Abstract
Background and Purpose
While the efficacy of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in reducing future vascular events for coronary heart disease patients is established, less is known about the precise benefit of these agents among stroke patients. We evaluated whether use of ACEIs or ARBs reduces future vascular events in persons with prior stroke.
Methods
We searched PUBMED, Cochrane Central Register of Controlled Trials, and bibliographies of relevant trials and recent review articles to identify randomized controlled trials. Relative risk (RR) with 95% confidence interval (CI) was used as a measure of the association between use of ACEIs or ARBs and risks of major vascular event (nonfatal stroke, nonfatal myocardial infarction, or death from cardiovascular causes) or stroke (ischemic or hemorrhagic), after pooling data across trials.
Results
Eight randomized controlled trials with 29667 participants were identified. Use of ACEIs or ARBs in persons with prior stroke was associated with lower risks of future major vascular events (RR 0.91, 95% CI 0.87 to 0.97, P=0.001, number needed to treat=71) and recurrent stroke (RR 0.93, 95% CI 0.86 to 0.99, P=0.03, number needed to treat=143). Heterogeneity was found among studies for end points of major vascular events (P=0.02, I2=61%) but not recurrent stroke (P=0.38, I2=6%). In subgroup analyses, there was generally no obvious heterogeneity among different study characteristics.
Conclusions
Treatment with an ACEI or ARB has a clear but rather modest effect on reducing vascular risk in persons with prior stroke.
Keywords: Renin angiotensin system, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, stroke, major vascular disease, randomized controlled trial, meta-analysis
INTRODUCTION
Regardless of hypertension history, blood pressure reduction is recommended for vascular risk reduction in persons with a stroke or transient ischemic attack who are beyond the first 24 hours.1 Blood pressure reduction and not antihypertensive agent class should be the main therapeutic focus of vascular risk reduction in these patients, but based on available data guidelines specifically mention the use of a diuretic or a diuretic-based regimen as ‘useful’.1 Still, there is continued interest in establishing the role of other antihypertensive agent classes in preventing recurrent vascular risk in stroke patients. Over the past years, the renin-angiotensin system (RAS) has become an important therapeutic target for vascular disease prevention. While pooled analyses of randomized trials have supported the efficacy of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in improving vascular outcomes for patients with coronary heart disease,2, 3 less is known about the precise benefit of these agents among patients with a more heterogeneous vascular disease entity like stroke.4
Substantially lower vascular event rates over the last five decades due to improved medical treatments, are making it increasingly difficult to demonstrate the beneficial effects of a drug for secondary stroke prevention, even in large randomized controlled trials.5 Furthermore, recommendations based on the results of single trials can sometimes be misleading due to the risk of false-positive and false-negative results.6 Therefore we undertook a systematic review and meta-analysis of randomized controlled trials to clarify whether use of ACEIs or ARBs reduces future vascular risks in persons with a history of cerebrovascular disease.
METHODS
The study was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement.7 We performed a systematic search of PUBMED and Cochrane Central Register of Controlled Trials (1966 to March 2011) using the search strategy: “stroke” or “cerebrovascular disease” or “cerebrovascular attack” or “cerebral infarct” or “intracranial hemorrhage” AND “angiotensin receptor blockers” or “angiotensin II receptor antagonists” or “T1-receptor antagonists” or “sartans” or “losartan” or “valsartan” or “candesartan” or “telmisartan” or “irbesartan” or “eprosartan” or “olmesartan” or “angiotensin-converting enzyme inhibitors” or “captopril” or “zofenopril” or “enalapril” or “ramipril” or “perindopril” or “quinapril” or “lisinopril” or “benazepril” or “fosinopril” or “trandolapril”. We restricted the search to human studies and clinical trials. There were no language restrictions. Manual searches of bibliographies of all relevant trials and recent review articles were reviewed and identified by two investigators (ML and KSH). We also contacted authors for data regarding subgroup of persons with prior stroke if it was not provided by published articles.
