Acute monoblastic and monocytic leukemia is a distinct subtype of acute myeloid leukemia (AML) (also known as AML-FAB subtype M5) and comprises ∼5 to 10% of all cases of AML. AML-M5 is currently treated with chemotherapy (such as anthracyclines) and/or with bone marrow transplantation. The development of more specific and effective therapies was hampered in part by the lack of proper animal models for this subtype of leukemia. Here, we report a mouse model for this entity. This is the first and the only relevant nonviral-based animal model for human monocytic leukemia.
One commonly used strategy for generating mouse solid tumor or leukemia models is to enhance the strength of tumor-promoting signals. One such signal is PtdIns(3,4,5)P3/Akt signaling pathway. PtdIns(3,4,5)P3 exerts its function by mediating protein translocation by binding to their pleckstrin homolog domains. PKB/Akt, a pleckstrin homolog domain containing serine/threonine protein kinase with oncogenic and anti-apoptotic activities, is one of the major downstream factors of PtdIns(3,4,5)P3. Hyperactivation of PtdIns(3,4,5)P3/Akt signaling pathway is frequently identified in a variety of cancers and is highly related to tumorigenesis. It has also been implicated in leukemia tumorigenesis. About 50–70% of AMLs display hyperactivation of this pathway.1 Accordingly, we examined whether monocytic leukemia can be induced by specifically enhancing PtdIns(3,4,5)P3 signaling in myeloid cells.
PtdIns(3,4,5)P3 signaling was elevated by disrupting the tumor suppressor phosphatase and tensin homolog (PTEN), a phosphatidylinositol 3′-phosphatase that converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2. Depletion of this lipid phosphatase led to accumulation of PtdIns(3,4,5)P3 on the plasma membrane and thus elevation of PtdIns(3,4,5)P3/Akt signaling. PTEN disruption in hematopoietic stem cells led to the development of both AML and acute lymphoblastic leukemia.2,3 To achieve myeloid-specific PTEN deletion, we used a conditional PTEN knockout (KO) mouse, in which two loxP sequences were inserted on either side of the exon 5 of PTEN encoding the phosphatase domain. We crossed this mouse with a myeloid-specific Cre line (The Jackson Lab, Bar Harbor, ME, USA), in which the Cre recombinase gene was inserted into the endogenous M lysozyme locus, and therefore is under the control of myeloid-specific lysozyme promoter. As a result, only in myeloid linage, the loxP site was cut by Cre recombinase, leading to the disruption of PTEN.4–6 Mice that are homozygous for this allele were viable, fertile, normal in size, had normal blood count and did not display any gross physical or behavioral abnormalities up to 3 months of age.4–6 Neutrophils isolated from these mice were more sensitive to chemoattractant stimulation and displayed prolonged survival. PTEN disruption also significantly enhanced neutrophil recruitment at sites of infection. In a bacterial pneumonia model, elevating PtdIns(3,4,5)P3 by disrupting PTEN enhanced recruitment of neutrophils to the inflamed lungs, delayed the apoptotic death and enhanced the bacteria killing capability of the recruited neutrophils.4–6
Leukemogenesis was observed in 11 of 18 PTEN KO mice examined. It occurred in mice older than 3 months. Peripheral blood smear showed numerous blasts (>20% of nucleated cells), which were intermediate to large in size, and had irregular to bilobed nuclei, dispersed chromatin, distinct nucleoli and moderate amount of cytoplasm (Figure 1a). Bone marrow aspirate smear also showed numerous blasts with similar morphological features (>20% of nucleated cells). Neutrophils and maturing myeloid precursors were markedly reduced. Erythroid precursors and megakaryocytes were present but markedly decreased in proportions (Figure 1a). The leukemic mice showed marked splenomegaly (Figure 1b), developed general weakness with or without subcutaneous mass and eventually died (Figure 1c). The above pathological features were detected in every animal that died. It is noteworthy that we never observed leukemogenesis in PTEN+/-mice. This is consistent with biochemical and cell biological studies conducted using these mice. In PTEN+/- neutrophils, phosphatidylinositol 3,4,5-trisphosphate/Akt was not hyperactivated and these cells did not show any defects in reactive oxygen species production and migration, as observed in PTEN-/- neutrophils. It appears that one copy of PTEN was sufficient to maintain the normal level of phosphatidylinositol 3,4,5-trisphosphate and the downstream signaling.4–6
Figure 1.
