Abstract
Neurotrophins such as ciliary neurotrophic factor (CNTF) and brain-derived neurotrophic factor (BDNF) and growth factors such as fibroblast growth factor (FGF-2) play important roles in neuronal survival and in axonal outgrowth during development. However, whether they can modulate regeneration after optic nerve injury in the adult animal is less clear. The present study investigates the effects of application of these neurotrophic factors on the speed, number, and distribution of regenerating axons in the frog Rana pipiens after optic nerve crush. Optic nerves were crushed and the factors, or phosphate-buffered saline, were applied to the stump or intraocularly. The nerves were examined at different times after axotomy, using anterograde labeling with biotin dextran amine and antibody against growth-associated protein 43. We measured the length, number, and distribution of axons projecting beyond the lesion site. Untreated regenerating axons show an increase in elongation rate over 3 weeks. CNTF more than doubles this rate, FGF-2 increases it, and BDNF has little effect. In contrast, the numbers of regenerating axons that have reached 200 µm at 2 weeks were more than doubled by FGF-2, increased by CNTF, and barely affected by BDNF. The regenerating axons were preferentially distributed in the periphery of the nerve; although the numbers of axons were increased by neurotrophic factor application, this overall distribution was substantially unaffected.
Keywords: regeneration, frog, neurotrophin, CNTF, FGF-2
Regeneration of the adult mammalian central nervous system (CNS) is very limited in part because of an unfavorable environment: the presence of inhibitory molecules (Schwab et al., 1985; Fawcett et al., 2012) and physical barriers at the lesion site (debris or astrocytic glial scar; Sandvig et al., 2004; Fawcett et al., 2012). Thus the mammalian optic nerve shows regeneration of its axons only after a peripheral nerve graft onto the cut end (So and Aguayo, 1985; Villegas-Perez et al., 1988). In contrast, lower vertebrates such as amphibians (Sperry, 1944; Scalia et al., 1985) and fish (Wanner et al., 1995; Ankerhold et al., 1998) regenerate successfully, partially because the environment of the CNS, and in particular the optic nerve, does not exhibit the same inhibitory properties. After injury in mammals, optic nerve axons are disconnected from their targets, and the supply of trophic factors is interrupted, resulting in a decline in their levels in the retina and eventual cell death (Barde, 1989; Raff et al., 1993; Mey and Thanos, 1993; Peinado-Ramon et al., 1996; Pettmann and Henderson, 1998; Lebrun-Julien and Di Polo, 2008). Fish retinal ganglion cells (RGCs) do not suffer the same fate, but in frogs there is approximately 50% cell loss (Scalia et al., 1985). We have investigated in previous studies the mechanisms by which the application of growth factors can increase this survival rate in the frog visual system (Blanco et al., 2000, 2008; Ríos-Muñoz et al., 2005). However, because of an the lack of inhibitory environment, this system is also a good model for exploring whether application of growth factors influences optic nerve regeneration.
Neurotrophic factors such as brain-derived neurotrophic factor (BDNF), fibroblast growth factor (FGF-2), and ciliary neurotrophic factor (CNTF) play important roles in neuron survival and in axonal outgrowth during development and in vitro. However, whether they can modulate regeneration after optic nerve injury in the adult animal is less clear.
In rats, BDNF transiently increases the outgrowth of regenerating axons from early developing RGCs and has a subtle effect on adult RGC explants (Avwenagha et al., 2003). BDNF application to zebrafish RGCs in culture increases axonal outgrowth and growth cone chemoattraction during development (Chen et al., 2013). FGF-2 application stimulates axonal growth during Xenopus visual system development (McFarlane et al., 1995, 1996). We have shown that in adult Rana pipiens FGF-2 increases the levels of the growth-associated protein 43 (GAP-43), a protein upregulated during axonal regeneration (Soto et al., 2003, 2006a). FGF-2 also upregulates the expression of BDNF and its receptor TrkB during axonal regeneration (Soto et al., 2006b; Blanco et al., 2008). For rats with optic nerve injury in vivo, it has been shown that FGF-2 gene delivery via recombinant adeno-associated viruses (AAV) stimulates axonal growth of a small number of axons (Sapieha et al., 2003).
Exogenous application of CNTF in rats induces a temporal enhancement in RGC survival in vivo (Mey and Thanos, 1993) and promotes regeneration of a few axons through peripheral nerve grafts (Cui et al., 1999; Cui and Harvey, 2000). CNTF can induce moderate axonal regeneration in vitro and in vivo but only when the effects of the inhibitory environment are suppressed (Lingor et al., 2008). The objectives of the present study were to take advantage of the permissive environment of the frog optic nerve to determine the effects that CNTF, BDNF, and FGF-2 have on the speed and number of regenerating axons after optic nerve crush.
MATERIALS AND METHODS
Animals
In total 80 adult frogs (Rana pipiens) of both sexes were used. They were obtained from commercial sources and kept in tanks with recirculating tap water at 18°C.
Surgical Technique for Optic Nerve Crush
With animals under 0.3% tricaine anesthesia, the right eyeball of a series of frogs was approached from the palate and an incision made; the extraocular muscles were teased aside, and the intraorbital section of the optic nerve was exposed. We avoided large blood vessels, and the nerve was crushed using Dumont No. 5 forceps. This leaves the meningeal sheath intact but creates a gap of approximately 1 mm that is completely free of axons. We have confirmed the lack of even small axons in this region by electron microscopic observation (data not shown); also, crushing in this manner perturbs RGC survival almost as effectively as cutting (Blanco et al., 2000). The incision was sutured, and the animals were allowed to recover for several hours in the laboratory under observation before replacing them in their tanks in the animal facility. All our protocols have been approved by the institutional animal care and use committee (IACUC) and follow the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Neurotrophic Factor Application
Optic nerve application
Immediately after the optic nerve was crushed, the proximal nerve stump was lifted and placed on a strip of Parafilm, and 5 µl of BDNF, FGF-2, or CNTF solution was applied directly to the crush lesion. The solution was left in place for 5 min; then, the Parafilm was removed and the palate sutured. Control applications consisted of 5 µl phosphate-buffered saline (PBS; 0.1 M). For BDNF (Alomone Labs, Jerusalem, Israel), a total of 100–125 ng was dissolved in 5 µl of 0.1 M PBS, pH 7.4. For FGF-2 (R&D Systems, Minneapolis, MN) and CNTF (Sigma, St. Louis, MO), 125 ng total was applied. A cocktail of BDNF, FGF-2, and CNTF was applied with the same final concentration and volume as each treatment alone.
To test the role of the receptors, neutralizing blocking antibodies, which block the binding of the neurotrophins to their receptors, were applied in a manner similar to the application of the factors described above. The antibodies were applied 10 min before subsequent BDNF, FGF-2, or CNTF applications. To inhibit the binding of BDNF to the TrkB receptor, 10 µl (1 µg) of neutralizing TrkB tyrosine kinase receptor polyclonal blocking antibody (Promega, Madison, WI) was applied directly into the optic nerve. Control applications consisted of 10 µl chicken IgY at the same concentration. To inhibit the binding of FGF-2 to the FGFR1, 10 µl (1.25 µg) of anti-FGFR1 IgM monoclonal antibody (Chemicon, Temecula, CA) against the receptor was applied directly into the optic nerve. Control applications consisted of 10 µl mouse IgM at the same concentration. For inhibition of the binding between CNTF and CNTFRα, 10 µl (1 µg) of an anti-CNTFRα blocking antibody was applied directly into the optic nerve. Control applications consisted of 10 µl rabbit IgG at the same concentration. In total 32 animals were used for these experiments.