Criteria for inclusion a study were as follow: (1) the study design was a randomized controlled trial; (2) participants had a history of stroke or transient ischemic attack; (3) the active treatment consisted of ACEIs or ARBs; (4) the follow-up duration was at least 6 months; and (5) total participants and the number of future major vascular events and/or recurrent stroke were reported separately for active treatment and comparator groups. Studies were excluded if (1) mandatory ACEIs or ARBs use in control groups or (2) the purpose of the study was to examine efficacy of ACEIs or ARBs in patients with acute stroke (i.e. adding ACEIs or ARBs within 48 hours of ictus). Data from eligible studies were abstracted independently by two investigators (ML and KSH). Discrepancies were resolved by discussion with a third investigator (BO) and by referencing the original report. The methodological quality of the trials was assessed on a 5-point Jadad scale.8
The primary outcomes of interest were the association of RAS modulator use and risk of major vascular events (i.e. composite of death from cardiovascular causes, nonfatal stroke and nonfatal myocardial infarction) and recurrent stroke (ischemic or hemorrhagic). The secondary outcomes of interest were risks of major coronary events, total death, death from cardiovascular causes, and hypotension. Also, since both calcium channel blockers and RAS modulators have been demonstrated to be beneficial for stroke prevention over other classes of antihypertensive drugs,9, 10 comparison was conducted between these two classes of antihypertensive drugs for vascular risk reduction in persons with prior stroke or transient ischemic attack.
Subgroup analyses were conducted according to different study characteristics: study population (prior stroke in entire original study population vs. post-hoc analysis of study subgroup with prior stroke), active treatment agent (ACEIs vs. ARBs), comparator agent (other antihypertensive drug vs. placebo), mean age at entry (< 65 years vs. ≥ 65 years), and hypertension at entry (hypertension as a recruitment criterion vs. hypertension not as a recruitment criterion).
Statistical Analysis
Data were analyzed according to the intention-to-treat principle. Relative risk (RR) with 95% confidence interval (CI) was used as a measure of the association between active treatment with ACEIs or ARBs and risk of future major vascular events or recurrent stroke. Heterogeneity was assessed by the probability value of χ2 statistics and I2, which describes the percentage of variability in the effect estimates that is due to heterogeneity rather than chance.11, 12 We regarded I2 of less than 40% as “heterogeneity might not be important” and more than 74% as “considerable heterogeneity” based on the suggestion of Cochrane Handbook for Systematic Review of Interventions. 13 We pooled data across trials using the fixed-effects model based on Mantel-Haenszel methods14 and compared the results with those obtained from a random-effects model. We also performed a sensitivity analysis to further explore the robustness of our results. To identify any study that may have exerted a disproportionate influence on the summary treatment effect, we removed each individual trial from the meta-analysis one at a time.13 The Cochrane Collaboration's Review Manager Software Package (RevMan 5) was used for the meta-analysis.
RESULTS
The literature review identified 30 full articles for detailed assessment, among which 15 were excluded because they did not report an end point of major vascular event or recurrent stroke among persons with prior stroke, and two because ARBs were added within 48 hours of index stroke. Our final analysis included 13 articles derived from 8 randomized controlled trials: end points were assessed in whole study population with prior stroke in 3 trials15-18 and in subgroup of persons with prior stroke in 5 trials10, 19-26 (Supplementary Figure 1). Table 1 shows the characteristics of the included studies with 29667 individuals. Post-enrollment major vascular events were reported in 7 studies and recurrent strokes were reported in 8 studies. Four trials compared RAS modulators15, 16, 20, 23 to placebo while the other 4 trials using other antihypertensive drug in a comparator group.10, 17, 19, 24 Mean baseline blood pressures varied from 144 to174/84 to 98 mmHg. The follow-up duration ranged from 2.5 to 5 years and the studies generally had a high quality (Jadad score ≥3).
Table 1.