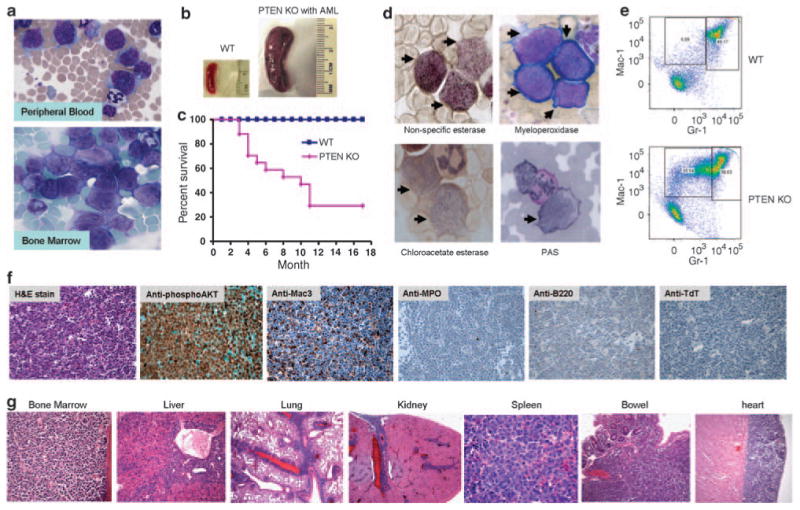
(a) Leukemic blasts in peripheral blood and bone marrow of myeloid-specific PTEN knockout mice. (b) Leukemic mice show enlarged spleen. (c) The death rate of myeloid-specific PTEN knockout mice. It was analyzed using the Kaplan–Meier and log-rank methods. The difference in survival was statistically significant (P<0.01 by log-rank test). (d) Cytochemical stains for indicated enzymes. Leukemic blasts are marked by black arrows. PAS, periodic acid-Schiff. The stain for myeloperoxidase is negative because only Wright–Giemsa counter stains were observed with no cytoplasmic reactivity. (e) Leukemic blasts are Mac-1 high and Gr-1 low cells. (f) Immunohistochemistry analysis using indicated antibodies. (g) Leukemic infiltration in multiple organs.
To determine the lineage of the blasts, we first performed cytochemical stains. The leukemic blasts exhibited intense diffuse cytoplasmic pattern with black granules in the cytoplasm to nonspecific esterase, a marker for monocytic differentiation, which is usually not expressed in myeloid blasts. In contrast, these cells were negative for myeloperoxidase and chloro-acetate esterase, which are markers of granulocytic elements, as well as cytoplasmic periodic acid-Schiff-positive large granules and globules, which are commonly associated with neoplastic erythroblasts and lymphoblasts (Figure 1d). The lineage was also analyzed using flow cytometry analysis. It showed an expanded Mac-1 high and Gr-1 low population, also indicative of monocytic differentiation (Figure 1e). These blasts also showed weak expression of CD34 (data not shown). A distinct tumor mass, almost exclusively comprised of blasts, was found in the pancreas of one KO mouse, which enables us to conduct immunohistochemistry assay using specific antibodies. The blasts exhibited nuclear and cytoplasmic staining for phospho-AKT, indicative of hyperactivation of AKT. Consistent with the results of cytochemical stains and flow cytometry, the blasts were positive for monocytic marker Mac-3 and negative for granulocytic marker myeloperoxidase, B-cell marker B220 and immature lymphoid marker terminal deoxynucleotidyl transferase (Figure 1f). This further confirmed the monocytic differentiation in the blasts.
Similar to what was observed in human monocytic leukemia, tissue sections of the leukemic PTEN KO mice also showed leukemic infiltrate in multiple organs, especially in bone marrow, spleen, liver, lung and heart (Figure1g and Supplementary Table 1). The bone marrow was diffusely infiltrated or almost replaced by the blasts. Normal hematopoietic elements such as erythroid precursors, maturing myeloid elements and megakaryocytes were markedly decreased. In spleen, sections predominantly showed red pulp expansion with leukemic infiltrate and markedly decreased normal hematopoietic elements. In liver, the blasts were located in the portal tracts and sinusoids. In lung, the leukemic infiltrate involved pleura and interstitium, with perivascular and peribronchiolar distribution.
The leukemic infiltrate in the heart showed a preference for pericardium. To a lesser extent, other organs had also been observed to be associated with leukemic infiltrate, including kidney, small and large intestine, brain, ovary, uterus, trachea, pancreas and lymph node. In kidney, the involvement was multifocal and usually included renal capsule, hilum and cortex. In small and large intestine, the involvement presented as multiple discrete tumor masses, involving lamina propria, submucosa and muscularis propria, but sparing epithelium in the mucosa. In brain, meningeal leukemic infiltrate was noted. Ovaries and uterus showed diffuse leukemic infiltrates (data not shown).