Intraocular application
For intraocular delivery of the factors, the tip of a 701LT Hamilton syringe needle (Hamilton Company, Reno, NV) was inserted into the temporal region of the right eye posterior to the ora serrata. Intraocular BDNF, FGF-2, or CNTF injections consisted of 10 µl PBS containing total amounts of 1,000 ng BDNF or 1,250 ng FGF-2 or CNTF. The injection was carried out slowly over the course of 5 min to minimize fluid reflux. If we assume a dilution within the eye of approximately 1 in 5, this probably delivers a concentration of the factors similar to that with optic nerve application. Control applications consisted of 10 µl of 0.1 M PBS. In addition, a combination of all factors was used to determine any differential effect on the axonal speed or number of regenerating axons for intraocular application followed the previous description. Again, 32 animals were used for these experiments.
Immunohistochemistry
After dissection, three to five eye cups with optic nerves were fixed for each control and experimental stage with buffered 2% paraformaldehyde solution for 1 hr. After PBS washing, the tissues were placed in 30% sucrose for cryoprotection at 4°C overnight, and, after being frozen, longitudinal cryostat sections of 20 µm were cut. The sections were washed twice (5 min each) in PBS containing 0.3% Triton X-100 + 0.5% bovine serum albumin (BSA) and incubated for 30 min in the same buffer containing 10% normal goat serum (NGS). They were then incubated with the monoclonal antibody against GAP-43 (1:500; Chemicon Millipore, Temecula, CA) diluted in 0.1 M PBS + 0.3% Triton X-100 + 0.5% BSA overnight at 4°C. After several washes in the same buffer solution, the sections were incubated with goat anti-mouse Cy2 (1:100; Jackson Immunoresearch, Werst Grove, PA) plus ExtrAvidin Cy3 (1:300; Sigma) for 2 hr at room temperature. The sections were rinsed in 0.1 M PBS six times, for 5 min each, and mounted in Polymount. Antibody specificity was previously tested by Western blot analysis and omitting the primary antibody (Soto et al., 2003). These procedures resulted in the absence of immunostaining.
Biotin Dextran Amine Anterograde Tracer
Nasal and temporal regions of the eye were injected with 10 µl 10% biotin dextran amine (BDA) diluted in distilled water (3,000 MW; Molecular Probes, Eugene, OR), approximately 96 hr before euthanization. To visualize the BDA signal, ExtrAvidin Cy3 was used as described above.
Measurements of Axonal Length, Number, and Distribution
Frozen sections of the whole optic nerve processed with GAP-43 were used to track the regenerating axons and to count the number and distribution of the fibers projecting beyond the injury. Alternating longitudinal sections through the nerve were analyzed to avoid overlap of data. Axonal regeneration was measured from confocal images obtained with a Zeiss Pascal laser scanning confocal microscope, using Zeiss LSM5 Image Browser Software. To quantify axonal extension, we used a modified version of a previously published method (Deng et al., 2009). The distance traversed by the 10 longest GAP-43- and BDA-positive axons, relative to the injury site, was measured for each section for a total of 10 sections per animal, tracking each axon through a confocal stack of images. In addition, we counted the total number of axons at 200 µm beyond the lesion site, because large numbers of axons were found to reach this point 2 weeks after axotomy. The experimenter was aware of the type of treatment at the time of quantification. For the axonal distribution, the first and last serial longitudinal sections were used for “peripheral” data, and the middle longitudinal sections of the nerve were used for the “central” data. Central sections are on average 1.4 times wider than peripheral, so peripheral counts were multiplied by this correction factor (1.4). The statistical analysis was performed in KyPlot software (KyensLab Inc.). The statistical significance was determined by using Student’s t-test, ANOVA, and Tukey-Kramer tests (*P < 0.05, **P < 0.01, ***P < 0.001).
Electron Microscopic Sections
Animals were euthanized 2 weeks after optic nerve crush and intraocular or optic nerve application of PBS, BDNF, FGF-2, or CNTF (n = 2 per treatment, for a total of 16 animals). The animals were perfused through the heart with a fixative solution containing 1% glutaraldehyde and 1% paraformaldehyde in 0.1 M cacodylate buffer, then the optic nerve and eyeball were dissected and left in fixative overnight. The proximal and distal stumps were then carefully dissected, postfixed with osmium, dehydrated, and embedded in Epon-Araldite for semithin and thin sectioning. Thin sections were examined with a JEOL JEM1011 electron microscope equipped with a Gatan digital camera.
RESULTS
Regenerating Optic Nerves Are Labeled With GAP-43 and BDA
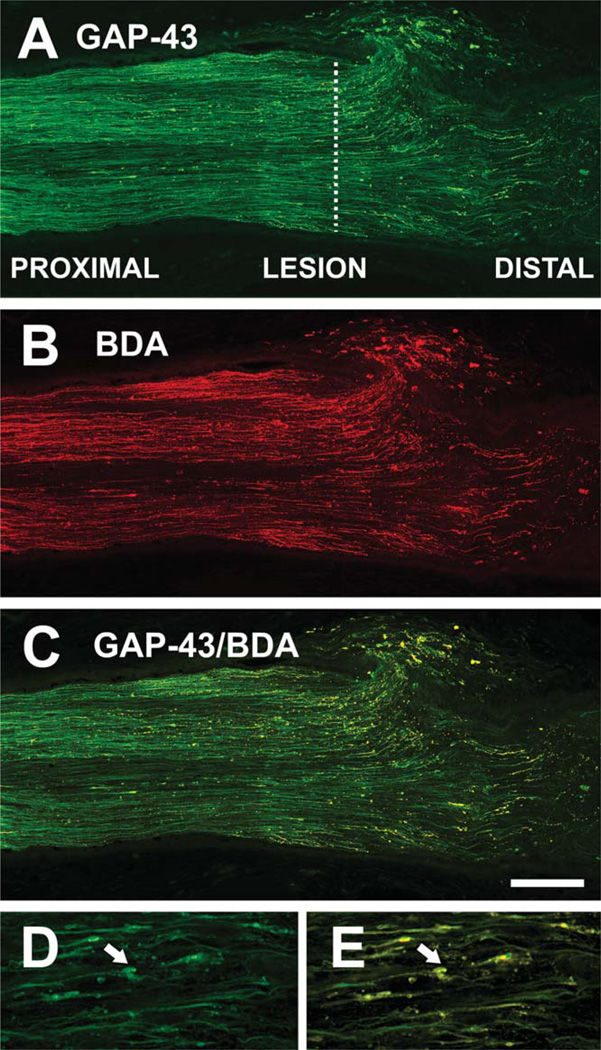
In the frog Rana pipiens, GAP-43 is expressed constitutively in adult RGCs and is upregulated during regeneration (Skene and Willard, 1981; Benowitz and Routtenberg, 1997; Soto et al., 2003). In the present study, 2 weeks after optic nerve crush, immunoreactivity for GAP-43 was observed in regenerating axons treated with PBS (Fig. 1A). The proximal region of the nerve also contained a large number of fibers anterogradely labeled with BDA that had been injected into the eyeball, and the lesion and distal areas showed many regenerating axons that had crossed into the distal portion of the nerve (Fig. 1B). Most, but not all, GAP-43-immunoreactive axons were double-labeled with BDA (Fig. 1C), indicating that the anterograde axonal transport machinery remains functional. All BDA-filled axons were GAP-43 positive, supporting the conclusion that GAP-43 is a reliable marker for all regenerating axons (Soto et al., 2003). Dilated bulbous structures at the tip of the regenerating axons in the distal region appeared labeled with GAP-43 and BDA (Fig. 1D,E), suggesting that these are growth cone-like structures, an important characteristic of active axonal elongation during regeneration.
Fig. 1.
