Abstract
The present in vitro microperfusion study compared the mechanism and rates of NaCl transport in neonatal and adult rabbit proximal straight tubules. In proximal straight tubules perfused with a late proximal tubular fluid and bathed in a serumlike albumin solution, the rate of volume absorption (JV) was 0.54 ± 0.10 and 0.12 ± 0.05 nl·mm−1·min−1 in adults and neonates, respectively (P < 0.05). With the addition of 10−5 M bath ouabain, JV decreased to 0.27 ± 0.07 and −0.03 ± 0.04 nl·mm−1·min−1 in adult and neonatal tubules, respectively (P < 0.05), consistent with lower rates of active and passive NaCl transport in the neonatal proximal straight tubule. The effect of luminal sodium and chloride removal on intracellular pH was used to assess the relative rates of Na+/H+ and Cl−/base exchange. The rates of Na+/H+ and Cl−/base exchange were approximately fivefold less in neonatal proximal straight tubules than adult tubules. In both neonatal and adult proximal straight tubules, the rate of Cl−/base exchange was not affected by formate, bicarbonate, or cyanide and acetazolamide, consistent with Cl−/OH− exchange. These data demonstrate an increase in proximal straight tubule NaCl transport during postnatal renal development.
Keywords: renal development, intracellular pH, sodium chloride transport, sodium/proton antiporter
The proximal convoluted tubule preferentially reabsorbs organic solutes and bicarbonate, leaving the luminal fluid with a higher chloride concentration than that in the peritubular plasma (15, 16). This chloride concentration gradient provides a driving force for passive chloride diffusion across the para-cellular pathway. In addition to passive chloride transport, there is active electroneutral transcellular NaCl transport by the proximal tubule (2, 7). In the adult rabbit proximal convoluted tubules perfused with a late proximal tubular fluid, approximately two-thirds of NaCl transport is active and one-third is passive (7).
The parallel operation of the Na+/H+ antiporter and Cl−/base exchange is thought to mediate net NaCl transport across the apical membrane of the proximal tubule (3, 5). There is evidence for Cl−/OH− or Cl−/ exchange on brush-border membrane vesicles (25); however, others have not found these exchangers (21). Brush-border membrane vesicles have a Cl−/formate exchanger (11, 12). Thus the parallel operation of Cl−/formate exchange and the Na+/H+ antiporter with formic acid recycling could mediate net NaCl transport (3, 12). Indeed, formate has been shown to stimulate NaCl transport when added to the apical and bathing solutions of proximal convoluted and straight tubules perfused in vivo and in vitro (14, 18, 19, 23, 24).
The rate of bicarbonate transport by the neonatal proximal tubule and apical membrane Na+/H+ antiporter activity are less in the neonatal proximal tubule than in the adult (4, 6, 20). Nonetheless, the luminal concentration of bicarbonate decreases along the length of the proximal tubule (20). The mechanism of NaCl transport in the neonate is unknown. The present in vitro microperfusion study examined the relative rates of active and passive NaCl transport in neonatal and adult proximal straight tubules. We also examined the rates of Na+/H+ antiporter and Cl−/base exchange in neonatal and adult proximal straight tubules.
METHODS
Isolated segments of superficial proximal straight tubules were perfused as previously described (6, 7, 9). Briefly, tubules were dissected in Hanks’ balanced salt solution containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2, 10 Tris hydrochloride, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. Tubules were transferred to a 1.2 ml temperature-controlled bath for flux studies and a 0.2-ml chamber in which the bathing solution was preheated to 38°C in intracellular pH (pHi) studies. The tubules were perfused using concentric glass pipettes.
In vitro microperfusion flux studies
Tubules were perfused at ~10 nl/min with a high-chloride solution simulating late proximal tubular fluid containing (in mM) 140 NaCl, 5 NaHCO3, 5 KCl, 4 Na2HPO4, 1 CaCl2, and 1 MgSO4. The bathing solution was a serumlike albumin solution containing (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 sodium acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, 5 alanine, and 6 g/dl bovine serum albumin. The osmolalities of these solutions were adjusted to 295 mosmol/kgH2O. The pH and osmolality of the bathing solution were maintained constant by continuously changing the bath at a rate of 0.5 ml/min.
Net volume absorption (JV, in nl·mm−1·min−1) was measured as the difference between the perfusion (VO) and collection (VL) rates (in nl/min) normalized per millimeter of tubular length (L). Exhaustively dialyzed [methoxy-3H]inulin was added to the perfusate at a concentration of 75 µCi/ml so that the perfusion rate could be calculated. The collection rate was measured with a 50-nl constant-volume pipette. The length (in mm) was measured with an eyepiece micrometer.
