Abstract
Tinnitus has been challenging to treat with consistently positive results. The Neuromonics Tinnitus Treatment is a newly available approach to the treatment of clinically significant, problematic tinnitus (and reduced sound tolerance) that was developed with the intention of simultaneously addressing the auditory, attentional, and emotional processes underlying the condition. It uses a prescribed acoustic stimulus, customized for each patient's individual audiometric profile, which provides a broad frequency stimulus to address the effects of auditory deprivation, promotes relief and relaxation with the intention of reducing engagement of the limbic system/amygdala and autonomic nervous system, and applies the principles of systematic desensitization to address the attentional processes. This article describes the underlying principles behind this approach. It also summarizes evidence for clinical efficacy from controlled clinical studies and from a private practice clinical setting, where it has been shown to provide consistently positive outcomes for patients meeting suitability criteria.
Keywords: tinnitus, acoustic, neural, stimulus
Context: Overview of the Neurological Processes That Contribute to Clinically Significant Tinnitus and Reduced Sound Tolerance
Characterization of tinnitus pathogenesis has been hampered by its heterogeneous nature—in terms of its initial causes, the mechanisms involved in its progression, its symptoms, and its effects on different patients—and by the lack of direct evidence for its causes and, hence, reliance on circumstantial evidence (Moller, 2007b). This notwithstanding, there is growing acceptance that for those patients with clinically significant tinnitus (i.e., tinnitus that is so intrusive and problematic that it warrants treatment), development of the condition typically involves neuro-plastic change within the auditory, attentional, and emotional processes within the brain (Kaltenbach, 2006; Georgiewa et al., 2006; Jastreboff, 2004; Moller, 2007a; Tyler, 2005). Cognitive processes appear to play a role in modulating the changes that occur in attentional and emotional systems (Jastreboff, 2004), and in some individuals, somatosensory, somato-motor, or visual-motor systems may also play a role (reviewed by Cacace, 2003).
In relation to the auditory processes leading to initial tinnitus perception (Konig, Schaette, Kempter, & Gross, 2006; Nuttall, Miekle, & Trune, 2004), functional studies have shown that auditory deprivation causes the auditory system to become more active and more sensitive to sound (Formby, Sherlock, & Gold, 2003; Heller & Bergman, 1953). Following peripheral hearing damage, for example, through noise insult or ototoxic drugs, there are changes in activity levels in the auditory nerves that appear to be centrally mediated (Eggermont & Roberts, 2004; Gerken, Saunders, & Paul, 1984; Kaltenbach, 2006). As a consequence, the auditory cortex receives more and/or different neural input, which it interprets as sound. Essentially, the cortex detects the amplified background neurological activity and interprets it as the sounds, such as ringing or buzzing, perceived in tinnitus. Changes in the auditory cortex have also been shown to involve reorganization of the tonotopic map (Muhlnickel, Elber, Taub, & Flor, 1998).
For the great majority of people who experience tinnitus, the tinnitus perception they experience as a result of these auditory processes is not especially bothersome. However, for those with clinically significant tinnitus, the perception becomes reinforced and more intrusive as a result of the involvement of attentional and emotional processes.
The attentional processes involve the perceptual filters that work on all senses to determine which sensory perceptions are brought to our conscious attention and which are not. These filters play an important role, as they ensure that conscious attention is focused on important stimuli while preventing us from being overwhelmed by sensory input. The filters recognize specific patterns of neural activity, which are constantly being updated and refined through experience. In the case of clinically significant tinnitus, these filters determine that attention should be applied to the specific patterns of neural activity associated with the tinnitus percept, such that it is constantly brought to the patient's conscious attention (Jastreboff, 2004; Searchfield, Morrison-Low, & Wise, 2007).
The limbic system of the forebrain (and the amygdala, in particular), certain sublimbic structures in the brainstem, and the autonomic nervous system (responsible for the “fight or flight” response) are involved in the control and expression of emotional states. In patients with clinically significant tinnitus, these systems become engaged in response to the awareness of tinnitus (Cacace, 2004; Kaltenbach, 2006; Lockwood et al., 1998; Muhlau et al., 2006). At its worst, this causes a stressful state of high arousal and anxiety in response to the tinnitus awareness, and this state has a significant effect on quality of life and general well-being.
The engagement of the autonomic system and amygdala not only causes a stress reaction, but also reinforces the other processes. That is, their engagement leads to further increases in the sensitivity of the auditory system and reinforcement of the attentional filters. This, in turn, leads to further increase in tinnitus loudness and awareness, which in turn increases the level of stress, and so on, in a self-perpetuating vicious cycle that sustains the tinnitus awareness and disturbance and can make the tinnitus progressively worse over time (Jastreboff, 2004).
Similar processes involving neuroplastic change in the auditory system and emotional centers of the brain are also believed to be involved in the development of conditions of reduced sound tolerance, such as hyperacusis and misophonia, which are commonly associated with tinnitus. Abnormally high gain within the auditory system and involvement of the limbic and autonomic nervous systems have been proposed as contributing factors in the development of reduced sound tolerance (Jastreboff & Hazell, 2004; Jastreboff & Jastreboff, 2004), and reinforcement by cognitive/attentional processes as well as social factors has been implicated (Baguley & Andersson, 2007). Functional studies in normal hearing people provide support for the involvement of auditory gain in the development of this condition (Formby et al., 2003).
Reduced sound tolerance can be debilitating for patients when severe, causing painful discomfort in response to moderately loud or specific sounds. This can result in the development of avoidance behaviors that can have a significant effect on quality of life (Baguley & Andersson, 2007).
This summarized overview of the processes involved in the development of clinically significant tinnitus is depicted in Figure 1. The key aspects of the Neuromonics Tinnitus Treatment, which address the auditory, attentional, and emotional processes in treatment of both tinnitus and conditions of reduced sound tolerance, appear at the bottom of the figure. A full description of the program follows.
Figure 1.
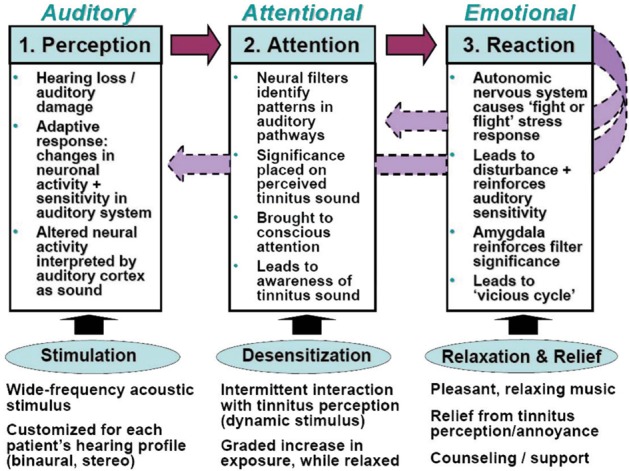
Schematic overview of key processes involved in the development of clinically significant tinnitus and how they are addressed by the Neuromonics Tinnitus Treatment.
