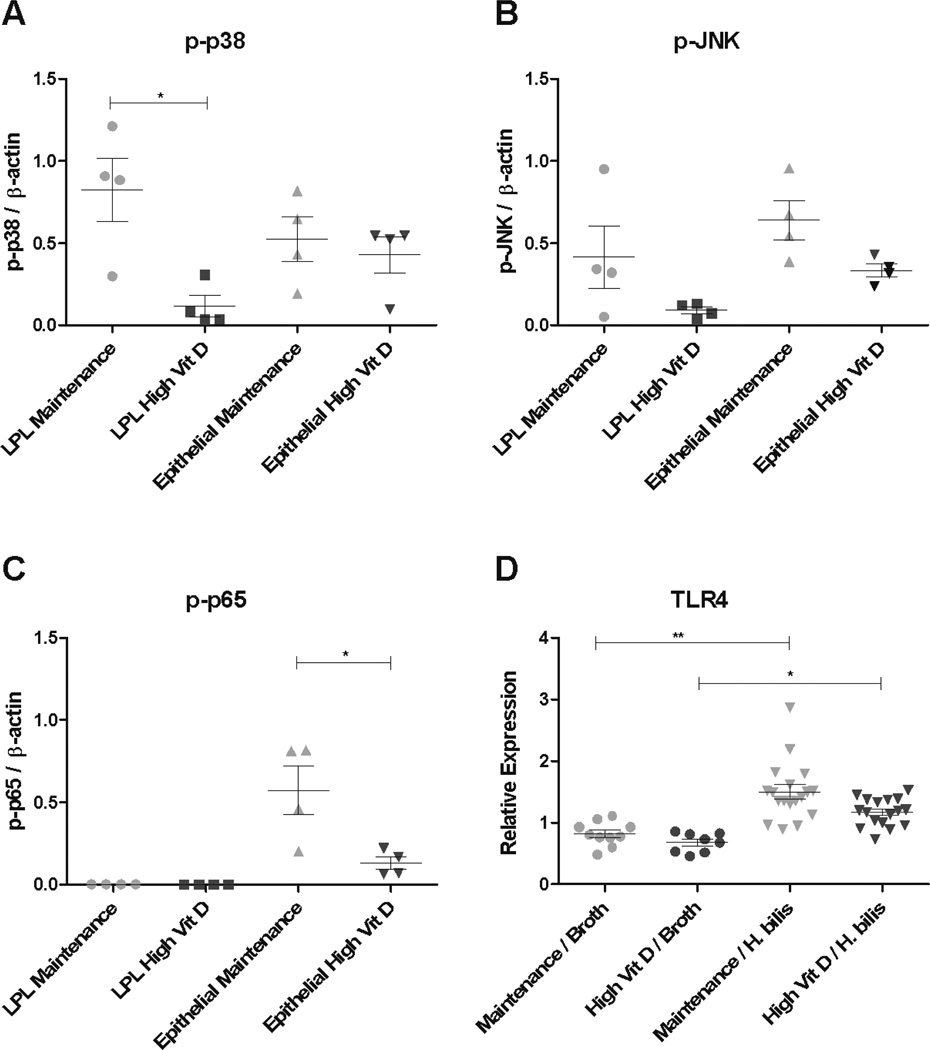
For changes in the MAPK pathway, relative levels of protein expression of activated forms of p38 (p-p38), JNK (p-JNK), and Erk1/2 (p-Erk) in LPL and colonic epithelial cell populations were determined. LPLs of Hb-infected animals fed increased dietary vitamin D had a 7-fold decrease in p-p38 compared to maintenance diet-fed animals (p=0.012, Fig 4A and Supplemental Fig 5). Interestingly, similar changes were not detected in colonic epithelial cells (Fig 4A and Supplemental Fig 5). There was a trend toward decreased p-JNK expression in colonic tissue from animals fed increased dietary vitamin D, however, these differences were not statistically significant (LPL: 4.5-fold decrease p= 0.14 and epithelial cells: 2-fold decrease p=0.052, Fig 4B and Supplemental Fig 5). There were no notable differences in p-Erk1/2, total P38 or total JNK in either cell population (data not shown).