The transepithelial potential difference (PD, in mV) was measured using the perfusion pipette as the bridge into the tubular lumen. The perfusion and bath solutions were connected to the recording and reference calomel half-cells, via bridges containing perfusion and an ultrafiltrate of the bathing solution, respectively, in series with a 3.6 M KCl/0.9 M KNO3 agarose bridge. This arrangement avoided direct contact of KCl/KNO3 agarose bridges with the solution that bathed the tubule. The recording and reference calomel half-cells were connected to the high- and low-impedance side, respectively, of an electrometer (model 602; Keithley Instruments, Cleveland, OH).
Tubules were incubated for at least 30 min before initiation of the control period. There were at least three collections in each period for measurement of volume absorption. The mean rate was used as the rate of volume absorption for that tubule. Ouabain (10−5 M) was then added to the bathing solution to inhibit active transport and repeat collections were performed after incubation for at least 10 min.
Measurement of pHi
The solutions used in these experiments are shown in Table 1. The fluorescent dye 2,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) was used to measure pHi as described previously (1, 4, 6, 23). Measurements of pHi were made using a Nikon inverted epifluorescent microscope attached to a PTI Ratiomaster at a rate of 30 measurements per second. A variable diaphragm was placed over the area to be measured. To calculate pH from the ratio of fluorescence (F500/F450), a nigericin calibration curve was performed as previously described (1, 6). There was no difference in the calibration curves of adult and neonatal proximal straight tubules.
Table 1.
Solutions used in pHi studies
| High Cl−, 0 Na |
0 Cl−, 0 Na |
0 Cl−, High Na+ |
0 Cl−, 0 Na+, +20 |
High Cl−, 0 Na+, +25 |
0 Cl−, 0 Na+, +25 |
|
|---|---|---|---|---|---|---|
| TMA-Cl | 140 | 115 | ||||
| TMA-OH | 140 | 120 | 115 | |||
| TMA-HCO3 | 25 | 25 | ||||
| NH4OH | 20 | |||||
| Na gluconate | 140 | |||||
| Gluconic acid lactone | 140 | 140 | 115 | |||
| K2HPO4 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| MgCl2 | 1 | 1 | ||||
| Mg gluconate | 1 | 1 | 1 | 1 | ||
| CaCl2 | 1 | 1 | ||||
| Ca gluconate | 12.5 | 12.5 | 12.5 | 10 | ||
| Glucose | 5 | 5 | 5 | 5 | 5 | 5 |
| l-Alanine | 5 | 5 | 5 | 5 | 5 | 5 |
| HEPES | 5 | 5 | 5 | 5 |
All constituents are in mM. All solutions were adjusted to an osmolality of 295 mosmol/kgH2O. -containing solutions were bubbled with 95% O2–5% CO2 and had a pH of 7.4. Non--containing solutions were bubbled with 100% O2 and had a pH of 7.4. pHi, intracellular pH; TMA, tetramethylammonium.
Tubules were incubated with the initial luminal and bathing solutions for at least 10 min and had a constant pHi for several minutes prior to measurement of transporter activity. dpHi/dt was measured from the slope of the change in pHi immediately after a luminal fluid exchange. Steady-state pHi values were achieved within 1 min after a luminal fluid exchange but were followed for several minutes to ensure a steady-state pHi was achieved.
Apparent buffer capacity (β) was measured as previously described using NH3/ (14, 17, 23). In the absence of HCO3, buffer capacity was 28.1 ± 5.0 mM/pH unit in neonatal proximal straight tubules and 43.0 ± 6.6 mM/pH unit in adult proximal straight tubules. Buffer capacity in the presence of HCO3 was estimated as the sum of the above buffer capacity and the HCO3 buffer capacity. The latter was calculated as 2.3·[HCO3]i (14, 17, 23), where [HCO3]i is the intracellular bicarbonate concentration. The buffer capacities in the presence of were 59.9 ± 7.4 and 71.4 ± 3.4 mM/pHi (not significant) in neonatal and adult proximal straight tubules, respectively.
Proton flux rates1 (JH, in pmol·mm−1·min−1) resulting from a luminal fluid change were calculated using the following formula JH = dpHi/dt·V/mm tubule·β. V was the tubular volume in liters, and β was the buffer capacity. Tubular volume was calculated from the measured inner and outer tubular diameters at ×400 magnification using an eyepiece reticle. The tubular volumes of neonatal and adult proximal straight tubules were 5.2 ± 0.2 × 10−10 and 10.5 ± 0.3 × 10−10 l/mm, respectively (P < 0.001).
Statistics
Data are expressed as means ± SE. Analysis of variance and the Student’s t-test for paired and unpaired data were used to determine statistical significance.