Description of the Neuromonics Tinnitus Treatment
The Neuromonics Tinnitus Treatment was developed with the intention of simultaneously addressing the auditory, attentional, and emotional processes underlying the condition. It involves use of a medical device for daily, at-home administration of an acoustic treatment, together with a comprehensive education, counseling, and support program that typically involves six face-to-face appointments with the clinician over a 6-month period. A key component of the approach is the use of an individually prescribed acoustic stimulus, which provides a broad frequency stimulus to address the effects of auditory deprivation, promotes relief and relaxation with the intention of reducing engagement of the amygdala and autonomic nervous system, and applies the principles of systematic desensitization to address the attentional processes. These key aspects of the Neuromonics Tinnitus Treatment are summarized in Figure 1.
A central tenet of the Neuromonics approach is that of customization for each patient's unique circumstances. In particular, it involves the use of an acoustic stimulus that is customized by spectral modification to account for each patient's hearing loss profile and administered in a manner that is tailored to his or her tinnitus profile. This is complemented with a counseling and support program that is collaboratively tailored to the specific needs of each individual. By customizing the treatment in this way, its effectiveness, efficiency, and user acceptability are maximized. Consistent with this philosophy, the acoustic stimulus is spectrally modified in a patient-specific fashion to account for each individual's measured hearing thresholds. This is accomplished using fairly standard audiological principles in terms of the proportion of the hearing loss that is accounted for—a variation of the “half gain” rule is applied, modified to account for loudness recruitment/sound tolerance issues that are commonplace among tinnitus sufferers, such that somewhat less than half gain is typically provided. This is accomplished for each ear, and then the signals for the two ears are combined in a manner that seeks to ensure a perceptually balanced listening experience across frequencies by applying a correction for the equal loudness contours, and across the two ears by applying a correction based on the measured asymmetry between the ears.
Hearing thresholds are measured using standard audiological procedures and, in the case of thresholds at 10 and 12.5 kHz, calibration according to ISO TR/389-5 (International Organization for Standardization, 1998). Spectral modification is then applied across the full frequency range up to 12.5 kHz. Psychoacoustic measures are used to characterize the tinnitus as a basis for tinnitus education and counseling, as well as (in the case of minimum masking level and loudness discomfort level) for monitoring of the patient's progress through treatment. Tinnitus pitch matching is performed using the two-alternative forced choice procedure from the Oregon Health Sciences University Tinnitus Clinic protocol (Vernon & Meikle, 1988). The minimum masking level procedure was adapted from the technique of Jastreboff, Hazell, and Graham (1994). Loudness discomfort level measurements are based on the classic procedure (Hawkins, Walden, Montgomery, & Prosek, 1987), but with some adaptations, as described by Davis, Paki, and Hanley (2007).
Patients initially use their customized treatment for 2 or more hours per day, especially at those times of the day (or night) when their tinnitus is most disturbing. In this way, while listening to the treatment, relief from the disturbing effects of the tinnitus perception is maximized in the early stage of treatment. This time does not need to be all in one session but can be split across several sessions per day as necessary to fit as conveniently as possible into the patient's daily routine. The acoustic stimulus is provided to patients via a proprietary, purpose-built medical device. This device has been specifically designed to facilitate convenient, efficient, and effective administration of the treatment. It includes features such as treatment dosage monitoring/reporting tools that facilitate compliant usage, as well as volume controls that encourage appropriate setting of volume when using the treatment. Use of the device also ensures the necessary control over the acoustic parameters of the treatment, including calibration for the frequency response of the earphones. The use of earphones with this device helps circumvent the high frequency free-field attenuation that is a practical limitation in other techniques, such as the use of hearing aids.
In addition to the use of a customized acoustic stimulus, the treatment also involves a structured rehabilitation program, typically provided over a 6-month period by a clinician specifically trained in tinnitus treatment (Davis, 2005). This program encompasses a comprehensive suite of elements: education concerning tinnitus and how it can be addressed; coaching and behavioral modification addressing such aspects as relaxation, sleep management, and reduction in exposure to factors that may exacerbate tinnitus; counseling to address any cognitive distortions relating to tinnitus and to assist with management of the emotional response to it; and monitoring of progress through measurement and feedback of various measures of tinnitus symptoms. All of these are delivered in a collaborative rather than directive fashion. Together with personalized treatment goal-setting, this approach serves to ensure that the treatment is focused as much as possible on the unique situation and needs of each individual patient.
How the Neuromonics Tinnitus Treatment Addresses the Neurological Processes Underlying Clinically Significant Tinnitus
Auditory Stimulation to Address the Effects of Auditory Deprivation
A key aspect is the objective of providing stimulation of the auditory neurons that have been deprived of stimulation because of hearing loss and/or decreased sound tolerance or other auditory system dysfunction. In this way, the treatment seeks to address the processes involving the auditory system's response to auditory deprivation that contribute to the tinnitus perception. This aspect of the treatment is consistent with recent studies that have shown that neuronal changes that result from hearing damage can be prevented by feeding sound into the auditory system in a manner that is specific to the frequencies of hearing loss (Norena & Eggermont, 2005, 2006). In the Neuromonics treatment, this is achieved through the use of a wide frequency stimulus that is spectrally modified to account for each patient's hearing thresholds.
The acoustic stimulus was created by combining a selection of commercially available and licensed music recordings with a specially designed broad frequency noise component at a predetermined signal-to-noise ratio. The signal-to-noise ratio was selected so as to allow the broad frequency noise component to be audible (it is commonly described by patients as being similar to a shower sound), alongside the high frequency content of the music, and thereby enhance the stimulus provided to the auditory cortex in the early stage of treatment, yet allow the noise component to not be so prominent that it detracts from the pleasantly relaxing listening experience provided by the music. This combination is then individually customized to account for each patient's audiometric profile by spectral modification using proprietary, patented digital sound processing algorithms (Davis, 2004, 2005). The frequency range over which stimulation is provided, which extends up to 12.5 kHz, contrasts with commonly used broadband noise generators, which in practice provide only a narrow band of effective stimulation, centered around the 0.5–2.0 kHz range (Baguley, Beynon, & Thornton, 1997).
