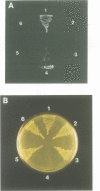
Abstract
Virulence of the plant pathogen Erwinia carotovora subsp. carotovora is dependent on the production and secretion of a complex arsenal of plant cell wall-degrading enzymes. Production of these exoenzymes is controlled by a global regulatory mechanism. A virulent mutants in one of the regulatory loci, expI, show a pleiotropic defect in the growth phase-dependent transcriptional activation of exoenzyme gene expression. The expI gene encodes a 26 kDa polypeptide that is structurally and functionally related to the luxI gene product of Vibrio fischeri. Functional similarity of expI and luxI has been demonstrated by reciprocal genetic complementation experiments. LuxI controls bioluminescence in V.fischeri in a growth phase-dependent manner by directing the synthesis of the diffusible autoinducer, N-(3-oxohexanoyl) homoserine lactone. E.c. subsp. carotovora expI+ strains or Escherichia coli harboring the cloned expI gene excrete a small diffusible signal molecule that complements the expI mutation of Erwinia as well as a luxI mutation of V.fischeri. This extracellular complementation can also be achieved by E.coli harboring the luxI gene from V.fischeri or by adding the synthetic V.fischeri autoinducer. Both the production of the plant tissue-macerating exoenzymes and the ability of the bacteria to propagate in planta are restored in expI mutants by autoinducer addition. These data suggest that the same signal molecule is employed in control of such diverse processes as virulence in a plant pathogen and bioluminescence in a marine bacterium, and may represent a general mechanism by which bacteria modulate gene expression in response to changing environmental conditions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andro T., Chambost J. P., Kotoujansky A., Cattaneo J., Bertheau Y., Barras F., Van Gijsegem F., Coleno A. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J Bacteriol. 1984 Dec;160(3):1199–1203. doi: 10.1128/jb.160.3.1199-1203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymeric J. L., Guiseppi A., Pascal M. C., Chippaux M. Mapping and regulation of the cel genes in Erwinia chrysanthemi. Mol Gen Genet. 1988 Jan;211(1):95–101. doi: 10.1007/BF00338398. [DOI] [PubMed] [Google Scholar]
- Bainton N. J., Bycroft B. W., Chhabra S. R., Stead P., Gledhill L., Hill P. J., Rees C. E., Winson M. K., Salmond G. P., Stewart G. S. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992 Jul 1;116(1):87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- Bainton N. J., Stead P., Chhabra S. R., Bycroft B. W., Salmond G. P., Stewart G. S., Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992 Dec 15;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu T. Secondary metabolites as chemical signals for cellular differentiation. Gene. 1992 Jun 15;115(1-2):159–165. doi: 10.1016/0378-1119(92)90554-3. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Choi S. H., Greenberg E. P. Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeri LuxR protein. J Bacteriol. 1992 Jun;174(12):4064–4069. doi: 10.1128/jb.174.12.4064-4069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-luxR protein regulatory circuit. J Bacteriol. 1988 Sep;170(9):4040–4046. doi: 10.1128/jb.170.9.4040-4046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987 Dec 23;15(24):10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. M., Greenberg E. P. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J Bacteriol. 1992 Jul;174(13):4384–4390. doi: 10.1128/jb.174.13.4384-4390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hinton J. C., Sidebotham J. M., Hyman L. J., Pérombelon M. C., Salmond G. P. Isolation and characterisation of transposon-induced mutants of Erwinia carotovora subsp. atroseptica exhibiting reduced virulence. Mol Gen Genet. 1989 May;217(1):141–148. doi: 10.1007/BF00330953. [DOI] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Isolation of Erwinia chrysanthemi mutants altered in pectinolytic enzyme production. Mol Microbiol. 1989 Nov;3(11):1587–1597. doi: 10.1111/j.1365-2958.1989.tb00144.x. [DOI] [PubMed] [Google Scholar]
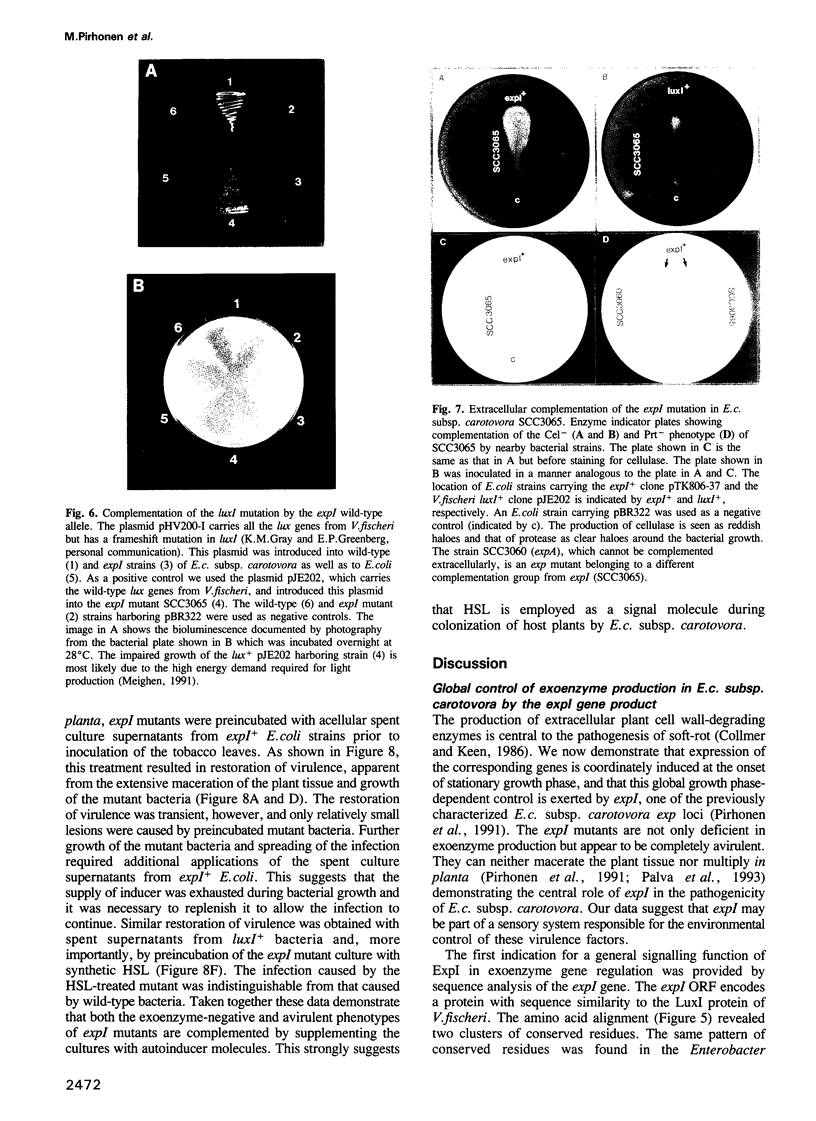
- Jones S., Yu B., Bainton N. J., Birdsall M., Bycroft B. W., Chhabra S. R., Cox A. J., Golby P., Reeves P. J., Stephens S. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993 Jun;12(6):2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985 Sep;163(3):1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M. B., Pirhonen M., Saarilahti H. T., Palva E. T. Molecular cloning of ompRS, a regulatory locus controlling production of outer membrane proteins in Erwinia carotovora subsp. carotovora. Mol Gen Genet. 1991 May;226(3):353–360. doi: 10.1007/BF00260646. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J Bacteriol. 1991 Jul;173(14):4474–4481. doi: 10.1128/jb.173.14.4474-4481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy J. L., Murata H., Chatterjee A. K. Genetic evidence for an activator required for induction of pectin lyase in Erwinia carotovora subsp. carotovora by DNA-damaging agents. J Bacteriol. 1992 Aug;174(16):5471–5474. doi: 10.1128/jb.174.16.5471-5474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen E. A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991 Mar;55(1):123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran F., Starr M. P. Metabolic regulation of polygalacturonic acid trans-eliminase in Erwinia. Eur J Biochem. 