Abstract
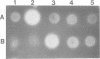
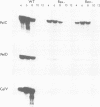
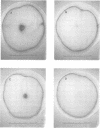
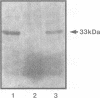
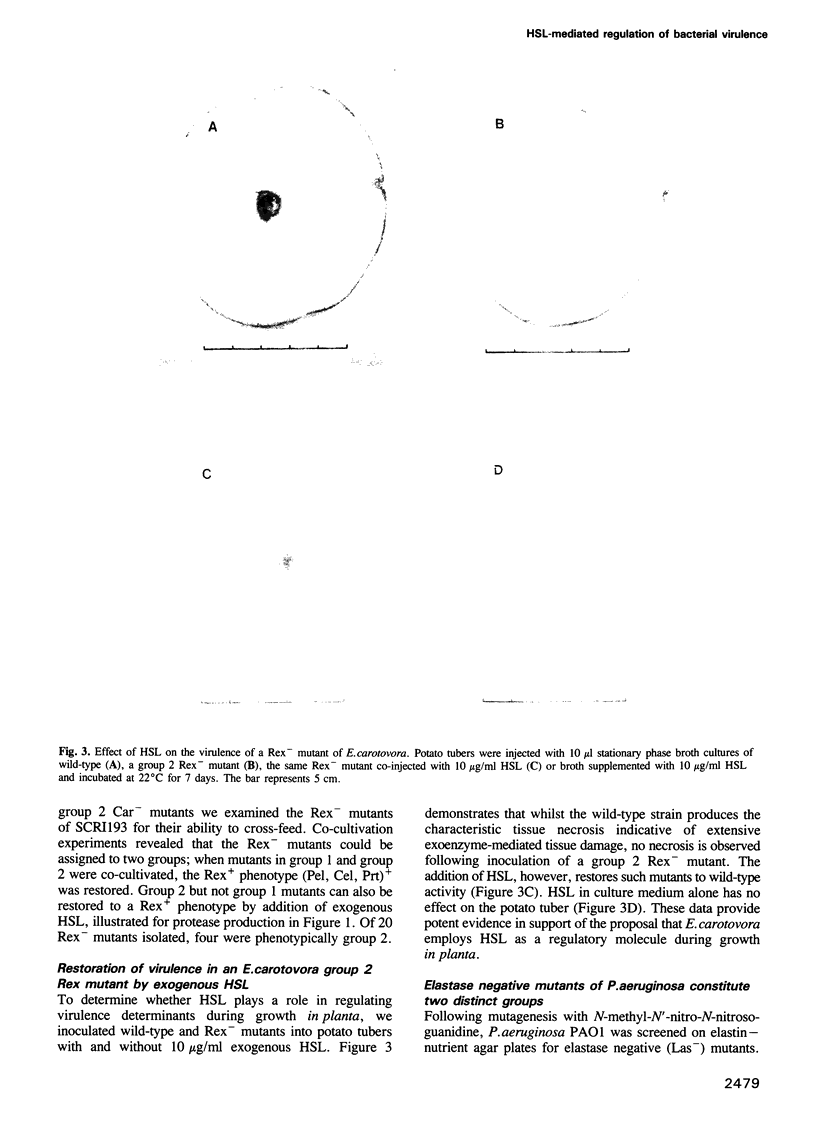
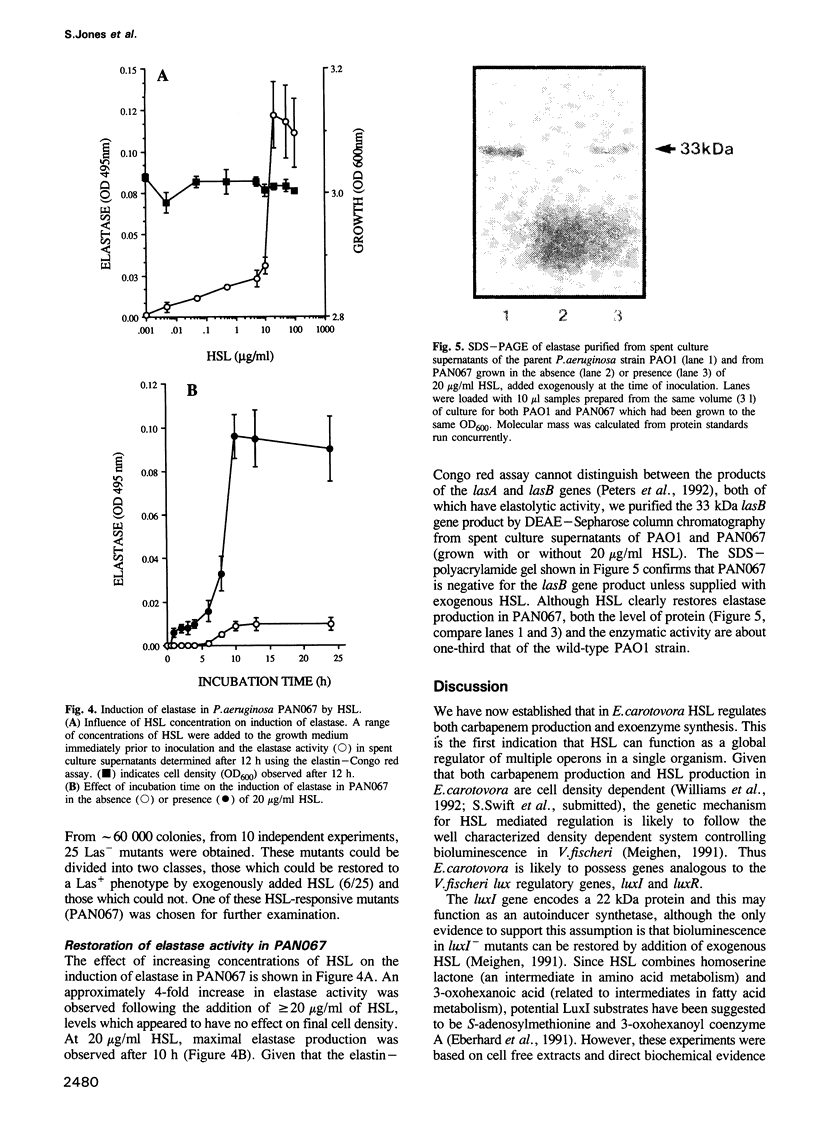
Erwinia carotovora and Pseudomonas aeruginosa secrete exoenzymes that contribute to the pathogenesis of plant and mammalian infections respectively. E.carotovora mutants defective in synthesis of the pectinase, cellulase and protease exoenzymes were isolated and classified into two groups. Group 2 mutants were found to be defective in the production of a small freely diffusible molecule, N-3-(oxohexanoyl)-L-homoserine, lactone (HSL), and were avirulent. Addition of exogenous HSL to these group 2 mutants restores synthesis of the exoenzymes and virulence in planta. Of the exoenzymes of P.aeruginosa the metalloprotease, elastase, is an established virulence determinant. Mutants of P.aeruginosa that are defective in elastase production have been isolated and were again found to fall into two groups. Analogous to the group 2 mutants of E.carotovora, group 2 mutants of P. aeruginosa are defective in the synthesis of HSL and exogenous HSL restores elastase production. HSL has now been linked to the control of bioluminescence in Vibrio fischeri, carbapenem antibiotic production of E.carotovora and the above exoenzyme virulence determinants. This information significantly enhances our understanding of the extent and nature of pheromone mediated gene expression control in prokaryotes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andro T., Chambost J. P., Kotoujansky A., Cattaneo J., Bertheau Y., Barras F., Van Gijsegem F., Coleno A. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J Bacteriol. 1984 Dec;160(3):1199–1203. doi: 10.1128/jb.160.3.1199-1203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton N. J., Bycroft B. W., Chhabra S. R., Stead P., Gledhill L., Hill P. J., Rees C. E., Winson M. K., Salmond G. P., Stewart G. S. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992 Jul 1;116(1):87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- Bainton N. J., Stead P., Chhabra S. R., Bycroft B. W., Salmond G. P., Stewart G. S., Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992 Dec 15;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik M. J., Storey D. G. Zinc and iron regulate translation of the gene encoding Pseudomonas aeruginosa elastase. Mol Microbiol. 1992 Feb;6(3):337–344. doi: 10.1111/j.1365-2958.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- Choi S. H., Greenberg E. P. Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeri LuxR protein. J Bacteriol. 1992 Jun;174(12):4064–4069. doi: 10.1128/jb.174.12.4064-4069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard F. M., Cabello A., Salmond G. P. Bacteriophage lambda-mediated transposon mutagenesis of phytopathogenic and epiphytic Erwinia species is strain dependent. Mol Gen Genet. 1989 Sep;218(3):491–498. doi: 10.1007/BF00332415. [DOI] [PubMed] [Google Scholar]