The digital sound processing algorithms (Davis, 2004) are applied separately for each ear and the reshaped ear-specific stimuli then combined in a manner that accounts for any asymmetry in hearing thresholds across the two ears. The customization process boosts intensity in areas where an individual has relatively poorer hearing and reduces the intensity in areas of relatively stronger hearing, thereby ensuring that an appropriate amount of stimulation is provided regardless of the nature and degree of each patient's individual hearing loss profile. As mentioned previously, the amount of hearing loss that is accounted for is consistent with the half gain rule, modified somewhat to take into account the abnormally low loudness tolerance levels often displayed by tinnitus patients (Stouffer & Tyler, 1990). Perceptual balance is further enhanced by applying the equal loudness contours, which adjust for perceptual differences in intensity across the frequency range. A further adjustment is made for the frequency response of the earphones used. To further facilitate a pleasant listening experience, intensity peaks are limited using soft compression limiting. The various adjustments are conducted using commonly accepted audiological practice, with some modification for application to the tinnitus patient population as outlined elsewhere (Davis et al., 2007). The digital sound processing is done using commonplace acoustic filtering and convolutional processes with sufficient resolution to provide a smooth frequency response that matches the audiometric measurements at the test frequencies.
An objective of the treatment is to stimulate the integrative pathways of the auditory system. This objective is addressed through the use of a stereo stimulus—one in which two discrete signals for the left and right ears are delivered in a manner in which the correlation between them is controlled. By adjusting for asymmetry between the ears and thereby balancing the perceptual loudness between the two ears, the treatment seeks to maximize the stereo effect. That this effect is achieved in practice is supported by the authors' observations in clinical practice. First, patients typically report that they do perceive a stereo effect and, indeed, enjoy what they often describe as an “engrossing listening experience through the entire head,” rather than at the level of the ear. Second, among patients who have benefited from the treatment to date are those with so-called “central tinnitus,” who describe their tinnitus as “inside their head” rather than in one ear or the other; these patients find that they are able to cover up or interact with their tinnitus using our treatment whereas they had not been successful in achieving such an effect with other binaural, uncorrelated stimuli (maskers or hearing aids). Finally, with patients who report tinnitus in a “dead” ear, it has been possible to provide treatment contralaterally through the ear with residual hearing; this is felt by the authors' to be a demonstration of the involvement of the integrative pathways in the auditory system.
Relaxation and Relief to Address the Aversive Reaction/Stress Response
Another key objective of the Neuromonics Tinnitus Treatment is to reduce the engagement of the limbic system/amygdala and autonomic nervous system, which are major contributors to tinnitus-related disturbance (Jastreboff & Hazell, 1993). This objective is addressed through the use of relaxing music as part of the acoustic stimulus as well as through the facilitation of a sense of relief from the tinnitus perception. These effects are reinforced and complemented by treatment-facilitated improvements in sleep, as well as by benefits arising from the broader counseling and support program.
The use of relaxing music draws on studies that have shown that relaxing music is as effective as progressive muscle relaxation training in generating a relaxation response (Kibler & Rider, 1983; Stoudenmire, 1975). To facilitate this effect, the music selected has a tempo that is similar to that of a relaxed heart beat (i.e., in the range of around 50 to 70 beats per minute) and incorporates minimal harsh percussion instruments or other distracting anomalies, which could detract from the relaxation experience. The absence of recognizable lyrics avoids evoking the linguistic areas of the brain. Selection of musical pieces for inclusion in the stimulus also took into account the need to have sufficient musical sophistication and richness to support repeated listening. In the current application of this approach, the musical content provided extends to 4 hours of acoustic stimulus, which is divided among four programs covering different music genres, to allow patients some choice among different styles according to their preference.
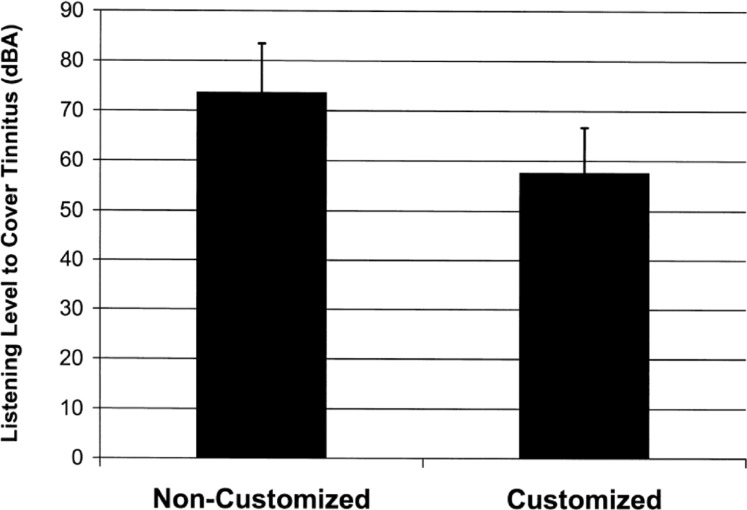
In relation to the relief aspect of the treatment (i.e., patients' ability to cover up—and hence get relief from—their tinnitus perception), the individually customized digital processing of the acoustic stimulus described above contributes to this effect by allowing patients in the initial phase of treatment to cover up their tinnitus at a low and therefore comfortable listening level. Figure 2 illustrates the degree of benefit in this regard that is provided by the customization process, as reported in a recent clinical study (Davis et al., 2007). Patients were asked to set the listening volume of matched precustomized and customized acoustic stimuli to the level that just covers up their tinnitus. Measurement of the average peak intensity then revealed a perceptually very large (16 dB) mean difference between the levels at which patients were able to gain relief from their tinnitus with the customized stimulus as opposed to the precustomized stimulus.
Figure 2.
Effect of customization on listening level that covers tinnitus perception.
Note: Solid bars represent mean peak intensity of acoustic stimulus at volume that patients reported was sufficient to cover up their tinnitus, with error bars corresponding to one standard deviation.
This observation is consistent with the authors' clinical experience that many tinnitus patients have unsuccessfully attempted to use regular (i.e., noncustomized) music to achieve relief from their tinnitus. Such patients commonly report that noncustomized music needs to be turned up to a volume that is uncomfortably loud before it generates any significant interaction with or masking of their tinnitus perception. This is consistent with the mismatch between the low-frequency emphasis of music and the high-frequency bias of the hearing loss commonly found in tinnitus patients (Davis, Wilde, & Steed, 2002). This mismatch is addressed as part of the Neuromonics customization procedure.
Commonly, patients with clinically significant tinnitus report that getting to sleep and returning to sleep after waking at night are especially problematic aspects of their condition. This makes sense in light of the auditory, attentional, and emotional processes in that (a) the tinnitus perception appears relatively more prominent in relatively quieter situations, (b) attention can become focused on the tinnitus perception in the absence of other cognitive distractions, and (c) the stress response generated by attention to the tinnitus perception is not conducive to sleepiness. As a result of the customized Neuromonics sound processing, relief and relaxation can be facilitated at a listening volume that is low enough to be used at those times when the patient is trying to get to sleep. Patients commonly use the treatment in this fashion and report early improvements in their sleep patterns. A number of features of the portable device have been designed with this usage in mind, including control buttons that can be navigated by touch, a lit LCD display, and a “sleep” setting that plays for an hour before shutting the device off with a gradual tapering in volume.