RESULTS
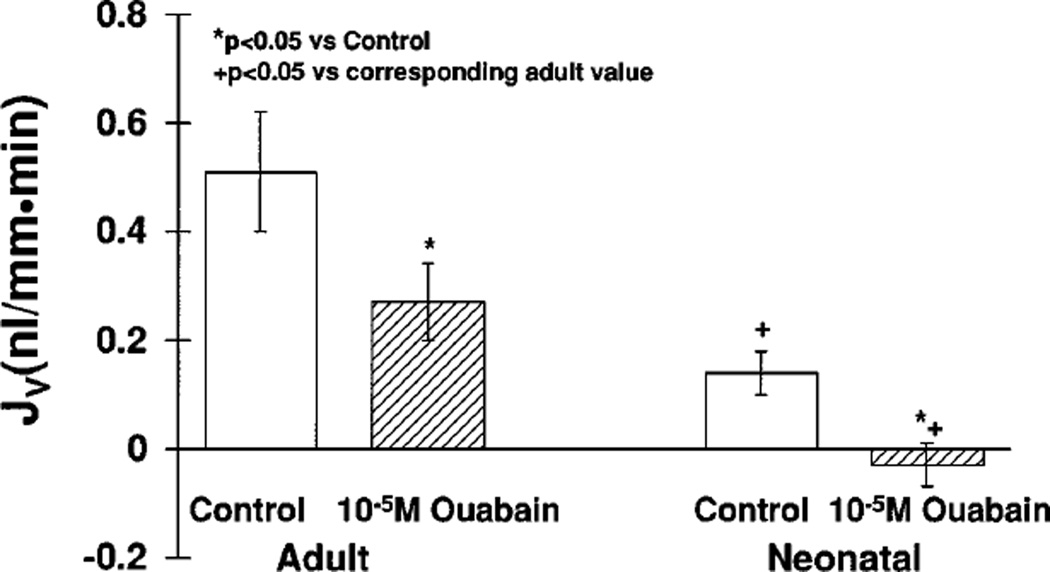
In the first series of experiments, we perfused neonatal and adult proximal straight tubules with a high-chloride solution simulating late proximal tubular fluid and bathed the tubules with a serumlike albumin solution. The tubular length in neonatal and adult tubules were 0.8 ± 0.1 mm. As shown in Fig. 1, the rates of volume absorption were significantly greater in adult proximal straight tubules than those measured in neonates. In both groups, 10−5 M ouabain was then added to the bathing solution so that the rate of passive transport could be determined. In adult proximal straight tubules, there was a 50% reduction in transport. However, in neonatal tubules the rate of volume absorption after addition of bath ouabain was not different from zero. These data are consistent with lower rates of both active and passive NaCl transport in neonatal compared with adult proximal straight tubules.
Fig. 1.
Effect of 10−5 M bath ouabain on adult and neonatal proximal straight tubule NaCl transport. Proximal straight tubules were perfused with a high-chloride solution simulating late proximal tubular fluid and bathed in a serumlike albumin solution. After measurements of volume absorption (JV) in the control period, 10−5 M bath ouabain was added to the bathing solution to inhibit active transport.
In tubules perfused with a high-chloride solution and bathed with a serumlike albumin solution, the transepithelial PD reflects the sum of any electrogenic sodium and proton transport and the chloride-bicarbonate diffusion potential. The PD was 3.0 ± 0.6 mV the control period and 3.1 ± 0.5 mV after the addition of ouabain (not significant). The PD of the neonatal tubules was only 0.2 ± 0.5 mV (P < 0.01 vs. adults) and increased significantly to 0.6 ± 0.5 mV after the addition of ouabain (P < 0.05).