Characteristics of included trials
| Pre-existing condition in original trials |
Active/Comparator | Patient number |
Mean age |
Time from stroke to trial, months |
Follow- up duration, years |
Baseline BP, mmHg |
Difference in BP at trial end,* mmHg |
Jadad score, 5- point maximum |
|
|---|---|---|---|---|---|---|---|---|---|
| CASE-J19 | High-risk hypertension | Candesartan/Amlodipine | 473 | 63.9 | > 6 | 3.2 | 163/92 | −1.7/−0.6 | 3 |
| HOPE20, 21, 26 | Vascular disease or DM plus one CV risk factor | Ramipril/Placebo | 1013 | 66.0 | > 2 | 5 | 151/79 | 10.0/4.0 | 5 |
| LIFE10, 22 | Hypertension with LVH | Losartan/Atenolol | 728 | 66.9 | > 6 | 4.8 | 174/98 | 1.3/−0.4 | 4 |
| MOSES17 | Hypertension with prior stroke or TIA | Eprosartan/Nitrendipine | 1352 | 67.9 | ≤24 | 2.5 | 151/87 | −1.5/−0.6 | 3 |
| PROFESS15 | Ischemic stroke | Telmisartan/Placebo | 20332 | 66.2 | ≤4 | 2.5 | 144/84 | 3.0/1.6 | 5 |
| PROGRESS16, 18 | Stroke | Perindopril/Placebo | 2561 | 64.0 | ≤60 | 3.9 | 144/84 | 4.9/2.8 | 5 |
| SCOPE23 | Aged hypertensive people | Candesartan + other antihypertensive drug/Placebo + other antihypertensive drug | 194 | 76.4 | NR | 3.7 | 166/90 | −0.3/1.5 | 5 |
| VALUE24, 25 | CV disease or high risk hypertensive people | Valsartan/Amlodipine | 3014 | 67.3 | ≥3 | 4.2 | 155/88 | −2.8/−1.6 | 5 |
BP: blood pressure, CV: cardiovascular, DM: diabetes mellitus, LVH: left ventricular hypertrophy, NR: not reported, TIA: transient ischemic attack
: comparator-active
Trial name: CASE-J: Candesartan Antihypertensive Survival Evaluation in Japan, HOPE: Heart Outcomes Prevention Evaluation, LIFE: Losartan Intervention For Endpoint reduction, MOSES: Morbidity and Mortality After Stroke, Eprosartan Compared With Nitrendipine for Secondary Prevention, PROFESS: Prevention Regimen for Effectively Avoiding Second Strokes trial, PROGRESS: Perindopril Protection against Recurrent Stroke Study, SCOPE: Study on Cognition and Prognosis in the Elderly, VALUE: Valsartan Antihypertensive Long-term Use Evaluation
Pooling all included randomized controlled trials showed that use of ACEIs or ARBs in persons with prior stroke or transient ischemic attack was associated with lower risks of future major vascular events (RR 0.91, 95% CI 0.87 to 0.97, P=0.001, number needed to treat=71) and recurrent stroke (RR 0.93, 95% CI 0.86 to 0.99, P=0.03, number needed to treat=143) (Figure 1). Heterogeneity was found among studies for end points of major vascular events (P=0.02, I2=61%) but not recurrent stroke (P=0.38, I2=6%) (Figure 1). The SCOPE study was distinct in terms of it's very small sample size (< 200 patients) and substantially large effect favoring the active treatment arm. Analysis excluding this trial showed similar benefit for RAS modulators in major vascular event reduction and the heterogeneity became insignificant (RR 0.92, 95% CI 0.87 to 0.97, P value for heterogeneity 0.13, I2=41%). There was no substantial asymmetric appearance on the funnel plots, implying substantial publication bias was unlikely (Supplemental figure 2A and 2B). The estimates from the random-effects model were similar to those of the fixed-effects model. The exclusion of any single study from the analysis did not alter the overall finding in a sensitivity test.
Figure 1.
Effect of renin-angiotensin system modulators on (A) the risk of major vascular events and (B) recurrent stroke in people with prior stroke
For secondary end points, 5 trials reported data on major coronary events,15-17, 20, 21, 25 2 on total death,15, 17 1 on death from cardiovascular causes,15 and 2 on hypotension.15, 17 ACEIs or ARBs use in persons with prior stroke showed non-significant reduction in major coronary events and death from cardiovascular causes. The risk of total death was similar between active and comparator groups. However, risk of hypotension was significantly higher in the RAS modulator group (Table 2).