Patients are instructed to use the treatment particularly at those times of the day when their tinnitus is usually most disturbing. It is a common complaint of patients that stress appears to make their tinnitus worse and it is a cruel irony that having tinnitus is in itself stressful, and the quiet times when others get to relax are often the worst times for tinnitus (Davis, 1995). Using the treatment at these times ensures that relief and relaxation benefits are maximized.
In addition to promoting relaxation and relief, the use of relaxing music has the added benefit that it makes the therapy pleasant for patients to use. These factors contribute to patient compliance with treatment. This, in turn, contributes to the speed and consistency with which benefits are achieved, given the dosage effect that is apparent with the acoustic stimulus used in the Neuromonics treatment (Davis et al., 2007).
As a complement to the use of the acoustic stimulus, the clinician administering the Neuromonics Tinnitus Treatment provides a comprehensive monitoring and support program, typically over a 6-month period (Davis, 2005). For those patients who present with relaxation and sleep issues, this program will include coaching and counseling to address these issues.
Systematic Desensitization to Address the Perceptual Filters That Lead to Attention to the Tinnitus
In the context of the relaxation response described above, the Neuromonics Tinnitus Treatment seeks to address the attentional processes underlying the condition using a novel application of the principles of systematic desensitization. A common behavior therapy technique, systematic desensitization uses a graduated exposure to increasing hierarchies of anxiety-provoking situations while a person is in a state of deep relaxation, which facilitates desensitization to the anxiety-provoking stimulus (Atkinson, Atkinson, & Hilgard, 1983). In applying these principles to the treatment of tinnitus, in light of the practical challenges also observed by others (Tyler, 1996), relaxing music was incorporated into the Neuromonics approach instead of the traditional progressive muscle relaxation training (Davis, 2005).
Other researchers (e.g., Jastreboff, 2004) have argued that for the brain to become habituated to the tinnitus perception, it needs to be exposed to it. The use of an acoustic stimulus can facilitate this outcome by providing partial perception while at the same time reducing its significance, especially when reinforced by appropriate counseling. The Neuromonics approach is consistent with that general principle but applies it in a novel fashion, drawing also on the principles of systematic desensitization. In contrast to the view advocated by some professionals that patients should not cover up their tinnitus completely (Jastreboff, 2004), in the Neuromonics approach, patients are permitted to do this as an interim measure in the early stages of treatment to maximize relief and relaxation and reduce the significance of the tinnitus. This initial stage (Stage 1) is also intended to maximize the amount of neurostimulation, in particular, the tonotopic representation of the frequencies otherwise under-represented due to hearing loss. Patients are then transitioned to the intermittent interaction stage (Stage 2) of treatment, in which complete covering up of their tinnitus is discouraged to facilitate desensitization.
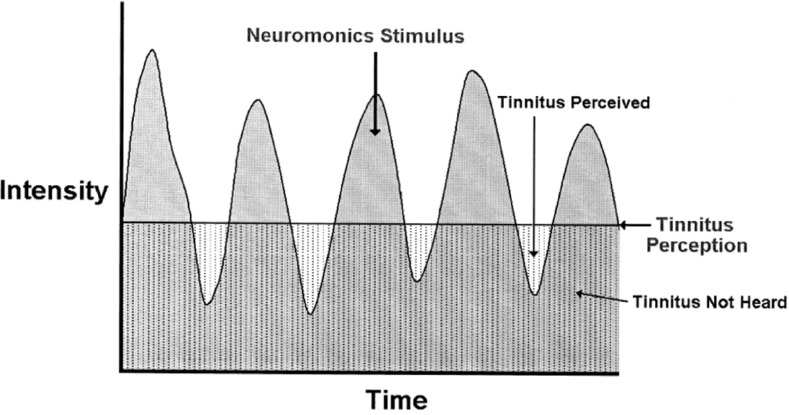
Because of the dynamics of the music, once customized for the patient's audiometric profile and delivered in a tightly controlled way, the stimulus allows the patient to cover the tinnitus in the peaks of intensity in the music, while allowing the tinnitus to be momentarily perceived in the intensity troughs (see Figure 3). In this way, the brain experiences repeated, momentary perception of the tinnitus while in a relaxed state. Patients are instructed not to continue to “tune in” to their tinnitus perception once they've set the volume appropriately at the beginning of the listening session. Rather, they are encouraged to strive to place it into the background of their conscious attention, and they are encouraged to engage in other activities while they are listening to it. By gradually increasing the degree of exposure to the tinnitus perception over time, the intention is to retrain the brain's attentional filters to perceive the tinnitus sound but not to pay particular attention to it and not to trigger the stress response in reaction to it.
Figure 3.
Schematic representation of intermittent interaction with tinnitus perception facilitated by the dynamic acoustic stimulus.
In the early stages of treatment, patients typically report that they are able to cover up or interact with their tinnitus perception to a large degree while listening to the treatment. However, when not listening to the treatment, their tinnitus perception is largely unchanged. Then, through the course of treatment, patients typically report that they find they become progressively less generally aware of their tinnitus, and less disturbed by it, between listening sessions (i.e., even when not actually listening to the stimulus). These observations of a benefit of treatment that is sustained beyond the periods of treatment usage, in the authors' view, provide strong support for the notion that the treatment is effecting change at a neuronal level, that is, that patients are becoming desensitized or habituated to the tinnitus perception due to changes within the auditory, attentional, and/or emotional processes described herein. These changes are typically mirrored by improvements in minimum masking levels and loudness discomfort levels (in the absence of any change in hearing thresholds), which provide corroborating support for the contention that the treatment is reversing the auditory gain aspect of their condition. Changes in minimum masking levels and loudness discomfort levels over the course of treatment have been previously reported by the authors (Davis et al., 2007).
Clinical Outcomes Achieved by the Neuromonics Tinnitus Treatment
By simultaneously addressing the auditory, attentional, and emotional processes implicated in clinically significant tinnitus, the Neuromonics Tinnitus Treatment aims to achieve consistent, rapid, and efficient results for suitable tinnitus patients. The intended clinical objective is, in the early stages of treatment, for patients to experience relief from their tinnitus and a sense of control, as well as improved relaxation and sleep in cases where relaxation and sleep have been problematic. Then, progressively over the course of treatment, it is intended that patients experience reduced awareness of their tinnitus, reduced tinnitus-related disturbance, and reduced effect from the tinnitus on their general well-being and quality of life.
The ability of the Neuromonics treatment to achieve these outcomes consistently has been validated in a series of controlled clinical studies, as well as through systematic evaluation of outcomes with large numbers of patients treated in private practice. Key results from these studies are described below.