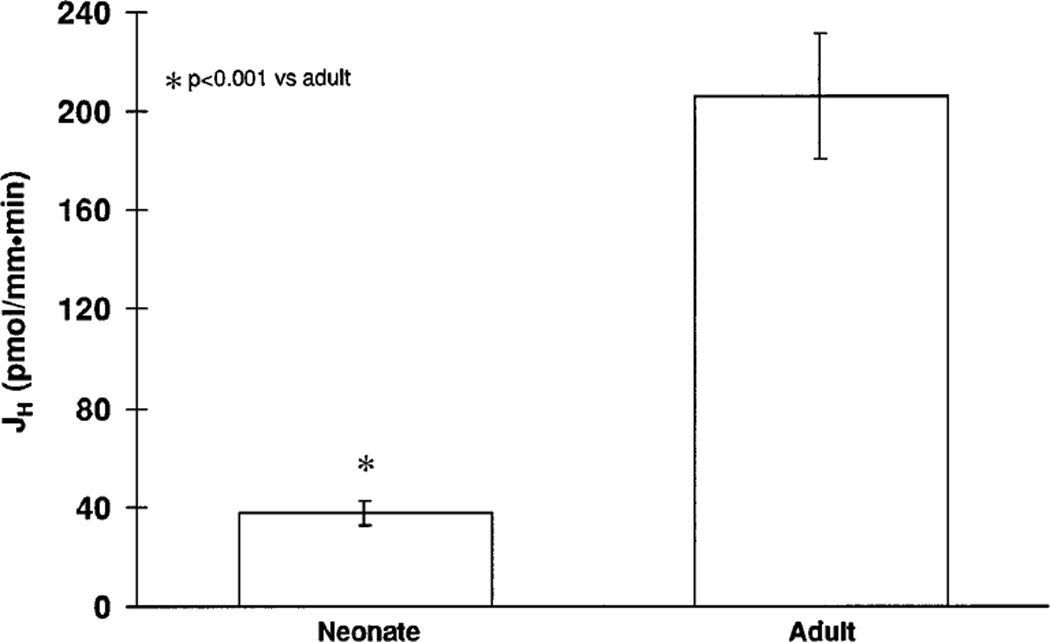
In adult proximal tubules, active NaCl transport is mediated by parallel Na+/H+ antiporter and Cl−/base exchange. To examine whether the lower rate of NaCl transport in neonates was due to a lower rate of Na+/H+ antiporter activity, we measured JH in response to addition of 140 mM luminal sodium in tubules initially perfused and bathed without sodium. The pHi for adult and neonatal tubules are shown in Table 2. As shown in Fig. 2, JH was significantly less in neonatal proximal tubules compared with that measured in adults. These data are consistent with a lower rate of Na+/H+ antiporter activity in neonatal compared with adult proximal straight tubules.
Table 2.
Effect on luminal Na+ on pHi in PST
| n | 0 Na+ | Na+ | 0 Na+ | |
|---|---|---|---|---|
| Adult PST | 6 | 7.32±0.12 | 7.81±0.10* | 7.23±0.18 |
| Neonatal PST | 8 | 7.26±0.16 | 7.66±0.15* | 7.13±0.16 |
Values are means ± SE; n = no. of experiments. PST, proximal straight tubule.
Na+ significantly different from 0 Na+ containing control and recovery (P<0.01).
Fig. 2.
Proton flux rates (JH) in response to addition of luminal sodium addition in neonatal and adult proximal straight tubules.
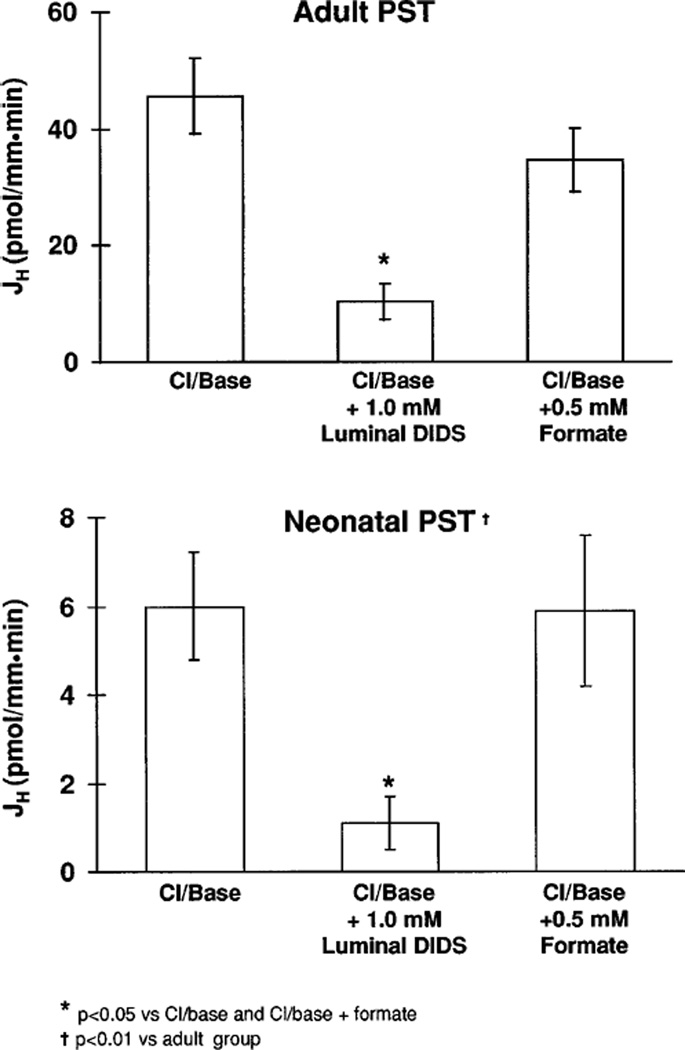
We next examined the rate of Cl−/base exchange in neonatal and adult proximal straight tubules. Tubules were initially perfused and bathed in a HEPES-buffered chloride containing solution without sodium. In the experimental period, luminal chloride was removed and JH was measured. pHi values are shown in Tables 3 and 4. As shown in Fig. 3, JH was significantly less in neonatal proximal straight tubules than in adult proximal straight tubules. In both adult and neonatal proximal straight tubules, Cl−/base exchange was inhibited by luminal 1.0 mM DIDS. As shown in Fig. 3, the rate of Cl−/base exchange was not affected by the addition of 0.5 mM formate to the luminal and bathing solutions. These data are consistent with a DIDS-inhibitable Cl−/base exchanger that was not affected by formate.