Table 2.
Effect of ACE inhibitors or ARBs on primary and secondary endpoints in people with a stroke history
| ACE inhibitors or ARBs, n/N (%) | Comparators, n/N (%) | RR (95% CI) | P value | |
|---|---|---|---|---|
| Major vascular events15-17, 19-21, 23-25 | 2074/14466 (14.3) | 2270/14473 (15.7) | 0.91 (0.87-0.97) | 0.001 |
| Recurrent stroke10, 15-17, 19-25 | 1328/14835 (9.0) | 1434/14832 (9.7) | 0.93 (0.86-0.99) | 0.03 |
| Major coronary events15-17, 20, 21, 24, 25 | 503/14121 (3.6) | 557/14151 (3.9) | 0.90 (0.80-1.01) | 0.08 |
| Total death15, 17 | 812/10827 (7.5) | 792/10857 (7.3) | 1.03 (0.94-1.13) | 0.57 |
| Death from cardiovascular causes15 | 223/10146 (2.2) | 263/10186 (2.6) | 0.85 (0.71-1.02) | 0.07 |
| Hypotension15, 17 | 481/10827 (4.4) | 257/10857 (2.4) | 1.87 (1.61-2.17) | < 0.001 |
Three trials compared RAS modulators and calcium channel blockers for risks of major vascular events and recurrent stroke in persons with prior stroke or transient ischemic attack.17, 19, 25 Compared to calcium channel blockers, RAS modulators reduced major vascular risk while recurrent stroke risk was not different between these two classes of antihypertensive drugs (Figure 2).
Figure 2.
Comparison between renin-angiotensin system modulators and calcium channel blockers for risks of (A) major vascular events and (B) recurrent strokes in people with prior stroke
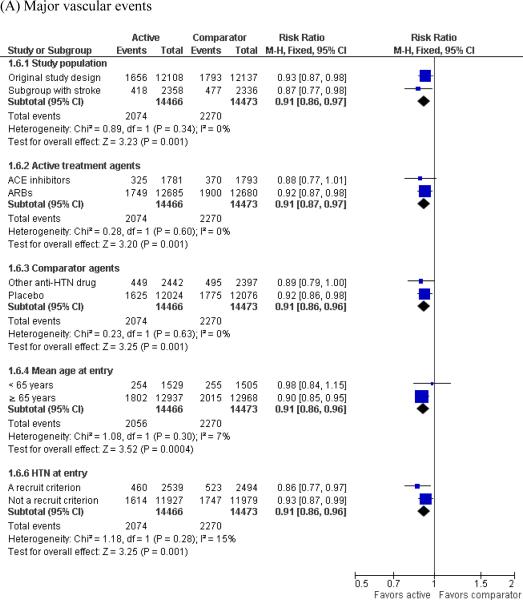
In subgroup analyses, use of RAS modulators was associated with a reduction or decreasing trend in the risk of subsequent major vascular events and recurrent strokes when we stratified the estimates by study population, active treatment agents, comparator agents, mean age at entry, and inclusion of hypertension as a study recruitment criterion. There was also no obvious heterogeneity among the different study characteristics (Figure 3).
Figure 3.
Subgroup analyses
DISCUSSION
In this meta-analysis of 8 randomized controlled trials of generally good quality, among almost 30,000 people with a history of symptomatic cerebrovascular disease, we found that use of ACEIs or ARBs were associated with a 9% relative risk reduction in overall vascular risk and a 7% relative risk reduction in recurrent stroke risk. Use of ACEIs or ARBs did not affect risk of all-cause mortality, but risk of hypotension was increased. Although the overall vascular protective effects of ACEIs and ARBs among patients with known stroke or transient ischemic attack seem definitive, it is relatively modest, when compared to other proven vascular risk reducing strategies. Nonetheless, this benefit was observed from clinical trials conducted mostly during a time of declining vascular event rates due to better background medical treatments and risk factor control,5 including active blood pressure control with other agents in the trial control arms.