Regenerating fibers after optic nerve crush. A: GAP-43 immunoreactivity in the optic nerve 2 weeks after injury, showing axons regenerating into the distal stump. The crush site is indicated by a dashed line. B: Intraocular application of biotin dextran amine (BDA) anterogradely labels regenerating axons in the same nerve. C: Super-imposition of A and B, showing colocalization of GAP-43 and BDA in the majority of regrowing axons. D,E: Higher magnification images of regenerating axons in the distal stump, showing growth-cone-like structures (arrows). Scale bar = 200 µm in C (applies to A–C); 50 µm for D,E.
Speed of Regeneration Increases With Time After Axotomy
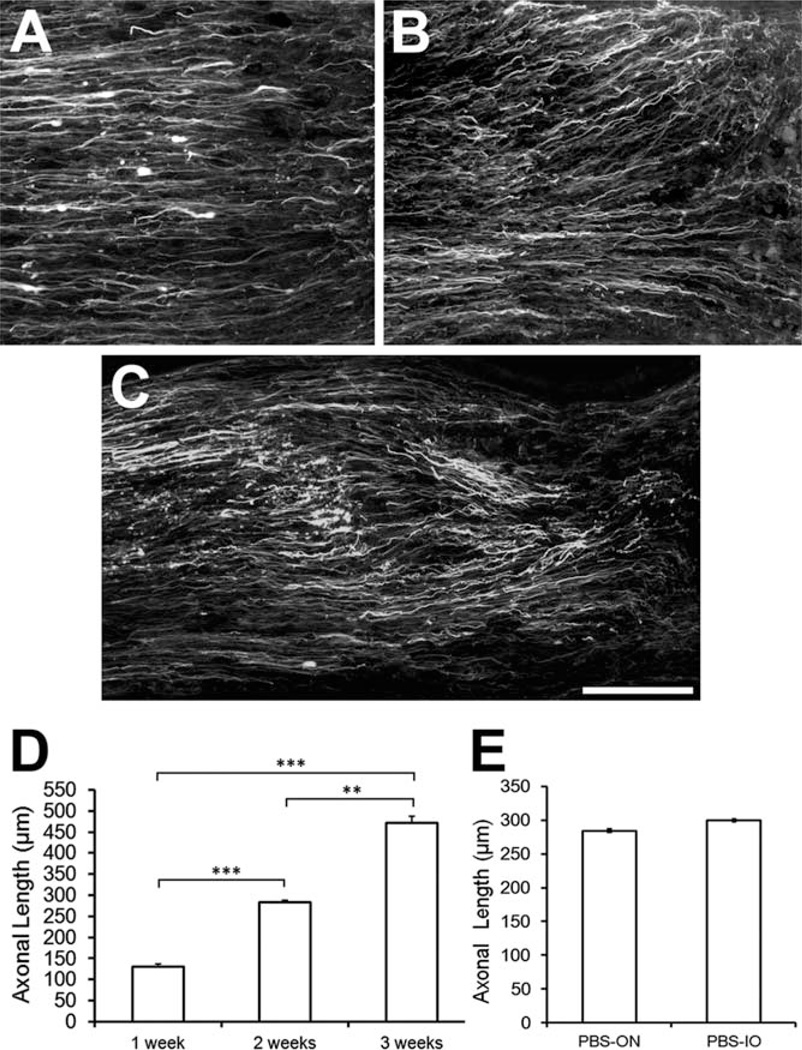
An intrinsic capacity for regeneration after optic nerve injury in frogs has long been established (Sperry, 1944). To study the effect of neurotrophic factors on axonal regeneration, we first have to know how fast the axons regrow in the absence of growth factors. Immunoreactivity for GAP-43 was observed in PBS-treated regenerating axons at 1, 2, and 3 weeks after axotomy (Fig. 2A – C). We measured the extension of these axons from the lesion site (Fig. 1), readily identified by abrupt termination of some of the axons, peripheral accumulation of BDA, and swelling of the distal area adjacent to the lesion. The 10 longest axons were traced in alternate serial sections from each nerve, and their lengths averaged. One week after axotomy these regenerating axons had extended 130 ± 7.4 µm (N = 5) beyond the lesion, at 2 weeks 283 ± 4.3 µm (N = 5), and by 3 weeks 472 ± 14.3 µm (N = 5; Fig. 2D). Thus, in the first week, the speed of regeneration was 130 µm/week, in the second 153 µm/week, and in the third 189 µm/week. Whether the rate of growth continued to increase after 3 weeks was not studied, partially because of problems of accessing the optic tract within the skull. To establish a baseline for the neurotrophin experiments, we then compared the effects of intraocular vs. optic nerve application of PBS at 2 weeks, because it has been reported that eye injury alone can modulate RGC survival and regeneration (Leon et al., 2000). We found no significant difference in axonal extension with the two methods of application (P > 0.05; Fig. 2E). We conclude that the site of application, whether intraocular or into the optic nerve, of the control saline solution does not affect the speed of regrowth after optic nerve crush.
Fig. 2.
Time course of axonal elongation after optic nerve. A–C: Regenerating GAP-43 labeled axons at 1, 2, and 3 weeks after optic nerve injury. D: Length of the 100 longest axons in each nerve, against time after injury. E: Mean axon length at 2 weeks, showing no significant difference between intraocular and nerve application of PBS. Values are mean ± SEM, n = 5 (1–3 weeks), **P < 0.01, ***P < 0.001. Scale bar = 100 µm.
CNTF, FGF-2, and BDNF Increase the Speed of Regeneration After Optic Nerve Crush
Optic nerve application
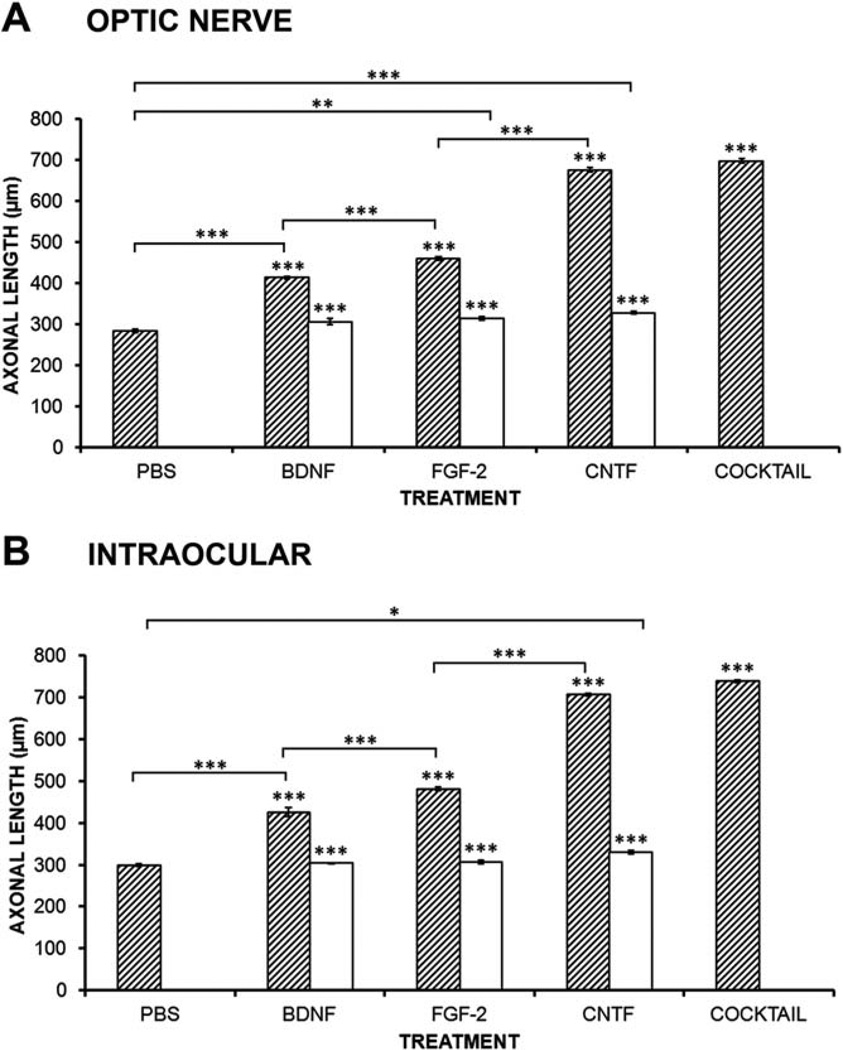
After the preliminary regeneration experiments described above, 2 weeks after axotomy was chosen as the time to compare the effects of the neurotrophins. BDNF application into the optic nerve increased the speed of the longest regenerating axons by 45% compared with PBS controls (Fig. 3A; P < 0.001, N = 5). Application of TrkB-receptor blocking antibody completely abolished this increase. Optic nerve application of FGF-2 increased the speed of the longest axons by 63% compared with controls (P < 0.001, N = 5), significantly more than BDNF, and concurrent application of FGFR1-blocking antibody blocked 90% of this effect (Fig. 3A). CNTF application was most effective in increasing the regeneration rate of the longest axons, with an increase of 138% compared with PBS controls. Antibody against CNTFRα blocked more than 90% of this effect (Fig. 3A). Finally, a mixture (cocktail) of all three neurotrophins significantly increased regeneration by 143%, but this was not significantly more effective than CNTF alone (Fig. 3A).