Table 3.
Effect of luminal Cl− on pHi in adult PST
| n | Cl− | 0 Cl− | Cl− | ||
|---|---|---|---|---|---|
| No formate | 10 | 7.00±0.06 | 7.32±0.06* | 7.07±0.05 | |
| Formate (0.5 mM) | 9 | 7.16±0.05 | 7.47±0.05* | 7.28±0.05 | |
| Luminal DIDS (1 mM) | 7 | 7.16±0.05 | 7.29±0.05† | 7.24±0.05 | |
| 14 | 7.09±0.04 | 7.32±0.05* | 7.17±0.05 | ||
| Cyanide (1 mM) + acetazolamide (0.1 mM) | 7 | 6.76±0.07‡ | 7.35±0.05* | 7.20±0.03 |
Values are means ± SE; n = no. of experiments.
0 Cl− significantly different from Cl− containing control and recovery (P<0.01).
0 Cl− significantly different from Cl− containing control (P<0.001).
Initial pHi significantly different from formate, luminal DIDS, and groups (P <0.05).
Table 4.
Effect of luminal Cl− on pHi in neonatal PST
| n | Cl− | 0 Cl− | Cl− | ||
|---|---|---|---|---|---|
| No formate | 8 | 7.05±0.11 | 7.33±0.14* | 7.09±0.13 | |
| Formate (0.5 mM) | 8 | 7.31±0.10 | 7.52±0.12* | 7.27±0.12 | |
| Luminal DIDS (1 mM) | 8 | 7.27±0.04 | 7.34±0.05† | 7.31±0.04 | |
| 8 | 7.08±0.10 | 7.36±0.10* | 7.20±0.09 | ||
| Cyanide (1 mM) + acetazolamide (0.1 mM) | 9 | 6.81±0.06‡ | 7.22±0.06† | 7.17±0.05 |
Values are means ± SE; n = no. of experiments.
0 Cl− significantly different from Cl− containing control and recovery (P<0.01).
0 Cl− significantly different from Cl− containing control (P<0.05).
Initial pHi significantly different from formate and luminal DIDS groups (P<0.01).
Fig. 3.
JH values in response to luminal chloride removal in adult and neonatal proximal straight tubules. These experiments were performed using HEPES-buffered solutions in the absence of sodium.
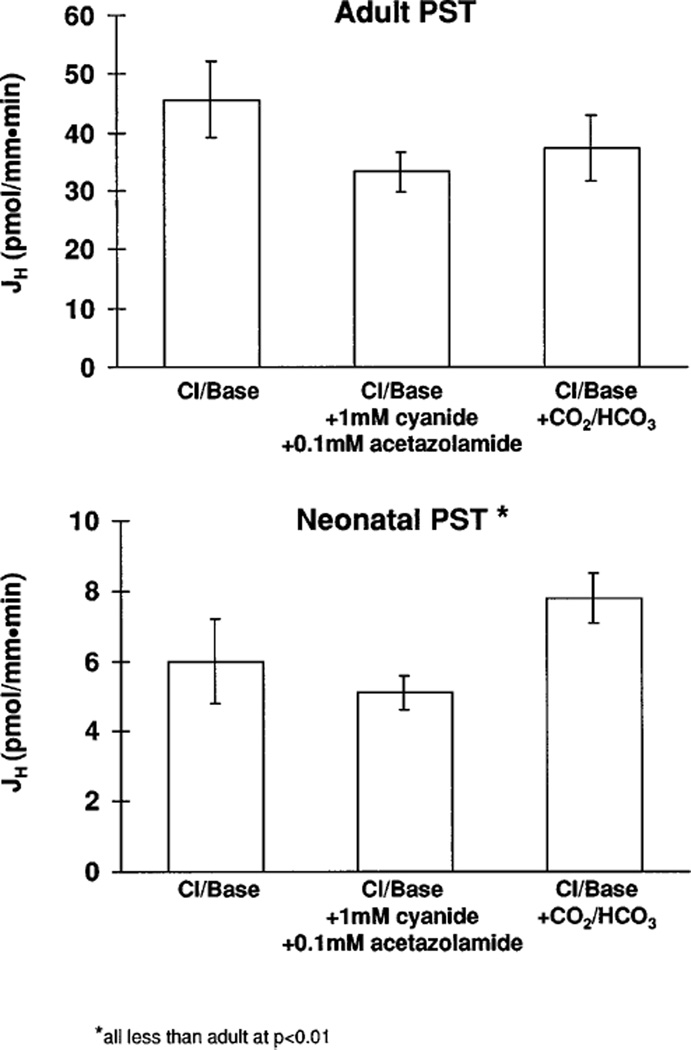
In the next series of experiments, we examined whether Cl−/base exchange in the neonatal and adult segment were mediated by Cl−/ or Cl−/OH− exchange. In these experiments, luminal chloride was removed in tubules perfused and bathed in the presence of 25 mM HCO3. As shown in Fig. 4, JH was not significantly different in the presence and absence of CO2/HCO3. Since proximal tubules can potentially generate bicarbonate and CO2 in HEPES-buffered solutions, we examined the effect of cyanide and acetazolamide on JH in tubules perfused and bathed in HEPES-buffered solutions. As shown in Fig. 4, the rate of Cl−/base exchange was comparable in the presence and absence of acetazolamide and cyanide in the neonate and adult proximal straight tubules. While some production of CO2 and HCO3 cannot be ruled out, these results are consistent with Cl−/OH− mediating Cl−/base exchange in both adult and neonatal proximal straight tubules. The rate of Cl−/OH− exchange increases severalfold during postnatal maturation.
Fig. 4.
JH values in response to luminal chloride removal with HEPES-buffered solutions, in absence and presence of lumen and bath cyanide and acetazolamide, and with bicarbonate-buffered solutions.
DISCUSSION