Figure 4. Increased dietary vitamin D decreases MAPK and NFκB signaling in Hb-infected Smad3−/− mice.
Whole cell lysates were isolated from proximal colon LPL and epithelial cells harvested 1 week post Hb infection. Expression levels of p-p38 (A), p-JNK (B), and p-p65 (C) were determined by western blot and densitometry. Densitometry results of each protein level were normalized to β-actin. Unpaired t test *p<0.05, **p<0.001. Hb-infected on maintenance diet (n=4) and Hb-infected on high vitamin D diet (n=4). (D) Colonic expression of TLR4 was determined by qRT-PCR from mice fed high vitamin D or maintenance diet and infected with and without Hb. Hb-infected/ maintenance diet (n=20), Hb-infected/ high vitamin D diet (n=20), broth-treated/ maintenance diet (n=10), and broth-treated/ high vitamin D diet (n=10). Kruskal-Wallis non-parametric test with Dunn’s post-hoc test (*p<0.05 and **P<0.001).
For alterations in the NFκB pathway, phosphorylated p65 (p-p65) was evaluated by western blot analysis in LPL and epithelial cell populations. Mice fed high vitamin D diet had a 4.5-fold decrease in p-p65 in colonic epithelial cells compared to mice fed maintenance diet (p=0.028, Fig 4C and Supplemental Fig 5). We did not detect p-p65 in LPL regardless of diet while IκBα, an inhibitor of NFκB activation, was present in both LPL and colonic epithelial cells. (Supplemental Fig 5).
To determine if the changes in p-P38 or p-P65 were associated with decreased TLR4 expression, qRT-PCR was performed on cecal tissues collected from mice 1week post Hb-infection. Average TLR4 expression in Hb-infected animals was modestly increased (1.3-fold) compared to broth-treated controls (p<0.01, Fig 4D). Although TLR4 expression in Hb-infected mice was lower when fed high vitamin D diet, the difference was not statistically significant.
High dietary vitamin D does not alter Hb colonization in Smad3−/− mice
Changes in the gut microbiome or changes in bacterial load can influence disease severity in both human patients (26) and animal models of IBD (26, 27). As TLR4 expression levels tend to be lower in high vitamin D fed Smad3−/− mice following Hb infection, we determined whether vitamin D alters H.bilis colonization in cecum and colon where they preferentially reside (16). Quantitative RT-PCR was used to compare the relative amount of Helicobacter organisms in cecal, proximal and distal colonic tissues collected 8-weeks post Hb-infection from mice fed either increased or maintenance levels of vitamin D. While cecal tissues had the highest concentration of Helicobacter as previously reported (16), no significant differences in Helicobacter colonization were associated with diet (Supplemental Fig 6).
High dietary vitamin D does not alter cecal expression of vitamin D receptor or enzymes involved in vitamin D metabolism
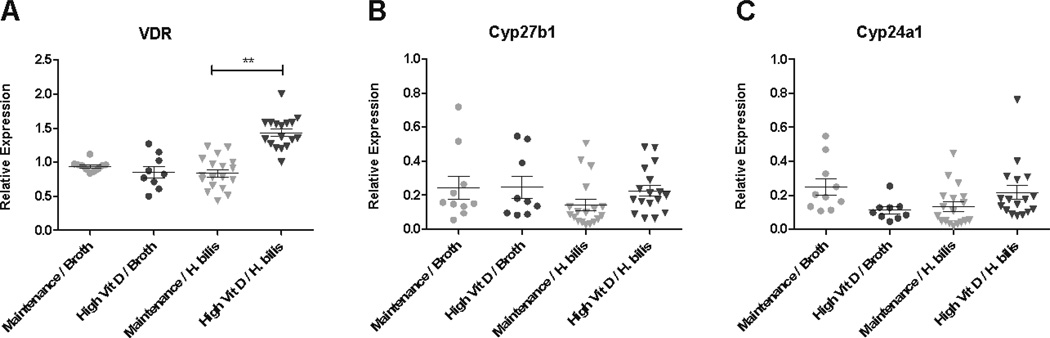
To determine whether the protective effect of increased dietary vitamin D was associated with changes in proteins involved in vitamin D signaling and/or metabolism, quantitative RT-PCR was used to evaluate RNA expression of vitamin D receptor (VDR) as well as two enzymes involved in conversion of vitamin D into its active and inactive forms, 25(OH)D3-1α-hydroxylase (Cyp27b1), and 1,25(OH)2D3 24 hydroxylase (Cyp24a1) in cecal tissue at one-week post Hb-infection. A small yet significant increase in VDR expression was detected in mice fed high vitamin D diet following Hb-infection (Fig 5A). However, there were no changes in mRNA levels of Cyp27b1 and Cyp24a1 associated with either diet or infection-status (Fig 5B–C). Although VDR mRNA was altered with diet, differences in VDR protein expression were not detected using western blot analysis of proximal colon tissue (data not shown).