Comparison of Neuromonics Tinnitus Treatment With Other Approaches for Tinnitus Treatment
An initial feasibility study found that the notion of individual spectral modification was viable and that music was clinically superior to equally modified noise (Davis & Wilde, 1995). A second clinical trial of the treatment was then undertaken to evaluate the effectiveness of the Neuromonics Tinnitus Treatment in comparison with two other types of treatment: (a) broadband noise plus counseling (noise+counseling) and (b) counseling only (Davis et al., 2002; Davis, Wilde, Steed, & Hanley, 2008).
Fifty patients who met suitability criteria were allocated alternately to receive Neuromonics treatment or one of the two control treatments. Suitability criteria included a clinically significant level of tinnitus disturbance, as indicated by a score on the Tinnitus Reaction Questionnaire (TRQ; Wilson, Henry, Bowen, & Haralambous, 1991) of 17 or greater (J. L. Henry & Wilson, 1991), and the absence of any of the following exclusion factors:
a significant hearing loss in the speech range, defined by a four-frequency (0.5, 1.0, 2.0, 4.0 kHz) average hearing threshold level worse than 70 dB in the best-hearing ear;
ongoing compensation claims related to tinnitus;
clinically significant psychosis, depression, cognitive incapacity, or insufficient English-language abilities;
maintenance of any significant factors that cause tinnitus to be aggravated such as loud noise exposure, ototoxic medication, and disease process;
concurrent treatment of tinnitus (including recent onset of hearing aid usage exceeding 1 hour per day).
All groups received the same amount of clinician contact time. Treatment was administered over a 6-month period for all groups, with outcomes assessed after 3 and 6 months. Patients receiving an acoustic stimulus were then advised that they could continue using their treatment or not, as they preferred, over a second 6-month period. All patients were reassessed at 12 months.
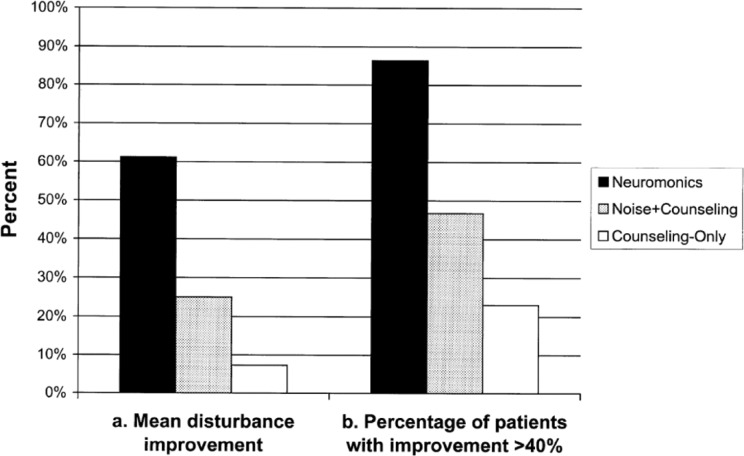
Patients receiving the customized stimulus (Neuromonics) reported significantly greater and more consistent improvements in tinnitus symptoms than patients who received an equivalent counseling and support program in combination with a broadband noise stimulus (noise+counseling) or with no acoustic stimulus (counseling only), as illustrated in Figure 4. After 6 months of treatment, 86% of Neuromonics patients had met the minimum criterion for clinical success, defined as an improvement in tinnitus disturbance of at least 40% (measured by TRQ score). As described elsewhere (Davis et al., 2007), this minimum threshold for clinical success was set so as to ensure that the improvement is clearly evident to the patient and, hence, is clinically significant. In the noise+counseling and counseling-only groups, 47% and 23% of patients, respectively, had reported a successful result by this criterion. Mean improvements in tinnitus disturbance after 6 months for patients receiving Neuromonics, noise+counseling, and counseling-only were 61%, 25%, and 7%, respectively. Differences between Neuromonics and control groups were statistically significant whereas differences between noise+counseling and counseling-only were not significant. Significant differences were also observed between treatment groups on other tinnitus measures. Patient reports of user acceptability were also more consistently positive for the Neuromonics stimulus than for the broadband noise stimulus.
Figure 4.
Comparison of clinical outcomes achieved with Neuromonics Tinnitus Treatment versus control treatments (tinnitus-related disturbance).
Note: Solid bars denote (a) mean improvement in tinnitus disturbance (as measured by a reduction in the Tinnitus Reaction Questionnaire [TRQ] score) or (b) the percentage of patients in each group who reported an improvement in the TRQ score of at least 40% after 6 months of treatment. Changes in TRQ score over time were statistically significant for Neuromonics (p<0.001) but not for Noise+Counseling or Counseling-Only.
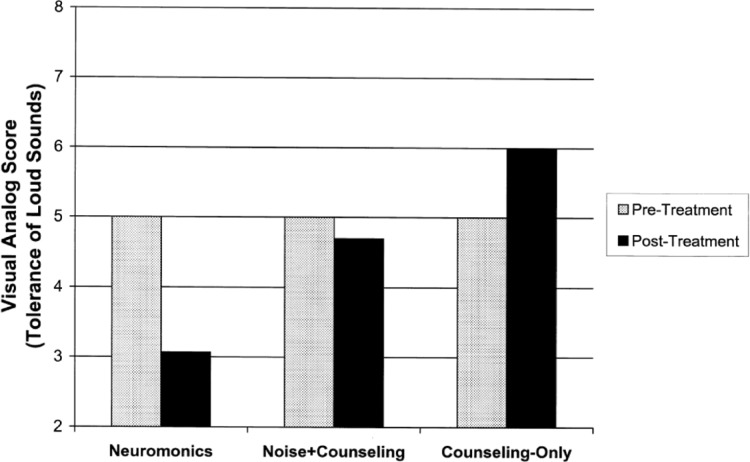
Patients receiving Neuromonics treatment also reported significantly greater and more consistent improvements in their tolerance of loud sounds than patients in the noise+counseling or counseling-only groups. Figure 5 displays Visual Analog Scale scores for loudness tolerance, rated on a 0- to 10-point scale, with pretherapy anchored at 5, improvements toward 0, and deterioration toward 10.
Figure 5.
Comparison of clinical outcomes achieved with Neuromonics Tinnitus Treatment versus control treatments (tolerance of loud sounds).
Note: Bars denote Visual Analog Scale scores rated on a scale from 0 to 10 prior to treatment (anchored at 5) and 12 months after commencement of treatment (improvements toward 0, deterioration toward 10). Changes were statistically significant for Neuromonics (p<0.001) but not for Noise+Counseling or Counseling-Only.