The findings of this meta-analysis are congruent with the largest individual stroke secondary prevention trial to date, PRoFESS.15 The hazard ratio point estimates in the meta-analysis and in PRoFESS are homogenous for reduction in vascular events (0.91 vs 0.94) and recurrent stroke (0.93 vs 0.95) are similar. PRoFESS alone was underpowered to detect benefits of this modest magnitude. Moreover, subjects in PRoFESS received more formidable background treatment with other vascular risk reduction therapies than most other trial cohorts (e.g. 47% patients received statin therapy, and 37% patients received ACEIs through the trial), and the low control event rate (3.7% per year) reduced the ability of add-on ARB treatment to demonstrate a significant benefit. Unlike PRoFESS alone, the meta-analysis had adequate power to detect the modest treatment effect exerted by add-on ACEI/ARB therapy.
The observed magnitude of vascular risk reduction for use of ACEIs or ARBs in people with stroke or transient ischemic attack was smaller than was seen among persons with known coronary heart disease.3 The reason for this difference in effect size between both vascular disease entities cannot be stated with certainty, but is likely explained by the substantial heterogeneity among mechanisms of stroke while coronary heart disease is generally caused by atherosclerosis. Thus, the varied anti-atherosclerotic actions of RAS modulators would be expected to be more robust in coronary heart disease patients.
Compared to calcium channel blockers, RAS modulators reduced major vascular disease by 11% in patients with prior stroke or transient ischemic attack while recurrent stroke risk was not different between these two classes of antihypertensive drugs. This findings was somewhat inconsistent with a recent study which suggested superiority of calcium channel blockers for stroke prevention due to low intraindividual variability in blood pressure.9 The discrepancy mostly derived from different study population since that study included diverse populations while the current study focused on persons with prior stroke or transient ischemic attack. Large randomized controlled trials comparing RAS inhibitors and calcium channel blockers in persons with prior stroke might be imperative to guide antihypertensive therapies in secondary stroke prevention.
There is evidence that diuretics which can have a stimulating effect on the RAS, reduce primary and recurrent stroke. 1 However, the exact mechanism through which thiazide and thiazide-like diuretics exert their antihypertensive/stroke preventive effect is not precisely known, but is it believed to overwhelm any untoward activation of the RAS.27 A similar argument is also made for the stroke preventive benefit of thiazide and thiazide-like diuretics in spite of their untoward glycemic effects (decreases in glucose tolerance) and lipid effects (increases in plasma levels of LDL cholesterol and triglycerides). In fact, one of the postulated reasons for the synergistic antihypertensive efficacy of RAS agent/thiazide combo treatments is that RAS agents ameliorate thiazide-induced activation of the renin-angiotensin-aldosterone system.27
Since we did not intend to examine the efficacy of blood-pressure lowering treatment with RAS modulators in patients with acute stroke, the results from this meta-analysis cannot be applied to this group of patients. In a recent large trial, adding an ARB in patients with acute stroke and raised blood pressure was associated with a non-significant increased risk of future vascular events.28 Mildly elevated blood pressure in the acute post-stroke period may be associated with a more favorable outcome, perhaps because it preserves perfusion to ischemic regions.29 Therefore it may be prudent to consider only adding ACEIs or ARBs after 48 hours of clinical stability in a fixed low dose (to be titrated upwards accordingly later on) or to delay initiation altogether in the first two weeks after the stroke. The challenge with the latter approach is that the drugs may end up being initiated in very delayed fashion or not at all, thereby also exposing the patient to unnecessary recurrent vascular risk.
There are limitations to this study. First, data were derived from subgroups of patients with prior stroke in 5 trials, which were not the original trial purpose. Although there was no obvious heterogeneity between studies (original design vs. host-hoc analysis), more randomized controlled trials focusing on the effect of using RAS modulators solely among persons with a history of stroke are warranted. Second, this study was a trial-level meta-analysis rather than an individual, patient-level pooled analysis. Detailed information such as recurrent stroke type (ischemic vs. hemorrhagic stroke), possibility of a specific drug effect that goes beyond the lowering of blood pressure, possibility of different RAS modulators effect on different smoking status, sex or race/ethnicity, or exact time from index stroke to use of RAS modulators was not available and further exploration for relevant results was not possible. A patient-level pooled analysis may better clarify these issues. Despite these limitations, the current meta-analysis is still informative with regard to providing insights into a viable secondary stroke prevention strategy. It suggests that use of ACEIs or ARBs modestly reduces the risks of major vascular events and recurrent stroke in persons with a history of cerebrovascular disease and may be a reasonable add-on therapy in the subacute or chronic stage following a stroke, if there are no contraindications.