Figure 5. Increased dietary vitamin D increases VDR mRNA expression but does not alter enzymes involved in vitamin D metabolism (Cyp27b1 and Cyp24a1).
Expression levels of VDR (A), Cyp27b1 (B) and (C) Cyp24a1 were determined with qRT-PCR using RNA from proximal cecum (3mm piece) isolated from mice 1 week post Hb-infection. Hb-infected/ maintenance diet (n=20), Hb-infected/ high vitamin D diet (n=20), broth-treated/ maintenance diet (n=10), and broth-treated/ high vitamin D diet (n=10). Kruskal-Wallis non-parametric test and Dunn’s post-hoc test to control for multiple comparisons. **p<0.001
A vitamin D deficient diet did not exacerbate colitis or colitis-associated colon cancer in Hb-infected Smad3−/− mice
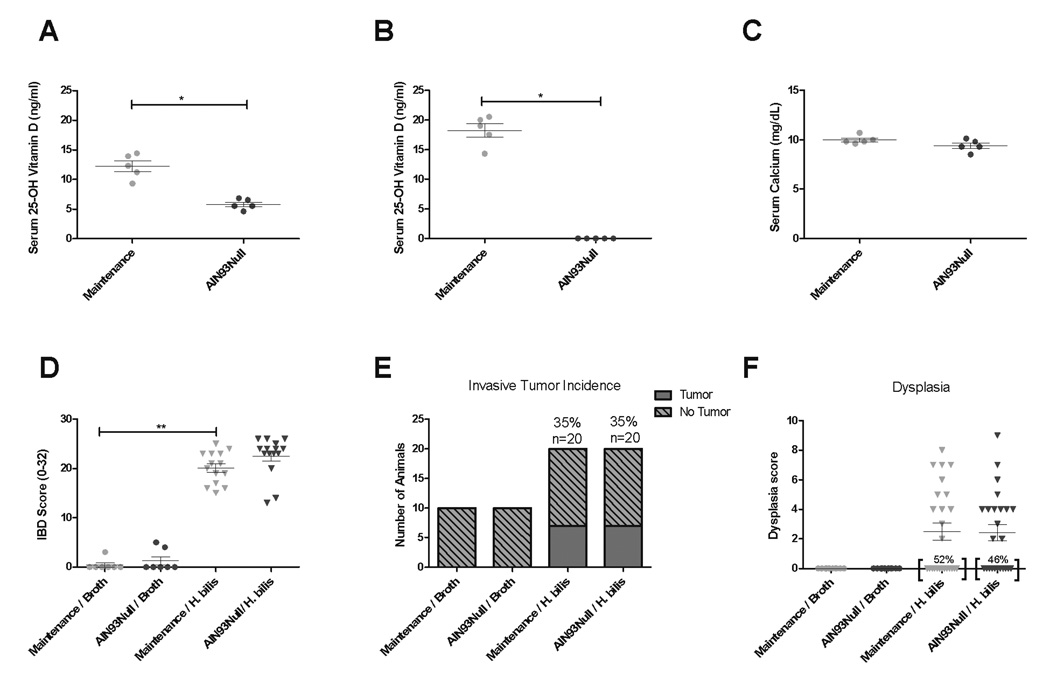
Because decreased serum vitamin D is associated with an increased risk for developing IBD as well as colon cancer in humans (4–7), we hypothesized that a diet deficient in vitamin D would exacerbate inflammation and potentially increase the incidence of colon tumors in Hb-infected Smad3−/− mice. We tested this hypothesis by feeding mice maintenance diet or diet devoid of vitamin D (AIN93Null) and induced inflammation by Hb infection. Serum 25-hydroxyvitamin D levels were significantly decreased in AIN93Null-fed mice compared to mice fed maintenance diet (mean: 5.7 vs 12.2 ng/ml, p=0.01) after two weeks on the diet and were below the limit of detection at the end of the 16 week study (Fig 6A–B). Despite decreased serum vitamin D levels, mice maintained on AIN93Null diet showed no differences in serum calcium compared to maintenance diet fed controls (Fig 6C). Clinical disease during the early inflammatory phase was assessed by monitoring subjective fecal scores and body weight change following Hb infection. Mice maintained on AIN93Null diet showed no differences in fecal scores or body weight change compared to maintenance diet fed controls (data not shown). To determine if decreased dietary vitamin D exacerbated IBD at early disease stages, proximal colon and cecal tissue of mice fed maintenance diet or AIN93Null were analyzed for inflammation one week post Hb infection. There were no differences in IBD associated with diet (Fig 6D). To further evaluate any effect of AIN93Null diet, proinflammatory cytokine expression in proximal colon tissues was measured to determine if decreased dietary vitamin D would exacerbate the inflammatory response induced by Hb. In correlation with the lack of effect on IBD scores, no differences were noted in cytokine gene expression associated with diet (Supplemental Fig 7). Consistent with the lack of altered inflammation early in disease, there were no differences in cancer incidence or dysplasia noted between AIN93Null fed mice compared with maintenance fed animals (Fig 6E–F) when necropsied after 16 weeks of Hb infection.