Comparison of Stage-Based Variants of Neuromonics Tinnitus Treatment
A recently reported follow-up study (Davis et al., 2007) aimed to determine which of two variations of the Neuromonics Tinnitus Treatment is more effective: a one-stage protocol, in which patients received intermittent interaction with their tinnitus perception throughout treatment, and a two-stage protocol, in which an initial 2-month stage involving a high level of interaction was followed by a 4-month stage of intermittent interaction. It was a repeated measures construction with random allocation of patients into one of two parallel groups. A total of 35 patients was included in the study. Suitability criteria were similar to those in the prior study.
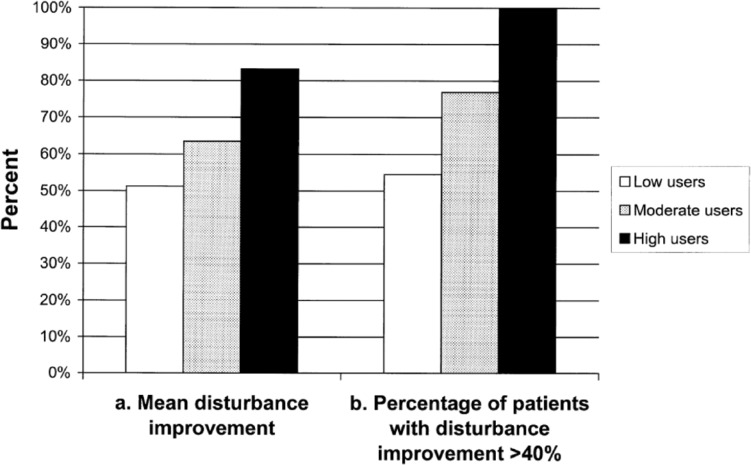
At 2, 4, 6, and 12 months after commencing treatment, both groups displayed clinically and statistically significant improvements in tinnitus distress, awareness, and minimum masking levels as well as loudness discomfort levels. Improvements increased with time over the first 6 months of therapy, at which time, 91% of all patients across the two groups reported an improvement in tinnitus disturbance (as measured by the TRQ) of at least 40%, with a mean improvement of 65%. Results were reported quickly, with significant benefits after only 2 months. Also, 80% of patients at 6 months reported a level of tinnitus disturbance that was no longer clinically significant. A relationship between reported treatment usage (hours per day) and clinical outcomes was observed (see Figure 6), suggesting that a dosage effect may apply with the stimulus provided. A very high proportion of patients reported sizeable benefits in sleep, relaxation, and general well-being, and more than 95% indicated that they found the treatment pleasant to listen to and would recommend it to others (Davis et al., 2007).
Figure 6.
Relation between clinical outcomes and treatment usage/dosage over first 4 months of treatment.
Note: Solid bars denote (a) mean improvement in tinnitus disturbance (as measured by a reduction in the Tinnitus Reaction Questionnaire [TRQ] score) or (b) the percentage of patients in each group who reported an improvement in the TRQ score of at least 40%. Low users reported usage of less than 1.5 hours per day, moderate users between 1.6 and 2 hours per day, and high users more than 2 hours per day. Differences between usage groups were statistically significant (p < .01).
Sizeable improvements were also reported in tolerance of loud sounds, as determined by increases in loudness discomfort levels, measured at 0.5, 1.0, 2.0, 4.0, and 6.0 kHz. For patients in the two-stage group, mean improvement in loudness discomfort level exceeded 10 dB.
Both of the stage-based variants of the treatment that were tested in this study were shown to yield consistently positive clinical outcomes. However, there was some evidence for a more consistent benefit within the two-stage group, and accordingly, it was that protocol that was adopted for application in the private practice setting.
Clinical Results Achieved in the Private Practice Clinical Setting
The Neuromonics Tinnitus Treatment was first made available to the general tinnitus patient population in a private practice setting in Australia in 2004 and has since become available in the United States, Singapore, and New Zealand. Through the phase of transition from the clinical trial environment to that of private practice, researchers associated with the development of this approach have formally tracked the clinical outcomes for patients undertaking treatment in Australian clinics.
A recent study that collated the clinical outcomes for more than 470 such patients (Hanley, Davis, Paki, Quinn, & Bellekom, 2008) assigned patients to one of three categories based on their degree of satisfaction of various criteria defining relative suitability for Neuromonics Tinnitus Treatment. These criteria had been defined based on clinical experience through the clinical trials conducted prior to the release of the treatment in a private practice setting. “Tier 1 suitability” patients, the most suitable patients, made up the largest group (almost half of the completing treatment). They included those patients who did not display any of the factors by which the other two categories were defined and largely paralleled the profile of patients who satisfied the eligibility criteria in the above-mentioned clinical studies. “Tier 2 suitability” patients exhibited one or more of the following: high apparent psychological disturbance, a low level of tinnitus-related disturbance, as indicated by a TRQ score below 17, or moderately severe or greater hearing loss in worst hearing ear (i.e., worse than 50 dB four-frequency average). “Tier 3 suitability” (the smallest category) patients exhibited one or more of the following: reactive tinnitus (i.e., tinnitus that is exacerbated by even low-level sound), multitone tinnitus, continued exposure to high levels of noise without effective hearing protection during the period of treatment, English-language comprehension difficulties, Meniere's disease, pulsatile tinnitus, actively pursuing compensation, or hearing loss worse than 50 dB four-frequency average in the best-hearing ear (i.e., in both ears).
Clinical outcomes were found to display a relation with patients' relative suitability for treatment according to defined criteria: among the most suitable patients, who made up the largest of the suitability categories, more than 90% of patients exceeded the minimum threshold for clinical success (defined as a reduction in tinnitus disturbance of at least 40%, as measured by the TRQ), and mean improvement in tinnitus disturbance exceeded 70%. Discontinuance rate (being the proportion of patients who opted to cease treatment and claim a refund) was low at 3%. For the other suitability categories, success rates and mean improvements were somewhat lower, and discontinuance rates higher. This study concluded that the treatment is effective in the private practice setting, yielding large reductions in tinnitus awareness and associated disturbance, as well as improved tolerance of loud sounds for suitable patients.
Patient Suitability and Other Considerations in Clinical Application of the Neuromonics Tinnitus Treatment
Based on collective experience to date within the clinical trial setting and in private practice, where to date more than 2,000 patients have undertaken treatment, it has been possible to define a number of criteria that clinicians may refer to in determining patient suitability for the treatment. Evaluation by an otolaryngologist is required to exclude certain conditions requiring medical intervention or other conditions that may warrant specialist intervention, such as temporo-mandibular dysfunction. Then, a comprehensive assessment of the patient's audio-metric and tinnitus profile as well as tinnitus history and general lifestyle is routinely conducted to determine suitability by these criteria. Based on this assessment, the best candidates for treatment are adults who display the following characteristics:
clinically significant level of tinnitus disturbance, which may be defined as a score of at least 17 on the TRQ or similar accepted threshold on equivalent measures of tinnitus disturbance, such as the Tinnitus Handicap Questionnaire (Kuk, Tyler, Russell, & Jordan, 1990) or Tinnitus Handicap Inventory (Newman, Sandridge, & Jacobson, 1998);
normal hearing or some hearing loss, but sufficient residual hearing loss in at least one ear such that four-frequency average hearing thresholds in at least one ear are better than 50 dB, and absence of conditions (such as Meniere's disease) in which hearing thresholds fluctuate;
normal or reduced tolerance of loud sounds, as shown by loudness discomfort levels measured using narrow band noise at several frequencies;
any psychological disturbance, such as anxiety or depression, is no worse than mild or moderate;
tinnitus is not reactive in nature (i.e., exacerbated by even low-level sounds), nor pulsatile, nor multi-tone in nature;
patient is not subject to continuing exposure to high levels of noise without adequate hearing protection;
patient is not actively pursuing compensation in relation to his or her tinnitus.