Supplementary Material
Acknowledgement
We deeply thank Professor Yasuno for kindly providing relevant data of CASE-J.
Footnotes
Disclosures: None for all authors
Competing interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–588. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 3.Baker WL, Coleman CI, Kluger J, Reinhart KM, Talati R, Quercia R, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors or angiotensin II-receptor blockers for ischemic heart disease. Ann Intern Med. 2009;151:861–871. doi: 10.7326/0003-4819-151-12-200912150-00162. [DOI] [PubMed] [Google Scholar]
- 4.Sokol SI, Portnay EL, Curtis JP, Nelson MA, Hebert PR, Setaro JF, et al. Modulation of the renin-angiotensin-aldosterone system for the secondary prevention of stroke. Neurology. 2004;63:208–213. doi: 10.1212/01.wnl.0000130360.21618.d0. [DOI] [PubMed] [Google Scholar]
- 5.Hong K, Yegiaian S, Lee M, Lee J, Saver J. Declining Stroke and Vascular Event Recurrence Rates in Secondary Prevention Trials over the Past 50 Years and Consequences for Current Trial Design. Circulation. 2011:2111–2119. doi: 10.1161/CIRCULATIONAHA.109.934786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peto R. Why do we need systematic overviews of randomized trials? Stat Med. 1987;6:233–244. doi: 10.1002/sim.4780060306. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 9.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 10.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. [Google Scholar]
- 14.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 15.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 17.Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES). Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 18.Effects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebrovascular disease. Eur Heart J. 2003;24:475–484. doi: 10.1016/s0195-668x(02)00804-7. [DOI] [PubMed] [Google Scholar]
- 19.Ogihara T, Nakao K, Fukui T, Fukiyama K, Fujimoto A, Ueshima K, et al. The optimal target blood pressure for antihypertensive treatment in Japanese elderly patients with high-risk hypertension: a subanalysis of the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial. Hypertens Res. 2008;31:1595–1601. doi: 10.1291/hypres.31.1595. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 21.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 22.Kizer JR, Dahlof B, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Stroke reduction in hypertensive adults with cardiac hypertrophy randomized to losartan versus atenolol: the Losartan Intervention For Endpoint reduction in hypertension study. Hypertension. 2005;45:46–52. doi: 10.1161/01.HYP.0000151324.05355.1c. [DOI] [PubMed] [Google Scholar]
- 23.Trenkwalder P, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Papademetriou V, et al. The Study on COgnition and Prognosis in the Elderly (SCOPE) - major CV events and stroke in subgroups of patients. Blood Press. 2005;14:31–37. doi: 10.1080/08037050510008823. [DOI] [PubMed] [Google Scholar]
- 24.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 25.Zanchetti A, Julius S, Kjeldsen S, McInnes GT, Hua T, Weber M, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: An analysis of findings from the VALUE trial. J Hypertens. 2006;24:2163–2168. doi: 10.1097/01.hjh.0000249692.96488.46. [DOI] [PubMed] [Google Scholar]
- 26.Sleight P, Yusuf S, Pogue J, Tsuyuki R, Diaz R, Probstfield J. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet. 2001;358:2130–2131. doi: 10.1016/S0140-6736(01)07186-0. [DOI] [PubMed] [Google Scholar]
- 27.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Sandset EC, Bath PM, Boysen G, Jatuzis D, Kõrv J, Lüders S, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 29.Yong M, Diener HC, Kaste M, Mau J. Characteristics of blood pressure profiles as predictors of long-term outcome after acute ischemic stroke. Stroke. 2005;36:2619–2625. doi: 10.1161/01.STR.0000189998.74892.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.