Figure 6. AIN93Null diet does not exacerbate Hb-induced disease in Smad3−/− mice.
Serum 25-hydroxyvitamin D was measured after (A) two weeks or (B) 18 weeks on diet. Serum calcium levels (C) were determined at 18 weeks after diet initiation. Mann-Whitney t test. *p<0.05, **p<0.001. One week post Hb-infection, cecum and proximal colon were histologically scored for inflammation (D). Hb-infected/ maintenance diet (n=14), Hb-infected/ AIN93Null diet (n=15), broth-treated/ maintenance diet (n=7), and broth-treated/ AIN93Null diet (n=7). Mann-Whitney t test. **p<0.001. 16 weeks post infection, colon and cecum were analyzed for cancer incidence (E) and dysplasia (F). Indicence of no dyplasia is indicated by percents near brackets (F). Hb-infected/ maintenance diet (n=20), Hb-infected/ AIN93Null diet (n=20), broth-treated/ maintenance diet (n=10), and broth-treated/ AIN93Null diet (n=10).
Discussion
Using Smad3−/− mice, we have shown that increased dietary vitamin D affords protection against the development of colon cancer. In this model, we have demonstrated that increased dietary vitamin D a) induces elevated serum 25-hydroxyvitamin D without causing hypercalcemia, b) significantly decreases inflammation, dysplasia and tumor incidence following infection with Hb, and c) is associated with decreased p- p38 (MAPK) and p-P65 (NFκB) expression during the acute inflammatory stage of disease. These studies provide evidence that the protective effect(s) of elevated dietary vitamin D supplementation in a model of inflammation-associated colon cancer are mediated through suppression of the inflammatory responses triggered following infection with colitogenic bacteria.
Animals fed increased concentrations of dietary vitamin D demonstrated significant protection against inflammation and tumor formation. It should be noted that though serum vitamin D levels are increased in mice fed high vitamin D diet compared to those fed maintenance diet, levels remain within the comparable recommended range for humans without reaching super-physiologic levels (28). Many animal and human studies that have shown anti-tumor effects of vitamin D administer metabolically active 1,25(OH)2D3 which results in hypercalcemia and vitamin D toxicities (10, 12, 15). Our studies show that dietary vitamin D supplementation offers a way to improve vitamin D status and provide protection from inflammation-associated colon cancer while avoiding vitamin D toxicity.