In this study the rate of volume absorption from a high-chloride solution simulating late proximal tubular fluid was significantly less in neonatal proximal straight tubules than that measured in adults. Passive transport made up 50% of the total transport in adult tubules, but was not different from zero in neonatal tubules. The lumen-positive PD was consistent with a chloride diffusion potential in adult proximal straight tubules. The fact that there was no change in the transepithelial PD with bath ouabain is in accord with an electroneutral NaCl transport in this segment as has been found in the proximal convoluted tubule (7). In the neonatal segment, there was a small lumen-positive potential which was not different from zero mV. Addition of bath ouabain resulted in a small increase in the transepithelial PD. The significance of this change in PD is unclear.
Previous studies had predicted that neonates would have a higher rate of passive proximal tubular transport than adults (13). Injection of inulin and sucrose into early proximal tubular segments resulted in the recovery of 100% of these compounds in the urine in both neonatal and adult guinea pigs. Only 92% of microinjected mannitol, a smaller molecule, was collected in the urine of neonates compared with the total recovery in adults (13). The reabsorption of mannitol by the neonatal proximal tubule was explained by a greater paracellular permeability due to the shorter length of the proximal tubular paracellular pathway in the neonate. However, there was no maturational difference in the width or length of the zonula occludens. We have previously examined the chloride permeability of adult and neonatal proximal convoluted tubules (22). Neonatal proximal convoluted tubules have a very low chloride permeability. The chloride permeability was higher in the adult juxtamedullary proximal convoluted tubules compared with that in the neonate. The data in the proximal straight tubule are in agreement with these results. The rate of volume absorption in the presence of a chloride gradient and bath ouabain provides an estimate of the importance of passive paracellular chloride transport in this segment. While 50% of transport remained in adult proximal straight tubules in the presence of ouabain, neonatal tubular transport was not different from zero.
We have previously compared the rate of Na+/H+ antiporter activity in neonatal and adult juxtamedullary proximal convoluted tubules (4, 6). The rate of Na+/H+ antiporter activity was approximately one-third that of adult proximal tubules (6). The abundance of NHE-3 mRNA and protein increases in concordance with that of the Na+/H+ antiporter (8). In the present study, we found that JH in response to luminal sodium addition was fivefold less in neonatal proximal straight tubules than that of the adult segment. These data demonstrate that there is maturation of proximal tubule Na+/H+ along the length of the proximal tubule.
A previous study examined the rate of renal Cl−/formate and Na+/H+ exchange activity in brush-border membrane vesicles of late fetal, 3- to 5-day-old newborn and adult guinea pigs (10). This study found low rates of Na+/H+ antiporter activity and Cl−/formate exchange in the fetal kidney. However, the rates of both transporters were comparable in the neonatal guinea pig as in the adult. The difference between this study and ours is likely due to the different rates of postnatal renal maturation in the rabbit and the guinea pig.