To test whether the leukemic cells are transplantable, we adoptively transferred them to a sub-lethally irradiated wild-type recipient mice (Figure 2a). A large amount of leukemic cells were found in the bone marrow and spleen of the recipient mice 5–6 months after the transplantation, suggesting successful engraftment of the transplanted cells in the host environment (Figure 2b). Consistently, fluorescence activated cell sorting analysis also showed a significant amount of leukemic cells (CD45.2+) in the bone marrow and the peripheral blood of the recipient mice (Figure 2c). Similar to the leukemic PTEN KO mice, the mice transplanted with leukemic cells also displayed marked splenomegaly (data not shown). We examined two leukemic donor mice. Cells isolated from one donor were injected into three recipient mice. Successful transplantation was observed in five of the six recipient mice.
Figure 2.
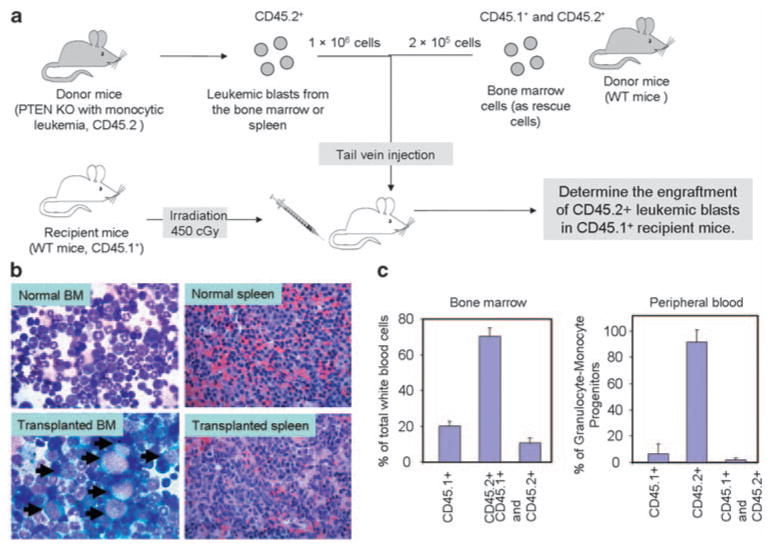
Monocytic leukemia developed in the PTEN knockout mice was transplantable. (a) Illustration of the strategy used for leukemic cell transfer. (b) Leukemic blasts are present in bone marrow and spleen of recipient mice. Leukemic blasts are marked by black arrows in BM. (c) FACS analysis showed a significant amount of leukemic cells (CD45.2+) in bone marrow and peripheral blood of the recipient mice.
Although 50–70% of AMLs display hyperactivation of PtdIns(3,4,5)P3 signal, the activity of this signaling pathway in monocytic leukemia has never been investigated. Thus, to validate our animal model as a relevant model for human mono-cytic leukemia, we further examined whether PtdIns(3,4,5)P3/Akt signaling pathway is indeed elevated in AML-M5 human patients. The activity of PtdIns(3,4,5)P3/Akt signaling pathway was assessed by the level of AKT phosphorylation, which is monitored by immunostaining using a phospho-AKT-specific antibody. As the bone marrow biopsies performed at Brigham and Women's Hospital were usually fixed in Zenker's solution, which interfered with phospho-AKT antibody, all the specimens selected in this study were formalin-fixed soft tissues to bypass this technical limitation. A total of 13 myeloid sarcoma and 7 leukemia cutis cases were retrieved by searching the surgical pathology files of the Department of Pathology of Brigham and Women's Hospital. Myeloid sarcoma, also termed as extra-medullary acute myeloid leukemia, extramedullary myeloid tumor, granulocytic sarcoma or chloroma, is a rare manifestation that is characterized by the occurrence of one or more tumor masses composed of myeloid blasts occurring at an anatomical site other than the bone marrow. A representative myeloid sarcoma case is shown in Figure 3a, and it shows diffuse proliferation of intermediate-sized blasts with round to irregular nuclei, dispersed chromatin, distinct nucleoli and moderate amount of cytoplasm. Leukemia cutis refers to the infiltration of leukemic cells into the epidermis, the dermis or the subcutis. A representative case of leukemia cutis is shown in Figure 3b and it shows dense diffuse infiltrate in the dermis, which is composed of intermediate to large blasts with round to irregular nuclei, dispersed chromatin, distinct nucleoli and moderate amount of cytoplasm. Occasional admixed small lymphocytes were seen. Of note, the epidermis was uninvolved by the leukemic infiltrate, which is a common feature in cases with leukemia cutis. In all the 20 monocytic leukemia cases, phospho-AKT antibody showed strong reactivity in the blasts, but only weak or negative reactivity in surrounding normal cells such as squamous epithelium in the epidermis (Figure 3).