Vitamin D supplementation decreases cancer incidence in several rodent models of colon cancer (12–15). These models rely on either genetic predisposition for the development of gastrointestinal neoplasias as is the case with APCMin/+ mice or treatment with a chemical mutagen such as azoxymethane (AOM) to simulate the adenoma to carcinoma progression that occurs during the development of sporadic colon cancer (8, 29). However, the molecular changes, disease progression, and pathology of inflammation-associated cancer are distinct from those changes observed in sporadic or familial colon cancers and therefore may influence the efficacy of vitamin D as well as mechanisms through which it promotes protection from tumor formation.
Chronic inflammation is believed to frequently play a key role in carcinogenesis (9). Links between inflammation and cancer have not only been observed in colon cancer in human patients with IBD (8) but also in liver, pancreatic, stomach, esophageal, and prostate cancers (9). Several studies utilizing mouse models of colitis have shown that vitamin D can be beneficial in preventing or ameliorating inflammation and clinical disease (30–32); however, these models do not typically progress to neoplasia. Recently, the DSS/AOM model, another model of inflammation associated cancer which does progress to dysplasia and tumor formation, was used to demonstrate that increasing concentrations of dietary vitamin D are protective against preneoplasic lesions in a dose-dependent manner (33). Consistent with these findings, we have shown that increased dietary vitamin D is effective at not only preventing inflammation and dysplasia, but subsequent invasive tumor formation as well.
Chronic inflammation is associated with increased production of proinflammatory cytokines including TNFα, IL1β, and IL-6 which contribute to carcinogenesis through influences on cell proliferation, apoptosis, differentiation, and angiogenesis (34). Dietary vitamin D supplementation significantly lowered inflammatory cytokines induced in response to Hb in Smad3−/− mice. In human patients with colon cancer, these proinflammatory cytokines are positively associated with increased cancer growth, higher neoplastic grade, and increased risk of mortality (34). Proinflammatory cytokines are also upregulated in IBD patients, even before the onset and progression to dysplasia or neoplasia (35), and vitamin D supplementation has been linked to decreased circulating proinflammatory cytokines in patients with colorectal adenomas (36). Epidemiologic evidence suggests that treatments which limit inflammation may be beneficial in reducing the incidence of inflammation-associated colon cancer in high risk populations (37). Together, these data suggest that dietary vitamin D may be effective at decreasing the proinflammatory milieu in IBD patients and serve as a useful adjunct treatment in certain populations.
The mechanism by which vitamin D suppresses colon cancer in Smad3−/− mice is not completely clear, although our data suggests the anti-inflammatory effects of vitamin D are important. Helicobacter species are Gram negative, microaerophilic bacteria that can induce local production of proinflammatory cytokines and chemokines through TLR4 signaling and subsequent activation of the MAPK and NFκB pathways (25). Both of these pathways have been shown to be upregulated in human patients with IBD (38) and are thought to be important links between inflammation and cancer (38–40). In vitro evidence suggests that vitamin D is able to suppress MAPK activity and subsequent proinflammatory cytokine production through the upregulation of MAPK phosphatase-1(41) and NFκB signaling through the upregulation of IκBα, an inhibitor of NFκB activation (42), or through decreased expression of the NFκB component RelB which can lead to inhibition of dendritic cell differentiation and maturation (43). During the early inflammatory disease phase in Hb-infected Smad3−/− mice, vitamin D high diet was associated with dramatic decreases in p-p38 in the lamina propria cells, decreased NFκB activation in epithelial cell populations, and suppressed proinflammatory cytokine expression compared to that observed in infected mice on maintenance diet. Based on these data, we propose a model where vitamin D suppresses inflammation by decreasing p38 MAPK activation in lamina propria cells, resulting in decreased proinflammatory cytokine production by those cells, which in turn decreases NFκB activation in colonic epithelial cells.