Fig. 3.
Effects of BDNF, FGF-2, and CNTF on the speed of regeneration. A: Histogram showing the effects of neurotrophic factor application to the optic nerve on the mean axonal length of the 100 longest axons. All factors show significant increases compared with PBS (hatched bars), and their receptor-blocking antibodies almost completely block these effects (open bars). A combination of factors (cocktail) is only as effective as CNTF. B: Histogram showing the effects of intraocular neurotrophic factor injection on the mean axonal length of the 100 longest axons. All factors show significant increases compared with PBS (hatched bars), and their receptor-blocking antibodies almost completely block these effects (open bars). The cocktail of factors is only as effective as CNTF alone. Bars represent mean ± SEM, with asterisks above indicating P values vs. PBS for growth factors (hatched bars) and vs. the respective growth factor for the blocking antibody (open bars). N = 5 for PBS, BDNF, FGF-2, and CNTF; N = 3 for blocking antibodies and cocktail applications. *P < 0.05, **P < 0.01, ***P < 0.001.
Intraocular application
BDNF application into the eyeball increased the speed of the longest regenerating axons by 42% compared with PBS controls (Fig. 3B; P < 0.001, N = 5). Application of TrkB-receptor blocking antibody completely blocked this increase. Intraocular application of FGF-2 increased the speed of the longest axons by 60% compared with controls (P < 0.001, N = 5), application of FGFR1-blocking antibody completely blocked this effect (Fig. 3B). Again, CNTF application was most effective in increasing the regeneration rate of the longest axons, with an increase of 136% compared with PBS controls, with antibody against CNTFRα blocking more than 90% of this effect (Fig. 3B). Again, the cocktail of all three neurotrophic factors was not significantly more effective than CNTF alone (Fig. 3B). Compared with the previous results, it is clear that in no case was intraocular application significantly different from optic nerve application.
CNTF and FGF-2 Increase the Total Number of Axons Projecting Beyond the Optic Nerve Crush
We selected a distance of 200 µm beyond the lesion site as a suitable point at which to count the total number of regenerating axons at 2 weeks after axotomy. A closer distance would include axons that had already regenerated by 1 week, whereas a distance longer than 300 µm would not include any axons at all. We counted the total number of axons at this point in alternate sections through the nerve, then summed these totals. This alternating count was made to avoid counting the same axon twice in different sections but means that this measure probably represents approximately half of the true total number of axons in the whole nerve.
Optic nerve application
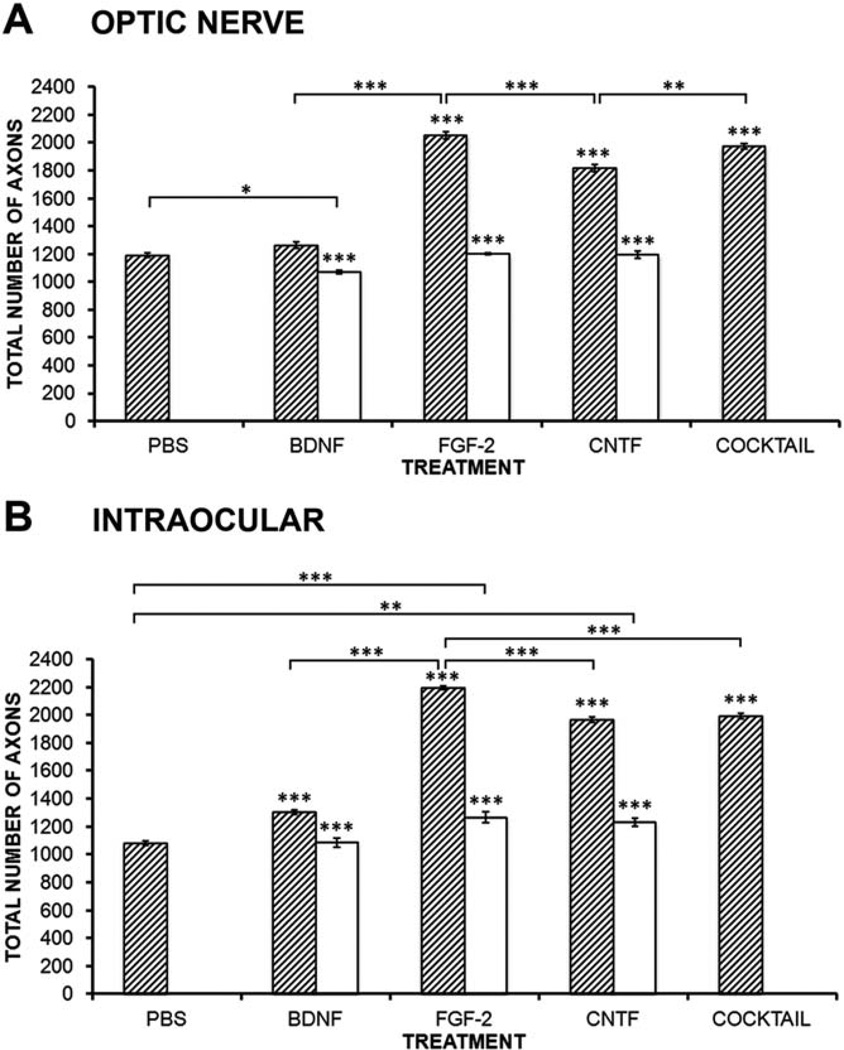
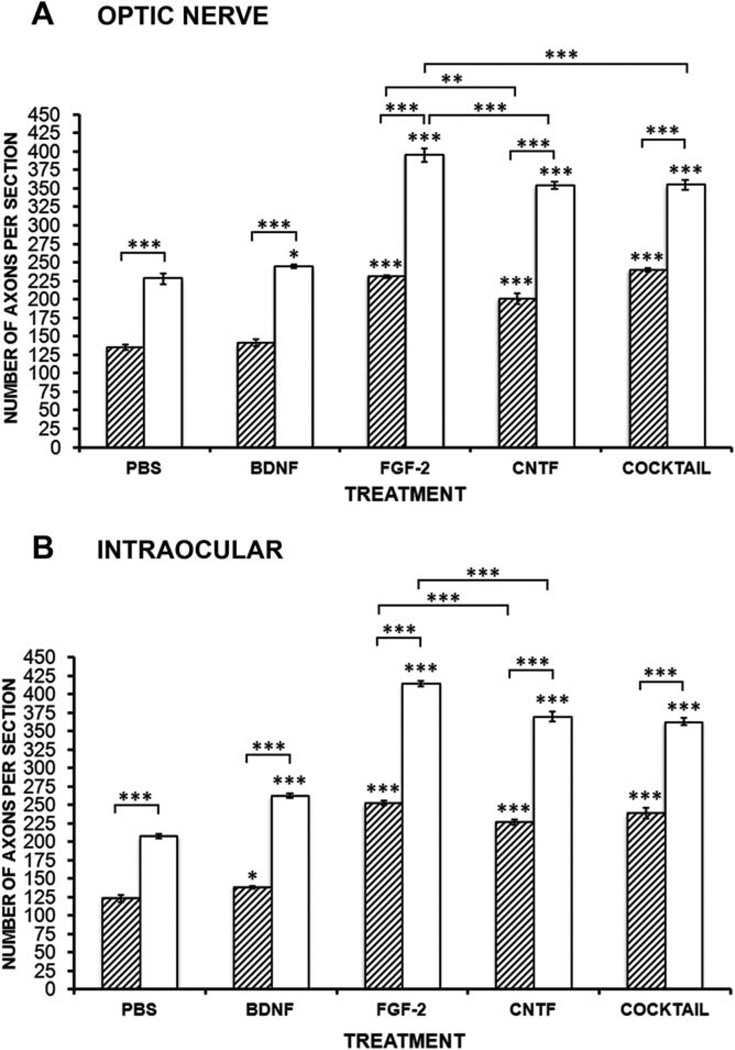