Figure 3.
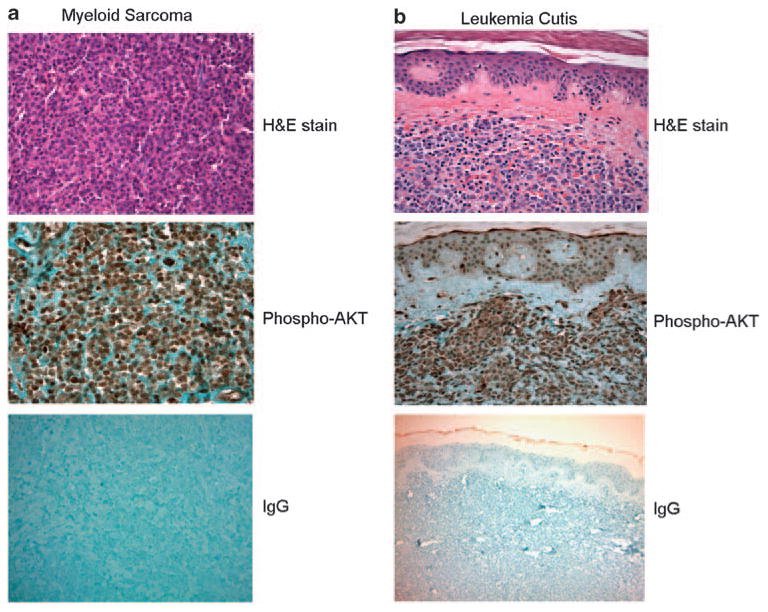
Akt hyperactivation was observed in all patients with acute monocytic leukemia examined in this study. Akt activation was assessed by immunohistochemistry using a rabbit phospho-AKT (Ser473) (736E11) monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA). Rabbit IgG was used for negative controls. Shown are representative pictures from a patient with myeloid sarcoma (a) and a patient with leukemia cutis (b).
In summary, we report that elevating PtdIns(3,4,5)P3 signaling in myeloid cells by PTEN disruption can induce acute monocytic leukemia. As hyperactivation of PtdIns(3,4,5)P3 signaling was observed in all human monocytic leukemia patients examined, this myeloid-specific PTEN KO mouse should be a relevant animal model for this human disorder. In two previous studies, using an inducible Mx-1-Cre recombinase mouse line, PTEN was disrupted in all hematopoietic stem cells. This led to the development of both AML and acute lymphoblastic leukemia, making it impossible to use these mice to specifically study acute monocytic leukemia. Although LysM-Cre could also lead to deletion of loxP-flanked gene in a small fraction of hematopoietic stem cells,7 we only observed acute monocytic leukemia in the LysM-Cre-PTEN KO mouse. Acute monocytic leukemia has also been elegantly modeled using activating Ras mutants, which were delivered into bone marrow cells using a retroviral vector.8 However, this model involves overexpression of Ras. In addition, the complex procedures for preparing and delivering viruses cannot be easily conducted by laboratories without necessary resources. Currently, the LysM-Cre-PTEN KO mouse is the only nonviral-based animal model for acute monocytic leukemia, and will greatly facilitate the investigation of the pathogenesis of and therapeutic treatments for this unique type of leukemia.
Supplementary Material
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells acts in lineage choice leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 4.Zhu D, Hattori H, Jo H, Jia Y, Subramanian KK, Loison F, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, et al. Tumor suppressor PTEN is a physiologic suppressor of chemo-attractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, et al. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood. 2009;113:4930–4941. doi: 10.1182/blood-2008-06-161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye M, Iwasaki H, Laiosa CV, Stadtfeld M, Xie H, Heck S, et al. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- 8.Parikh C, Subrahmanyam R, Ren R. Oncogenic NRAS rapidly and efficiently induces CMML- and AML-like diseases in mice. Blood. 2006;108:2349–2357. doi: 10.1182/blood-2004-08-009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