We have shown that while increased dietary vitamin D affords protection against the development of colon cancer, decreased dietary vitamin D was not sufficient to exacerbate disease in Smad3−/− mice. Because epidemiologic studies (5, 7, 30) as well as studies utilizing mouse models of colitis suggest that vitamin D deficiency or lack of vitamin D signaling can exacerbate IBD (44–46), we hypothesized that decreased dietary vitamin D would exacerbate disease in the Smad3−/− mouse model. Interestingly, although the AIN93Null diet significantly depleted circulating serum 25-hydroxyvitamin D levels, we did not see exacerbation of Hb- induced inflammation or subsequent inflammation-associated colon cancer. These findings are consistent with the idea that modulation of inflammation is likely responsible for the protection afforded by increased dietary vitamin D.
In conclusion, increased dietary vitamin D suppresses acute inflammation and consequently neoplastic development in a mouse model of bacterial-driven colon cancer. While additional studies are needed to elucidate the molecular mechanisms through which vitamin D and TGFβ interact to afford protection in this model, these findings suggest that vitamin D supplementation may prove useful in the treatment of IBD or potentially the prevention of inflammation-associated cancer by limiting inflammation early in disease development.
Supplementary Material
Acknowledgments
Financial support: This research was supported by AICR 09A136-Rev, NIH 5T32DK007742-17 and NIH R21 CA149995-01A1.
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Gill S, Sinicrope FA. Colorectal cancer prevention: is an ounce of prevention worth a pound of cure? Semin Oncol. 2005;32(1):24–34. doi: 10.1053/j.seminoncol.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Lofano K, Principi M, Scavo MP, Pricci M, Ierardi E, Di Leo A. Dietary Lifestyle and Colorectal Cancer Onset, Recurrence, and Survival: Myth or Reality? J Gastrointest Cancer. 2012 doi: 10.1007/s12029-012-9425-y. [DOI] [PubMed] [Google Scholar]
- 3.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 4.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9(3):227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 5.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012;142(3):482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi: 10.3389/fimmu.2012.00107. PMCID: 3347037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 10.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr. 2004;134(12 Suppl):3463S–3471S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]
- 11.Fichera A, Little N, Dougherty U, Mustafi R, Cerda S, Li YC, et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J Surg Res. 2007;142(2):239–245. doi: 10.1016/j.jss.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Akhter J, Chen X, Bowrey P, Bolton EJ, Morris DL. Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Dis Colon Rectum. 1997;40(3):317–321. doi: 10.1007/BF02050422. [DOI] [PubMed] [Google Scholar]
- 13.Murillo G, Mehta RG. Chemoprevention of chemically-induced mammary and colon carcinogenesis by 1alpha-hydroxyvitamin D5. The Journal of steroid biochemistry and molecular biology. 2005;97(1–2):129–136. doi: 10.1016/j.jsbmb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Wali RK, Bissonnette M, Khare S, Hart J, Sitrin MD, Brasitus TA. 1 alpha,25-Dihydroxy-16-ene-23-yne-26,27-hexafluorocholecalciferol, a noncalcemic analogue of 1 alpha,25-dihydroxyvitamin D3, inhibits azoxymethane-induced colonic tumorigenesis. Cancer research. 1995;55(14):3050–3054. [PubMed] [Google Scholar]
- 15.Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, et al. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) Mouse. Cancer research. 2002;62(3):741–746. [PubMed] [Google Scholar]
- 16.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66(2):828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauchemin N, Huot J. Dordrecht. London: Springer; 2010. Metastasis of colorectal cancer. [Google Scholar]
- 18.Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G764–G778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- 19.Maggio-Price L, Treuting P, Bielefeldt-Ohmann H, Seamons A, Drivdahl R, Zeng W, et al. Bacterial infection of Smad3/Rag2 double-null mice with transforming growth factor-beta dysregulation as a model for studying inflammation-associated colon cancer. Am J Pathol. 2009;174(1):317–329. doi: 10.2353/ajpath.2009.080485. PMCID: 2631344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall D, Cameron J, Lightwood D, Lawson AD. Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflamm Bowel Dis. 2007;13(2):219–224. doi: 10.1002/ibd.20055. [DOI] [PubMed] [Google Scholar]