BDNF applied into the optic nerve does not increase the total number of axons and does not show any statistical difference compared with control values (P > 0.05, N = 5; Fig. 4A). FGF-2 application caused a marked 72% increase in the number of axons (P < 0.001, N = 5; Fig. 4A), whereas CNTF was somewhat less effective (52% increase, P < 0.001, N = 5; Fig. 4A). For each neurotrophic factor, the appropriate receptor-blocking antibody completely abolished the increase in number of axons. In the case of the TrkB receptor-blocking antibody, the number of axons was actually decreased by 8%, suggesting that this receptor plays a role in promoting normal regeneration. Application of the cocktail again did not have an effect significantly greater than the most effective single treatment, in this case, FGF-2. It should be noted that in PBS-treated animals no more than 16% of the RGCs have died at 2 weeks after axotomy (Blanco et al., 2000), so most of these increases in axon numbers must be due to effects on axon regrowth rather than differential RGC survival.
Fig. 4.
Effects of BDNF, FGF-2, and CNTF on the number of regenerating axons. A: Histogram showing the effects of neurotrophic factor application to the optic nerve on the number of axons that reach 200 µm in length. FGF-2 and CNTF show significant increases compared with PBS (hatched bars), and their respective receptor-blocking antibodies almost completely block these effects (open bars). The cocktail of factors is only as effective as FGF-2 alone. B: Histogram showing the effects of intraocular neurotrophic factor injection on the number of axons that reach 200 µm in length. All factors show significant increases compared with PBS (hatched bars), and their receptor-blocking antibodies almost completely block these effects (open bars). The cocktail of factors is only as effective as CNTF alone. Bars represent mean ± SEM, with asterisks above indicating P values vs. PBS for growth factors (hatched bars) and vs. the respective growth factor for the blocking antibody (open bars). N = 5 for PBS, BDNF, FGF-2, and CNTF; N = 3 for blocking antibodies and cocktail applications. *P < 0.05, **P < 0.01, ***P < 0.001.
Intraocular application
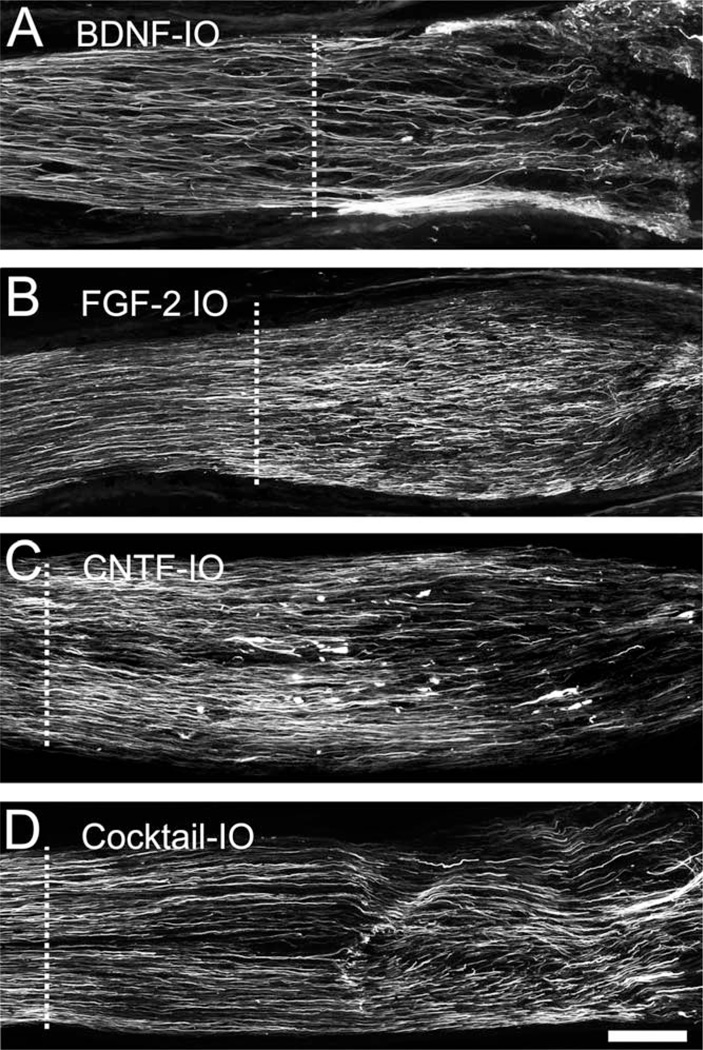
In contrast to optic nerve application, application of BDNF into the eyeball did indeed increase the total number of axons by 20% compared with control values (P > 0.001, N = 5; Figs. 4B, 5A). FGF-2 application again caused the largest increase in the number of axons (102%, P < 0.001, N = 5; Figs. 4B, 5B), and intraocular CNTF increased the number by 82% (P < 0.001, N = 5; Figs. 4B, 5C). For BDNF, the TrkB antibody completely blocked the increase in number of axons, whereas the blocking antibodies for FGFR1 and CNTFRα blocked about 90% of the effect (Fig. 4B). Application of the cocktail intraocularly was strangely less effective (20%) than FGF-2 alone, being equivalent to CNTF. In the case of the axon counts, unlike the measurements of axonal speed, it is clear that intraocular application of each growth factor alone is much more effective than nerve application.
Fig. 5.
Effects of neurotrophic factors on axonal regeneration. A–D: GAP-43 immunoreactivity at 2 weeks after axotomy, qualitatively illustrating the effects of intraocular application of the growth factors on axons numbers and extension. Dashed line indicates crush site. Scale bar = 100 µm.