- Galloway D. R. Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol. 1991 Oct;5(10):2315–2321. doi: 10.1111/j.1365-2958.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Pelzel S. E., Latimer J., Muller-Eberhard U., Hansen E. J. Identification of a genetic locus of Haemophilus influenzae type b necessary for the binding and utilization of heme bound to human hemopexin. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1973–1977. doi: 10.1073/pnas.89.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Wallace J. C., Brown J. P. Finding protein similarities with nucleotide sequence databases. Methods Enzymol. 1990;183:111–132. doi: 10.1016/0076-6879(90)83009-x. [DOI] [PubMed] [Google Scholar]
- Hinton J. C., Sidebotham J. M., Hyman L. J., Pérombelon M. C., Salmond G. P. Isolation and characterisation of transposon-induced mutants of Erwinia carotovora subsp. atroseptica exhibiting reduced virulence. Mol Gen Genet. 1989 May;217(1):141–148. doi: 10.1007/BF00330953. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Meighen E. A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991 Mar;55(1):123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Cryz S. J., Iglewski B. H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980 Jun;142(3):836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. E., Park S. J., Darzins A., Freck L. C., Saulnier J. M., Wallach J. M., Galloway D. R. Further studies on Pseudomonas aeruginosa LasA: analysis of specificity. Mol Microbiol. 1992 May;6(9):1155–1162. doi: 10.1111/j.1365-2958.1992.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Pirhonen M., Flego D., Heikinheimo R., Palva E. T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993 Jun;12(6):2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Williams P. lux genes and the applications of bacterial bioluminescence. J Gen Microbiol. 1992 Jul;138(7):1289–1300. doi: 10.1099/00221287-138-7-1289. [DOI] [PubMed] [Google Scholar]
- Wang X. D., de Boer P. A., Rothfield L. I. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 1991 Nov;10(11):3363–3372. doi: 10.1002/j.1460-2075.1991.tb04900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Bainton N. J., Swift S., Chhabra S. R., Winson M. K., Stewart G. S., Salmond G. P., Bycroft B. W. Small molecule-mediated density-dependent control of gene expression in prokaryotes: bioluminescence and the biosynthesis of carbapenem antibiotics. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):161–167. doi: 10.1111/j.1574-6968.1992.tb14035.x. [DOI] [PubMed] [Google Scholar]