- 21.Torrence AE, Brabb T, Viney JL, Bielefeldt-Ohmann H, Treuting P, Seamons A, et al. Serum biomarkers in a mouse model of bacterial-induced inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(4):480–490. doi: 10.1002/ibd.20347. [DOI] [PubMed] [Google Scholar]
- 22.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124(3):762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 23.Myles MH, Dieckgraefe BK, Criley JM, Franklin CL. Characterization of cecal gene expression in a differentially susceptible mouse model of bacterial-induced inflammatory bowel disease. Inflammatory bowel diseases. 2007;13(7):822–836. doi: 10.1002/ibd.20138. [DOI] [PubMed] [Google Scholar]
- 24.Ericsson AC, Myles M, Davis W, Ma L, Lewis M, Maggio-Price L, et al. Noninvasive detection of inflammation-associated colon cancer in a mouse model. Neoplasia. 2010;12(12):1054–1065. doi: 10.1593/neo.10940. PMCID: 3003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathak SK, Basu S, Bhattacharyya A, Pathak S, Banerjee A, Basu J, et al. TLR4-dependent NF-kappaB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to Helicobacter pylori peptidyl prolyl cis-, trans-isomerase (HP0175)-mediated induction of IL-6 release from macrophages. Journal of immunology. 2006;177(11):7950–7958. doi: 10.4049/jimmunol.177.11.7950. [DOI] [PubMed] [Google Scholar]
- 26.Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. PMCID: 3032643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez GA, Appleyard CB. Bacterial load in animal models of acute and chronic 'reactivated' colitis. Digestion. 2003;67(3):161–169. doi: 10.1159/000071296. [DOI] [PubMed] [Google Scholar]
- 28.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med. 2004;351(15):1548–1563. doi: 10.1056/NEJMcpc049016. [DOI] [PubMed] [Google Scholar]
- 29.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. PMCID: 3072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130(11):2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 31.Ryz NR, Patterson S, Zhang Y, Ma C, Huang T, Bhinder G, et al. Active Vitamin D(1,25-Dihydroxyvitamin D3)Increases Host Susceptibility to Citrobacter rodentium by Suppressing Mucosal Th17 Responses. American journal of physiology Gastrointestinal and liver physiology. 2012 doi: 10.1152/ajpgi.00320.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1,25(OH)2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12(1):57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummel DM, Thiem U, Hobaus J, Mesteri I, Gober L, Stremnitzer C, et al. Prevention of preneoplastic lesions by dietary vitamin D in a mouse model of colorectal carcinogenesis. The Journal of steroid biochemistry and molecular biology. 2012 doi: 10.1016/j.jsbmb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumm JB, Oft M. Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene. 2008;27(45):5913–5919. doi: 10.1038/onc.2008.275. [DOI] [PubMed] [Google Scholar]
- 35.Talero E, Sanchez-Fidalgo S, Villegas I, de la Lastra CA, Illanes M, Motilva V. Role of different inflammatory and tumor biomarkers in the development of ulcerative colitis-associated carcinogenesis. Inflammatory bowel diseases. 2011;17(3):696–710. doi: 10.1002/ibd.21420. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, et al. Effects of supplemental vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: a randomized, controlled clinical trial. Cancer Prev Res (Phila) 2011;4(10):1645–1654. doi: 10.1158/1940-6207.CAPR-11-0105. PMCID: 3188339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 38.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115(2):357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 39.Slattery ML, Lundgreen A, Wolff RK. MAPKinase genes and colon and rectal cancer. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of immunology. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. PMCID: 3368346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291(2):E315–E322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 43.Griffin MD, Dong X, Kumar R. Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation. Archives of biochemistry and biophysics. 2007;460(2):218–226. doi: 10.1016/j.abb.2007.01.034. PMCID: 1945094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151(6):2423–2432. doi: 10.1210/en.2010-0089. PMCID: 2875827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, Yamaori S, Tanabe T, Johnson CH, Krausz KW, Kato S, et al. Implication of intestinal VDR deficiency in inflammatory bowel disease. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbagen.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17(12):2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.