Differential Effects of Neurotrophins on the Distribution of Axons in the Optic Nerve
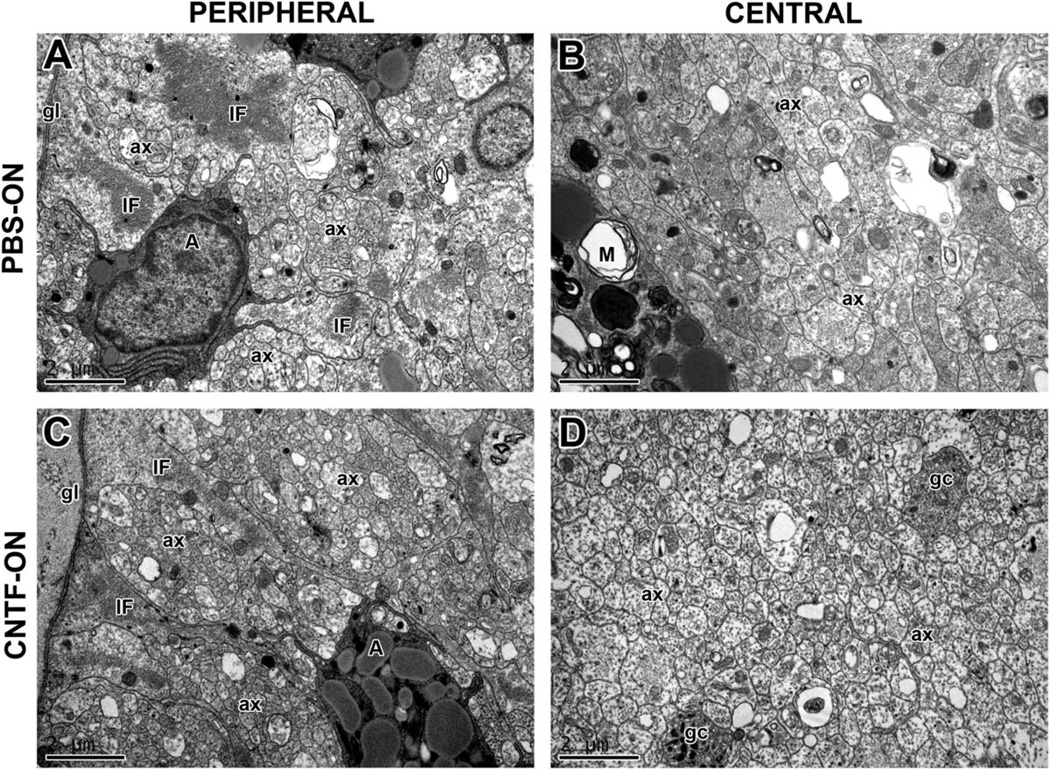
In control nerves, there were significantly more axons in the peripheral region than in the central region (Fig. 6A: 68% more, P < 0.001, N = 5; Fig. 6B: 69%, P < 0.001, N = 5). Electron micrographs of PBS-treated nerves show small groups of fibers in close proximity to peripheral astrocytes and the border of the glia limitans (Fig. 7A; Blanco and Orkand, 1996). In the central region of the nerve are some scattered regenerating axons but also vesicular profiles, some of which were surrounded by disintegrating myelin sheaths and debris (Fig. 7B). We then investigated whether growth factor application to the two different sites had any effects on the differential distribution of axons and the microenvironment within the nerve.
Fig. 6.
Effects of neurotrophic factors on the regional distribution of regenerating axons. A,B: Histograms showing the effects of neurotrophic factor application on the number of axons that reach 200 µm in length, divided into peripheral (open bars) and central (hatched bars) regions of the nerve. In all cases, there are more axons peripherally than centrally. A: Optic nerve application of FGF-2 and CNTF increases the numbers of peripheral and central axons. Cocktail application is only as effective as CNTF alone. B: Intraocular application of all three factors increases the numbers of peripheral and central axons. Cocktail application is only as effective as CNTF alone. N = 5 for PBS, BDNF, FGF-2, and CNTF; N = 3 for cocktail applications. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 7.
Electron micrographs of the regenerating optic nerve. Transverse sections taken approximately 200–300 µm beyond the lesion 2 weeks after injury. A,B: Peripheral and central regenerating axons of PBS-treated control optic nerves. A: Bundles of unmyelinated axons (ax) are in close proximity to astrocytic processes of the glia limitans (gl), identified by their intermediate filaments (IF). Other, darker cell bodies showing secretory activity are visible (A). B: Regenerating axons (ax) are visible, alongside microglia with engulfed debris (M). C,D: Peripheral and central regenerating axons of CNTF-treated optic nerve. C: Large axonal bundles (ax) are surrounded by astrocytic processes with intermediate filaments (IF). Dark secretory cells are also visible. D: Central region showing many regenerating axons, including growth cones (gc). Scale bars = 2 µm.
Optic nerve application
Optic nerve application of BDNF had only a small, but significant, effect on peripheral axons (Fig. 6A), causing a 7% increase in their numbers (P < 0.05, N = 5). FGF-2 increased the numbers of both peripheral and central axons equally, by 71% and 74%, respectively (Fig. 6A; P < 0.001, N = 5). In contrast, CNTF preferentially increased the peripheral axons (56% increase, somewhat greater than the 48% increase seen centrally, P < 0.05). Application of the mixture of neurotrophic factors had the opposite effect, preferentially increasing the numbers of central axons as opposed to the periphery (77% increase, vs. 55%, P < 0.001). In the periphery, the cocktail was only as effective as CNTF alone, less than FGF-2. However, centrally, the cocktail was as effective as FGF-2 alone, more so than CNTF.
Intraocular application
Intraocular application of BDNF had a somewhat greater effect on axon numbers in both areas, increasing central axons by 12% (P < 0.05) and peripheral axons by 26% (P < 0.001). Likewise, FGF-2 was more effective, but it caused similar increases in both peripheral and central regions (100% vs. 106% increases). For intraocular application, CNTF had a greater effect on the numbers of centrally located axons (85% increase) compared with the periphery (78% increase, P < 0.05), the opposite of its effects when applied to the nerve. Cocktail application intraocularly was more effective than optic nerve application overall, but it still was only as effective as CNTF peripherally and as effective as FGF-2 centrally.
Electron microscopic observations were made of nerves in which neurotrophic factors were applied intraocularly or to the nerve. BDNF-treated nerves appeared similar to the controls shown in Figure 7A,B, whereas FGF-2- and CNTF-treated nerves were similar in appearance, independently of the site of application. In these nerves, large bundles of axons in the periphery were associated with astrocytes, and large numbers of regenerating axons were present centrally (Fig. 7C,D). These qualitative observations support the conclusion from the axon counts that there are more axons in the neurotrophin-treated nerves but do not suggest that there are any obvious differences in the microenvironment.
DISCUSSION
This study establishes a model for optic nerve regeneration in the frog Rana pipiens, first to measure the intrinsic capabilities of regeneration and second to test how axonal extension and the number of regenerating fibers are affected when exogenous neurotrophic factors are applied. This model using experimental nerve crush simulates some aspects of real clinical conditions that damage optic nerve axons, such as traumatic optic neuropathy (Wu et al., 2008), ischemic optic neuropathy (Hayreh, 2009), optic neuritis (Guy, 2008), and glaucoma (Lebrun-Julien and Di Polo, 2008). In this as in previous studies (Ríos-Muñoz et al., 2005; Soto et al., 2006b), we have used a single application of growth factor to the nerve or eyeball. It is not known how long BDNF, CNTF, and FGF-2 persist at these sites in the frog, but the half-life of CNTF in mammalian eyeball has been measured in minutes (Dittrich et al., 1994), although BDNF in rodent brain can have a half-life of 3 hr in embryos (Fukumitsu et al., 2006) or last for up to 4 days in adults (Nawa et al., 1995). However, it is certain that the prolonged effects of growth factor application that we observe here, and have observed in the past, must far outlast the persistence of the actual factors themselves. Whether methods for sustained delivery will greatly potentiate these effects remains to be investigated.