Among patients who fall outside of the profile defined by the above-mentioned characteristics, many achieve positive outcomes with treatment (Hanley et al., 2008). However, with such patients, some modification to the standard treatment protocol is often required, success rates are somewhat lower, and discontinuance rates higher. Accordingly, clinicians are advised to manage expectations appropriately with such patients.
For patients presenting with a level of tinnitus disturbance below the threshold for clinical significance, or with recent-onset tinnitus (less than 6 months), clinicians may be advised to offer some basic education and support/counseling in the first instance and encourage the patients to return if symptoms persist. However, with recent-onset patients in a distressed state, a comprehensive program such as Neuromonics may be considered; there is some evidence in published reports that early intervention may be associated with better response to treatment in some cases (Kleinjung et al., 2007).
In some cases, concurrent treatment with other treatment modalities may be beneficial. For instance, concurrent psychological intervention for patients with high levels of psychological disturbance may be advisable. Concurrent use of hearing aids should be recommended for patients whose hearing loss is sufficiently severe that communication is impaired and they regularly strain to hear.
Comparison With Other Treatment Programs
A recent report (Davis et al., 2007) provided a comparison between clinical outcomes achieved with the Neuromonics Tinnitus Treatment and those reported by separate studies for other treatment programs. Although direct comparisons are limited by study differences, mean improvement in tinnitus disturbance and the proportion of patients reporting significant improvements were much greater in the case of the Neuromonics treatment (65% mean improvement and 91% reporting significant improvements after 6 months) when compared with Tinnitus Retraining Therapy (26% mean improvement and 29% reporting significant improvements after 6 months) or Tinnitus Masking (17% mean improvement and 19% reporting significant improvements after 6 months) (J. A. Henry et al., 2005) and with Cognitive Behavioral Therapy (41% mean improvement for moderate disturbance patients and 31% mean improvement for severe disturbance patients after 6 months) (Hiller & Haerkotter, 2005). Davis et al. (2007) also reported that a very high proportion of patients using the Neuromonics Tinnitus Treatment reported that they found it pleasant to use. This compares favorably with the use of other tinnitus treatment devices, which have been associated with reports of high discontinuance rates (J. A. Henry, Schechter, Nagler, & Fausti, 2002; Hiller & Haerkotter, 2005). In the authors' private practice clinical experience, high patient acceptance ratings and treatment continuance rates have been observed among patient populations in which a high proportion of patients have previously tried other treatments (including hearing aids and tinnitus maskers) without success (Hanley et al., 2008). Benefits reported by patients also include facilitation of sleep and relaxation, aspects for which other tinnitus maskers and hearing aids are not generally regarded to provide much benefit. In the authors' experience, anecdotal reports from clinicians indicate that this treatment approach also offers convenience and efficiency (i.e., modest amount of clinical time per patient) benefits for clinicians, when compared with the more counseling-intensive alternative approaches, such as Cognitive Behavioral Therapy.
Further Directions
The Neuromonics Tinnitus Treatment is the subject of ongoing and planned further research. Clinical studies are under way and planned to investigate the relative efficacy of the treatment for various patient subcategories, including patients whose tinnitus is the result of military service and sufferers of hyperacusis. Brain imaging studies are also under way that aim to identify the neural correlates of patient responsiveness to the treatment. In addition, ongoing product development efforts seek to further enhance the treatment's efficacy, efficiency, and user acceptability, as well as the breadth of the treatment's application across various clinical subcategories with the diverse tinnitus patient population.
Summary
The Neuromonics Tinnitus Treatment was designed to simultaneously address the critical neurological processes that contribute to clinically significant tinnitus: the auditory, attentional, and emotional aspects of the condition. By doing so, it aims to achieve larger, more rapid, and more consistent clinical outcomes, with an intervention that is more pleasant for the patient and more convenient for the administering clinician than previously available alternatives. Evidence of clinical efficacy in controlled clinical studies, as well as in the private practice clinical setting, has demonstrated that this approach is clinically effective, efficient, and acceptable to patients, in particular among those patients who satisfy various suitability criteria. The relation between patient fit on defined suitability criteria and clinical outcomes achieved is instructive to health care professionals as to what patients might expect from treatment depending on their degree of suitability.
References
- Atkinson R. L., Atkinson R. C., Hilgard E. R. (1983). Introduction to psychology (8th ed., pp. 500–501). New York: Harcourt, Brace, Jovanovich [Google Scholar]
- Baguley D. M., Andersson G. (2007). Hyperacusis: Mechanisms, diagnosis, and therapies. Oxford: Plural Publishing [Google Scholar]
- Baguley D. M., Beynon G. J., Thornton F. (1997). A consideration of the effect of ear canal resonance and hearing loss upon white noise generators for tinnitus retraining therapy. Journal of Laryngology and Otology, 111, 810–813 [DOI] [PubMed] [Google Scholar]
- Cacace A. T. (2003). Expanding the biological basis of tinnitus: Crossmodal origins and the role of neuroplasticity. Hearing Research, 175, 112–132 [DOI] [PubMed] [Google Scholar]
- Cacace A. T. (2004). The limbic system and tinnitus. In Snow J. B. (Ed.), Tinnitus: Theory and management (pp. 162–170). Hamilton, Ontario: BC Decker [Google Scholar]
- Davis P. B. (1995). Living with tinnitus. Sydney: Gore & Osment [Google Scholar]
- Davis P. B. (2004). Tinnitus rehabilitation device and method. U.S. Patent No. 6,682,472.