We have previously examined Cl−/base exchange on the apical membrane of adult proximal convoluted tubules using similar solutions and techniques as that in this study (23).We found that in both superficial and juxtamedullary proximal convoluted tubules, there was a significant rate of DIDS-inhibitable Cl−/base exchange in the absence of formate. There was no difference in JH upon luminal chloride removal in the absence of and presence of CO2/HCO3, consistent with Cl−/OH exchange in both segments. In superficial but not juxtamedullary proximal convoluted tubules, JH was significantly higher in the presence of formate, consistent with Cl−/formate exchange. In tubules perfused with a high-chloride solution and bathed with a serumlike albumin solution, addition of formate to the lumen and bathing solution increased the rate of volume absorption. In juxtamedullary proximal convoluted tubules, addition of formate did not stimulate volume absorption. These data are consistent with the presence of a Cl−/formate exchanger on the apical membrane of superficial, but not juxtamedullary proximal convoluted tubules.
The mechanism of apical membrane Cl−/base exchange has previously been examined in adult rabbit proximal straight tubules perfused in vitro (14). This study found that luminal chloride removal in the absence of lumen and bath sodium resulted in comparable rates of Cl−/base exchange in the present study. Addition of formate had no effect on the rate of Cl−/base exchange in their studies, consistent with the absence of a Cl−/formate exchanger in this segment. Our results in neonatal and adult proximal straight tubules are in agreement with their findings. Neither addition of 25 mM bicarbonate nor the addition of acetazolamide and cyanide in the presence of HEPES-buffered solutions affected JH, consistent with Cl−/OH exchange in this segment. Our studies in adult proximal straight tubules are also consistent with these findings. However, the pHi changes in adult and neonatal proximal straight tubules could also be explained by HCl cotransport. The previous findings of Cl−/formate exchange in the superficial proximal convoluted tubule and the present findings showing that the rate of Cl−/base exchange was not affected by formate in the proximal straight tubule suggest that there is axial heterogeneity for the mechanism of Cl−/base exchange. The physiological significance of these findings is unclear.
The present study examined the mechanism of Cl−/base exchange in neonatal proximal straight tubules. We found that, as in the adult, Cl−/base exchange is mediated by Cl−/OH exchange. Unlike our findings in the superficial proximal convoluted tubule, there was no evidence for Cl−/formate exchange in the proximal straight tubule. The rate of Cl−/OH exchange was approximately sevenfold less in neonatal proximal straight tubules compared with adult proximal straight tubules.
In summary, the present in vitro microperfusion study compared the rates of NaCl transport in neonatal and adult proximal straight tubules. In tubules perfused with a high-chloride solution simulating late proximal tubular fluid, the rate of active and passive NaCl transport was less in neonatal proximal tubule than in the adult segment. Our data demonstrate that parallel Na+/H+ and Cl−/OH− exchange mediate apical membrane NaCl transport in the neonate and the adult proximal straight tubule. The rates of both transporters are significantly less in the neonate.
Acknowledgments
We are grateful for the secretarial assistance of Janell McQuinn.
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grants DK-41612 (to M. Baum) and DK-02232 (to R. Quigley).
Footnotes
All proton fluxes are presented as absolute values and expressed as JH, in pmol·mm−1·min−1.
REFERENCES
- 1.Alpern RJ. Mechanism of basolateral membrane H+/OH−/ transport in the rat proximal convoluted tubule. J. Gen. Physiol. 1985;86:613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpern RN, Howlin KJ, Preisig PA. Active and passive components of chloride transport in the rat proximal convoluted tubule. J. Clin. Invest. 1985;76:1360–1366. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am. J. Physiol. 1997;273:F179–F192. doi: 10.1152/ajprenal.1997.273.2.F179. (Renal Physiol. 42) [DOI] [PubMed] [Google Scholar]
- 4.Baum M. Developmental changes in rabbit juxtamedullary proximal convoluted tubule acidification. Pediatr. Res. 1992;31:411–414. doi: 10.1203/00006450-199204000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Baum M. Evidence that parallel Na+-H+ and Cl−- (OH−) antiporters transport NaCl in the proximal tubule. Am. J. Physiol. 1987;252:F338–F345. doi: 10.1152/ajprenal.1987.252.2.F338. (Renal Fluid Electrolyte Physiol. 21) [DOI] [PubMed] [Google Scholar]