Elongation of Rana pipiens Axons After Optic Nerve Crush
Lower vertebrates such as frogs and fish possess the ability to regenerate the optic nerve. In the case of fish, all RGCs survive and regenerate their axons after axotomy (Wanner et al., 1995; Ankerhold et al., 1998), but, in the case of Rana pipiens, only 50% of the RGCs survive after axotomy, and these are capable of regenerating and restoring visual function (Sperry, 1944; Blanco et al., 2000). The present study measured the length of the longest 100 axons over different time periods after injury. These measurements suggest that Rana pipiens axons show a small acceleration in their regrowth, increasing in speed from 130 µm/week to 189 µm/week at 3 weeks. These regeneration speeds are consistent with our previous studies, which have shown optic axons innervating the tectum at 6 weeks (Soto et al., 2006a). This seems somewhat slower than the results reported from other studies (Stelzner et al., 1986; Humphrey, 1988); however, in those cases the animals were kept at higher, more variable temperatures, and the axon lengths were not measured directly, as in this study.
BDNF Increases Elongation of Regenerating Axons
Among the three growth factors tested, BDNF has the least effect. It has no significant effect on the numbers of regrowing axons when applied to the nerve, but it elicits a small (20%) increase when applied to the cell bodies. In frogs, few RGCs have died by 2 weeks after axotomy (Blanco et al., 2000), so this axon count is a reasonably accurate measure of regenerating RGCs, with the caveat that regenerating axons can produce collateral branches (Stelzner et al., 1986). In addition, the growth factor increases the rate of elongation of the fastest regrowing axons irrespective of the site of application. There are some reports of BDNF having stimulatory effects on regenerating axon outgrowth in rats, pigs, and even humans (Sawai et al., 1996; Takano et al., 2002; Bonnet et al., 2004); however, in other cases BDNF may promote RGC survival but prevent outgrowth (Pernet and Di Polo, 2006).
FGF-2 Doubles the Number of Regenerating Axons and Increases Their Rate of Elongation
FGF-2 is the most effective of the three neurotrophic factors in increasing the number of regenerating axons, more than doubling them when applied intraocularly and increasing them by 72% when applied to the nerve. It is also effective at increasing the elongation rate of the fastest regrowing axons, although in this case the site of application makes no difference.
FGF-2 has been implicated as a trophic factor that promotes survival and axonal regeneration. FGF-2 application upon nerve crush potentiates the injury-evoked upregulation of growth-associated protein (GAP-43) levels in RGCs (Soto et al., 2003). Also, previous observations have shown that FGF-2 application upregulates mRNA for BDNF and its receptor TrkB after optic nerve injury (Soto et al., 2006b; Blanco et al., 2008). It has long been known that FGF-2 stimulates axonal growth of Xenopus RGCs during development (McFarlane et al., 1995, 1996), but there is less evidence for effects in adult RGCs. However, it has been shown that viral delivery of FGF-2 to RGCs in rats with optic nerve injury stimulates axonal elongation, but only in a few of the surviving neurons (Sapieha et al., 2003).
CNTF Doubles the Speed of Regeneration
CNTF, applied either to the cut axons or to the cell bodies, elicits a very potent effect on axonal elongation, more than doubling the speed of regeneration of the fastest-growing axons. CNTF was also quite effective in increasing the numbers of regrowing axons, but more so when applied intraocularly. In adult mice, two intraocular injections of CNTF greatly increase the numbers of RGC axons that have reached the end of a peripheral nerve graft 3 weeks after nerve crush (Cui and Harvey, 2000), and a continuous supply via adenoviral transfection of the neurons themselves, or of neighboring Müller glia, is even more effective (Leaver et al., 2006; Pernet et al., 2013). However, in these studies, the numbers of axons at different distances were counted, making it difficult to distinguish effects on the rate of axonal elongation from increases in the numbers of surviving, regenerating RGCs.
Differential Effects of the Neurotrophic Factors Possibly Indicate the Involvement of Different Signaling Pathways
Concomitant application of blocking antibodies against the appropriate receptors blocks at least 90% of the effects of the neurotrophic factors on both axon elongation and axon numbers. In the case of BDNF, the TrkB antibody blocks completely, whereas the antibodies against CNTFRα and FGR1 are only 90% effective. For FGF-2, this perhaps indicates that the response could also be partially mediated by FGFR3, which is present in the RGCs (Duprey-Díaz et al., 2012); for CNTF, it is not clear what other receptors may be present in this system.
For axonal elongation, the order is CNTF > > FGF-2 > BDNF, whereas, for axon numbers, it is FGF-2 > CNTF > > BDNF. These differential effects of the growth factors on axon elongation and regenerating axon numbers perhaps suggest that different downstream signaling pathways are involved in these different processes. However, our observation that the neurotrophic cocktail is no more effective than CNTF alone in the first case or FGF-2 in the second indicates that there is also some degree of overlap or cross-talk of the signaling pathways, so that a single growth factor can saturate the response.
CNTF is known to activate three signaling pathways in RGCs: the Janus kinase/signal transducer and activators of transcription (JAK/STAT), mitogen-activated protein kinase (MAPK), and phosphatidylinositide 3-kinase (PI3/ Akt) signaling pathways (Park et al., 2004; Lingor et al., 2008; Fischer and Leibinger, 2012; Wen et al., 2012). FGF-2 activates MAPK and PKA pathways (Soto et al., 2006b), and BDNF is known to activate MAPK and Akt signaling (Nakazawa et al., 2002; Bonnet et al., 2004). There is a good deal of potential overlap in these pathways, and it is not possible to point out which is clearly responsible for CNTF’s strong effect on axonal elongation or FGF-2’s predominant effect on axon numbers. It has been suggested that only the JAK/STAT and PI3/Akt, and not the MAPK, pathways are directly involved in stimulating axon growth (Müller et al., 2009), which would account for the strong effect of CNTF that we observe but does not explain the effects of FGF-2. In contrast, the stimulatory effect of virally delivered FGF-2 on rat RGC axon regrowth was shown to be due mostly to MAPK activation (Sapieha et al., 2006). However, BDNF also activates MAPK in RGCs, yet has rather small effects on axon outgrowth unless potentiated by cyclic AMP activation (Hu et al., 2010). Clearly there is a need to investigate in more detail the signaling pathways involved in promoting axon elongation and numbers of regenerating axons.
We conclude that both CNTF and FGF-2 have strong facilitating effects on axon regeneration in the frog optic nerve. Future studies will concentrate on elucidating the intracellular signaling pathways involved.
ACKNOWLEDGMENTS
The authors thank Clarissa del Cueto for her expert technical assistance. G.S.V.-M. thanks the UPR-RCM Teaching and Research Graduate Assistantship Program and the MBRS-RISE award R25GM061838 for their economic support during this research project. We are also grateful for the use of the confocal microscope facilities (Zeiss LSM Pascal) at the Institute of Neurobiology, supported by NSF DBI-0115825 and DoD-52680-LS-ISP grants.
Contract grant sponsor: NIH, Contract grant number: GM093869 (to R.E.B.); Contract grant number: SC1NS081726 (to J.M.B.); RCMI-G12RR03051 (to R.E.B., J.M.B.); Contract grant sponsor: NSF, Contract grant number: DBI-0959225 (to R.E.B.)