1969 Dec;11(2):291–295. doi: 10.1111/j.1432-1033.1969.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Nasser W., Reverchon S., Robert-Baudouy J. Purification and functional characterization of the KdgR protein, a major repressor of pectinolysis genes of Erwinia chrysanthemi. Mol Microbiol. 1992 Jan;6(2):257–265. doi: 10.1111/j.1365-2958.1992.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Pirhonen M., Heino P., Helander I., Harju P., Palva E. T. Bacteriophage T4 resistant mutants of the plant pathogen Erwinia carotovora. Microb Pathog. 1988 May;4(5):359–367. doi: 10.1016/0882-4010(88)90063-0. [DOI] [PubMed] [Google Scholar]
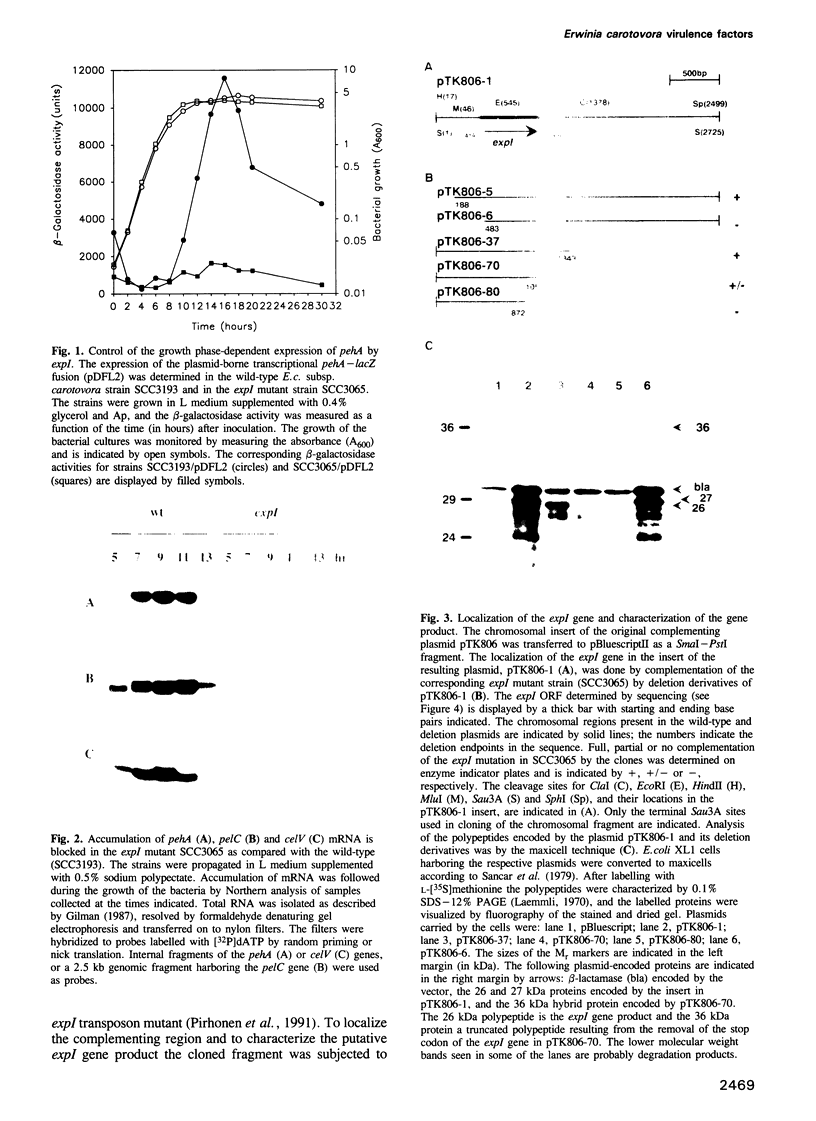
- Saarilahti H. T., Heino P., Pakkanen R., Kalkkinen N., Palva I., Palva E. T. Structural analysis of the pehA gene and characterization of its protein product, endopolygalacturonase, of Erwinia carotovora subspecies carotovora. Mol Microbiol. 1990 Jun;4(6):1037–1044. doi: 10.1111/j.1365-2958.1990.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Saarilahti H. T., Pirhonen M., Karlsson M. B., Flego D., Palva E. T. Expression of pehA-bla gene fusions in Erwinia carotovora subsp. carotovora and isolation of regulatory mutants affecting polygalacturonase production. Mol Gen Genet. 1992 Jul;234(1):81–88. doi: 10.1007/BF00272348. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhorn H. E., Stones V. L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol. 1992 Jul;174(14):4769–4776. doi: 10.1128/jb.174.14.4769-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Stark T. F., Beattie W. G., Moses R. E. Multiple control elements for the uvrC gene unit of Escherichia coli. Nucleic Acids Res. 1986 Mar 11;14(5):2301–2318. doi: 10.1093/nar/14.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Studnicka G. M. Nucleotide sequence homologies in control regions of prokaryotic genomes. Gene. 1987;58(1):45–57. doi: 10.1016/0378-1119(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Wang X. D., de Boer P. A., Rothfield L. I. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 1991 Nov;10(11):3363–3372. doi: 10.1002/j.1460-2075.1991.tb04900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Young K. Y., Edlin G. J., Konigsberg W. High-frequency generalised transduction by bacteriophage T4. Nature. 1979 Jul 5;280(5717):80–82. doi: 10.1038/280080a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zink R. T., Engwall J. K., McEvoy J. L., Chatterjee A. K. recA is required in the induction of pectin lyase and carotovoricin in Erwinia carotovora subsp. carotovora. J Bacteriol. 1985 Oct;164(1):390–396. doi: 10.1128/jb.164.1.390-396.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]