- 6.Baum M. Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J. Clin. Invest. 1990;85:499–506. doi: 10.1172/JCI114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum M, Berry CA. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit proximal tubule. J. Clin. Invest. 1984;74:205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum M, Biemesderfer D, Gentry D, Aronson P. Ontogeny of rabbit renal cortical NHE-3 and NHE-1: effect of glucocorticoids. Am. J. Physiol. 1995;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. (Renal Fluid Electrolyte Physiol. 37) [DOI] [PubMed] [Google Scholar]
- 9.Burg M, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am. J. Physiol. 1966;120:1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- 10.Guillery EN, Huss DJ. Developmental regulation of chloride/formate exchange in guinea pig proximal tubules. Am. J. Physiol. 1995;269:F686–F695. doi: 10.1152/ajprenal.1995.269.5.F686. (Renal Fluid Electrolyte Physiol. 38) [DOI] [PubMed] [Google Scholar]
- 11.Karniski LP, Aronson PS. Anion exchange pathways for Cl− transport in rabbit renal microvillus membranes. Am. J. Physiol. 1987;253:F513–F521. doi: 10.1152/ajprenal.1987.253.3.F513. (Renal Fluid Electrolyte Physiol. 22) [DOI] [PubMed] [Google Scholar]
- 12.Karniski LP, Aronson PS. Chloride/formate exchange with formic acid recycling: a mechanisms of active chloride transport across epithelial membranes. Proc. Natl. Acad. Sci. USA. 1985;82:6362–6365. doi: 10.1073/pnas.82.18.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaskel FJ, Kumar AM, Lockhart EA, Evan A, Spitzer A. Factors affecting proximal tubular reabsorption during development. Am. J. Physiol. 1987;252:F188–F197. doi: 10.1152/ajprenal.1987.252.1.F188. (Renal Fluid Electrolyte Physiol. 21) [DOI] [PubMed] [Google Scholar]
- 14.Kurtz I, Nagami G, Yanagawa N, Li L, Emmons C, Lee I. Mechanisms of apical and basolateral Na+-independent Cl−/base exchange in the rabbit superficial proximal straight tubule. J. Clin. Invest. 1994;94:173–183. doi: 10.1172/JCI117304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F-Y, Cogan MG. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am. J. Physiol. 1984;247:F816–F821. doi: 10.1152/ajprenal.1984.247.5.F816. (Renal Fluid Electrolyte Physiol. 16) [DOI] [PubMed] [Google Scholar]
- 16.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am. J. Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. (Renal Fluid Electrolyte Physiol. 13) [DOI] [PubMed] [Google Scholar]
- 17.Roos A, Boron WF. Intracellular pH. Physiol. Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 18.Schild L, Giebisch G, Karniski LP, Aronson PS. Chloride transport in the mammalian proximal tubule. Pflügers Arch. 1986;407:S156–S159. doi: 10.1007/BF00584945. [DOI] [PubMed] [Google Scholar]
- 19.Schild L, Giebisch G, Karniski LP, Aronson PS. Effect of formate on volume reabsorption in the rabbit proximal tubule. J. Clin. Invest. 1987;79:32–38. doi: 10.1172/JCI112803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Evan AP. Development of solute transport in rabbit proximal tubule. I. and glucose absorption. Am. J. Physiol. 1983;245:F382–F390. doi: 10.1152/ajprenal.1983.245.3.F382. (Renal Fluid Electrolyte Physiol. 14) [DOI] [PubMed] [Google Scholar]
- 21.Seifter JL, Knickelbein R, Aronson PS. Absence of Cl−/OH exchange and NaCl cotransport in rabbit renal microvillus membrane vesicles. Am. J. Physiol. 1984;247:F753–F759. doi: 10.1152/ajprenal.1984.247.5.F753. (Renal Fluid Electrolyte Physiol. 16) [DOI] [PubMed] [Google Scholar]
- 22.Sheu J-N, Baum M, Bajaj G, Quigley R. Maturation of rabbit proximal convoluted tubule chloride permeability. Pediatr. Res. 1996;39:308–312. doi: 10.1203/00006450-199602000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Sheu J-N, Quigley R, Baum M. Heterogeneity of chloride/base exchange in rabbit superficial and juxtamedullary proximal convoluted tubules. Am. J. Physiol. 1995;268:F847–F853. doi: 10.1152/ajprenal.1995.268.5.F847. (Renal Fluid Electrolyte Physiol. 37) [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Giebisch G, Aronson PS. Effects of formate and oxalate on volume absorption in rat proximal tubule. Am. J. Physiol. 1992;263:F37–F42. doi: 10.1152/ajprenal.1992.263.1.F37. (Renal Fluid Electrolyte Physiol. 32) [DOI] [PubMed] [Google Scholar]
- 25.Warnock DG, Yee FJ. Chloride uptake by brush border membrane vesicles: coupling to proton gradients and K+ diffusion potentials. J. Clin. Invest. 1981;67:103–115. doi: 10.1172/JCI110002. [DOI] [PMC free article] [PubMed] [Google Scholar]