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- Ankerhold R, Leppert CA, Bastmeyer M, Stuermer CA. E587 antigen is upregulated by goldfish oligodendrocytes after optic nerve lesion and supports retinal axon regeneration. Glia. 1998;23:257–270. [PubMed] [Google Scholar]
- Avwenagha O, Campbell G, Bird MM. The outgrowth response of the axons of developing and regenerating rat retinal ganglion cells in vitro to neurotrophin treatment. J Neurocytol. 2003;32:1055–1075. doi: 10.1023/B:NEUR.0000021902.65233.8d. [DOI] [PubMed] [Google Scholar]
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Blanco RE, Orkand PM. Astrocytes and regenerating axons at the proximal stump of the severed frog optic nerve. Cell Tissue Res. 1996;286:337–345. doi: 10.1007/s004410050703. [DOI] [PubMed] [Google Scholar]
- Blanco RE, Lopez-Roca A, Soto J, Blagburn JM. Basic fibroblast growth factor applied to the optic nerve after injury increases long-term cell survival in the frog retina. J Comp Neurol. 2000;423:646–658. [PubMed] [Google Scholar]
- Blanco RE, Soto I, Duprey-Díaz M, Blagburn JM. Upregulation of brain-derived neurotrophic factor by application of fibroblast growth factor-2 to the cut optic nerve is important for long term survival of retinal ganglion cells. J Neurosci Res. 2008;86:3382–3392. doi: 10.1002/jnr.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Garcia M, Vecino E, Lorentz JG, Sahel J, Hicks D. Brain-derived neurotrophic factor signaling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res. 2004;1007:142–151. doi: 10.1016/j.brainres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lee H, Henle SJ, Cheever TR, Ekker SC, Henley JR. Primary neuron culture for nerve growth and axon guidance studies in zebrafish (Danio rerio) PLoS One. 2013;8:e57539. doi: 10.1371/journal.pone.0057539. (1–11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11:3999–4002. doi: 10.1097/00001756-200012180-00019. [DOI] [PubMed] [Google Scholar]
- Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- Deng K, He H, Qiu J, Lorber B, Bryson B, Filbin MT. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J Neurosci. 2009;29:9545–9552. doi: 10.1523/JNEUROSCI.1175-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich F, Thoenen H, Sendtner M. Ciliary neurotrophic factor: pharmacokinetics and the acute-phase response in rat. Ann Neurol. 1994;35:151–163. doi: 10.1002/ana.410350206. [DOI] [PubMed] [Google Scholar]
- Duprey-Díaz MV, Blagburn JM, Blanco RE. Changes in fibroblast growth factor-2 and FGF receptors in the frog visual system during optic nerve regeneration. J Chem Neuroanat. 2012;46:35–44. doi: 10.1016/j.jchemneu.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Schwab ME, Montani L, Brazda N, Müller HW. Defeating inhibition of regeneration by scar and myelin components. Hdbk Clin Neurol. 2012;109:503–522. doi: 10.1016/B978-0-444-52137-8.00031-0. [DOI] [PubMed] [Google Scholar]
- Fischer D, Leibinger M. Promoting optic nerve regeneration. Prog Ret Eye Res. 2012;31:688–701. doi: 10.1016/j.preteyeres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26:13218–13230. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J. Optic nerve degeneration in experimental autoinmmune encephalomyelitis. Ophthalmic Res. 2008;40:212–216. doi: 10.1159/000119879. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Ischemic optic neuropathy. Prog Ret Eye Res. 2009;28:34–62. doi: 10.1016/j.preteyeres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cho S, Goldberg JL. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:1747–1754. doi: 10.1167/iovs.09-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey MF. A morphometric study of the retinal ganglion cell response to optic nerve severance in the frog Rana pipiens . J Neurocytol. 1988;17:293–304. doi: 10.1007/BF01187852. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Di Polo A. Molecular and cell-based approaches for neuroprotection in glaucoma. Optom Vis Sci. 2008;85:417–424. doi: 10.1097/OPX.0b013e31817841f7. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingor P, Tönges L, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M. ROCK inhibition and CNTF interact on intrinsic signaling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- McFarlane S, McNeill L, Holt CE. FGF signaling and target recognition in the developing Xenopus visual system. Neuron. 1995;15:1017–1028. doi: 10.1016/0896-6273(95)90091-8. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Cornel E, Amaya E, Holt CE. Inhibition of FGF receptor activity in retinal ganglion cell axons causes errors in target recognition. Neuron. 1996;17:245–254. doi: 10.1016/s0896-6273(00)80156-7. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Müller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cel Neurosci. 2009;41:233–246. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3 signaling pathways. Invest Ophthalmol Vis Sci. 2002;43:3319–3326. [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Park K, Luo JM, Hisheh S, Harvey AR, Cui Q. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci. 2004;24:10806–10815. doi: 10.1523/JNEUROSCI.3532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3 and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129:1014–1026. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, Schaffer DV, Flannery JG, Schwab ME. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis. 2013;51:202–213. doi: 10.1016/j.nbd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Pettmann B, Henderson CE. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Ríos-Muñoz W, Soto I, Duprey-Díaz MV, Blagburn JM, Blanco RE. Fibroblast growth factor 2 applied to the optic nerve after axotomy increases Bcl-2 and decreases Bax in ganglion cells by activating the extracellular signal-regulated kinase signaling pathway. J Neurochem. 2005;93:1422–1433. doi: 10.1111/j.1471-4159.2005.03129.x. [DOI] [PubMed] [Google Scholar]
- Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24:656–672. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Sapieha PS, Hauswirth WW, Di Polo A. Extracellular signal-regulated kinases 1/2 are required for adult retinal ganglion cell axon regeneration induced by fibroblast growth factor-2. J Neurosci Res. 2006;83:985–995. doi: 10.1002/jnr.20803. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci. 1996;16:3887–3894. doi: 10.1523/JNEUROSCI.16-12-03887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Arango V, Singman EL. Loss and displacement of ganglion cells after optic nerve regeneration in adult Rana pipiens. Brain Res. 1985;344:267–280. doi: 10.1016/0006-8993(85)90804-2. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH, Willard M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J Cell Biol. 1981;89:86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So KF, Aguayo AJ. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985;328:349–354. doi: 10.1016/0006-8993(85)91047-9. [DOI] [PubMed] [Google Scholar]
- Soto I, Marie B, Baro DJ, Blanco RE. FGF-2 modulates expression and distribution of GAP-43 in frog retinal ganglion cells after optic nerve injury. J Neurosci Res. 2003;73:507–517. doi: 10.1002/jnr.10673. [DOI] [PubMed] [Google Scholar]
- Soto I, López-Roca T, Blagburn JM, Blanco RE. Changes in nNOS and NADPH diaphorase in frog retina and tectum after axotomy and FGF-2 application. Brain Res. 2006a;1103:65–75. doi: 10.1016/j.brainres.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Soto I, Rosenthal JJ, Blagburn J, Blanco RE. Fibroblast growth factor 2 applied to the optic nerve after axotomy upregulates BDNF and TrkB in ganglion cells by activating the Erk and PKA signaling pathways. J Neurochem. 2006b;96:82–96. doi: 10.1111/j.1471-4159.2005.03510.x. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Optic nerve regeneration with return of vision in anurans. J Neurophysiol. 1944;7:57–69. [Google Scholar]
- Stelzner DJ, Bohn RC, Strauss JA. Regeneration of the frog optic nerve. Comparisons with development. Neurochem Pathol. 1986;5:255–288. doi: 10.1007/BF02842939. [DOI] [PubMed] [Google Scholar]
- Takano M, Horie H, Iijima Y, Dezawa M, Sawada H, Ishikawa Y. Brain-derived neurotrophic factor enhances neurite regeneration from retinal ganglion cells in aged human retina in vitro. Exp Eye Res. 2002;74:319–323. doi: 10.1006/exer.2001.1118. [DOI] [PubMed] [Google Scholar]
- Villegas-Perez MP, Vidal-Sanz M, Bray GM, Aguayo AJ. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner M, Lang DM, Bandtlow CE, Schwab ME, Bastmeyer M, Stuermer CA. Reevaluation of the growth-permissive substrate properties of goldfish optic nerve myelin and myelin proteins. J Neurosci. 1995;15:7500–7508. doi: 10.1523/JNEUROSCI.15-11-07500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Ret Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Yin ZQ, Wang Y. Traumatic optic neuropathy therapy: an update of clinical and experimental studies. J Intern Med Res. 2008;36:883–889. doi: 10.1177/147323000803600503. [DOI] [PubMed] [Google Scholar]