- Davis P. B. (2005). Music and the acoustic desensitization protocol for tinnitus. In Tyler R. (Ed.), Tinnitus treatments (pp. 146–160). New York: Thieme [Google Scholar]
- Davis P. B., Paki B., Hanley P. J. (2007). The Neuromonics Tinnitus Treatment: Third clinical trial. Ear and Hearing, 28, 242–259 [DOI] [PubMed] [Google Scholar]
- Davis P. B., Wilde R. A. (1995). Clinical trial of a new tinnitus masking technique. In Reich G. E., Vernon J. A. (Eds.), Proceedings of the Fifth International Tinnitus Seminar (pp. 305–309). Portland, OR: American Tinnitus Association [Google Scholar]
- Davis P. B., Wilde R. A., Steed L. (2002). Clinical trial findings of a neurophysiologically-based tinnitus rehabilitation technique using tinnitus desensitization music. In Patuzzi R. (Ed.), Proceedings of the Seventh International Tinnitus Seminar (pp. 74–77). Perth: University of Western Australia [Google Scholar]
- Davis P. B., Wilde R. A., Steed L., Hanley P. J. (2008). Treatment of tinnitus with a customized acoustic neural stimulus: A controlled clinical study. Ear, Nose, & Throat Journal, 87(6), 330–339 [PubMed] [Google Scholar]
- Eggermont J. J., Roberts L. E. (2004). The neuroscience of tinnitus. Trends in Neurosciences, 27, 676–682 [DOI] [PubMed] [Google Scholar]
- Formby C., Sherlock L. P., Gold S. L. (2003). Adaptive plasticity of loudness induced by chronic attenuation and enhancement of the acoustic background. Journal of the Acoustical Society of America, 114, 55–58 [DOI] [PubMed] [Google Scholar]
- Georgiewa P., Klapp B. F., Fischer F., et al. (2006). An integrative model of developing tinnitus based on recent neurobiological findings. Medical Hypotheses, 66, 592–600 [DOI] [PubMed] [Google Scholar]
- Gerken G. M., Saunders S. S., Paul R. E. (1984). Hypersensitivity to electrical stimulation of auditory nuclei follows hearing loss in cats. Hearing Research, 13, 249–259 [DOI] [PubMed] [Google Scholar]
- Hanley P. J., Davis P. B., Paki B., Quinn S. A., Bellekom S. R. (2008). Treatment of tinnitus with a customized acoustic neural stimulus: Clinical outcomes in general private practice (in press). [DOI] [PubMed]
- Hawkins D., Walden B., Montgomery A., Prosek R. (1987). Description and validation of an LDL procedure designed to select SSPL90. Ear and Hearing, 8, 162–169 [DOI] [PubMed] [Google Scholar]
- Heller M. F., Bergman M. (1953). Tinnitus aurium in normally hearing persons. Annals of Otology, Rhinology, & Laryngology, 62, 73–83 [DOI] [PubMed] [Google Scholar]
- Henry J. A., Schechter M. A., Loovis C., Zaugg T., Kaelin C., Montero M. (2005). Clinical management of tinnitus using a “progressive intervention” approach. Journal of Rehabilitation Research and Development, 42, 95–116 [DOI] [PubMed] [Google Scholar]
- Henry J. A., Schechter M. A., Nagler S. M., Fausti S. H. (2002). Tinnitus retraining therapy and masking: How do they compare? In Patuzzi R. (Ed.), Proceedings of the Seventh International Tinnitus Seminar (pp. 247–254). Perth: University of Western Australia [Google Scholar]
- Henry J. L., Wilson P. H. (1991). Psychological management of tinnitus: An evaluation of cognitive interventions. In Aran J.-M., Dauman R. (Eds.), Proceedings of the Fourth International Tinnitus Seminar (pp. 477–480). New York: Kugler [Google Scholar]
- Hiller W., Haerkotter C. (2005). Does sound stimulation have additive effects on cognitive-behavioural treatment of chronic tinnitus? Behaviour Research and Therapy, 43, 595–612 [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization. (1998). Acoustics: Reference zero for the calibration of audiometric equipment, Part 5: Reference equivalent threshold sound pressure levels for pure tones in the frequency range 8 kHz to 16 kHz. Geneva: Author [Google Scholar]
- Jastreboff P. (2004). The neurophysiological model of tinnitus. In Snow J. B. (Ed.), Tinnitus: Theory and management (pp. 96–107). Hamilton, Ontario: BC Decker [Google Scholar]
- Jastreboff P., Hazell J. (1993). A neurophysiological approach to tinnitus: Clinical implications. British Journal of Audiology, 27, 7–17 [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J., Hazell J. (2004). Tinnitus retraining therapy: Implementing the neurophysiological model. Cambridge, UK: Cambridge University Press [Google Scholar]
- Jastreboff P. J., Hazell J.W.P., Graham R. L. (1994). Neurophysiological model of tinnitus: Dependence of the minimal masking level on treatment outcome. Hearing Research, 80, 216–232 [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J., Jastreboff M. M. (2004). Decreased sound tolerance. In Snow J. B. (Ed.), Tinnitus: Theory and management (pp. 8–15). Hamilton, Ontario: BC Decker [Google Scholar]
- Kaltenbach J. A. (2006, June-July). The dorsal cochlear nucleus as a participant in the auditory, attentional and emotional components in tinnitus. Hearing Research, 216–217, 224–234 [DOI] [PubMed] [Google Scholar]
- Kibler V. E., Rider M. S. (1983). The effect of progressive muscle relaxation and music on stress as measured by finger temperature response. Clinical Psychology, 39(2), 213–215 [DOI] [PubMed] [Google Scholar]
- Kleinjung J., Steffens T., Sand P., Murthum T., Hajak G., Strutz J., Langguth B., Eichhammer P. (2007). Which tinnitus patients benefit from transcranial magnetic stimulation? Otolaryngology—Head and Neck Surgery, 137(4), 589–595 [DOI] [PubMed] [Google Scholar]
- Konig O., Schaette R., Kempter R., Gross M. (2006). Course of hearing loss and occurrence of tinnitus. Hearing Research, 221, 59–64 [DOI] [PubMed] [Google Scholar]
- Kuk F. K., Tyler R. S., Russell D., Jordan H. (1990). The psychometric properties of a tinnitus handicap questionnaire. Ear and Hearing, 11(6), 434–445 [DOI] [PubMed] [Google Scholar]
- Lockwood A. H., Salvi R. J., Coad M. L., Towsley M. L., Wack D. S., Murphy B. W. (1998). The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology, 50, 114–120 [DOI] [PubMed] [Google Scholar]
- Moller A. (2007a). The role of neural plasticity in tinnitus. Progress in Brain Research, 166, 37–46 [DOI] [PubMed] [Google Scholar]
- Moller A. (2007b). Tinnitus: Presence and future. Progress in Brain Research, 166, 3–16 [DOI] [PubMed] [Google Scholar]
- Muhlau M., Rauschecker J. P., Oestreicher E., et al. (2006). Structural brain changes in tinnitus. Cerebral Cortex, 16, 1283–1288 [DOI] [PubMed] [Google Scholar]
- Muhlnickel W., Elber T., Taub E., Flor H. (1998). Reorganization of auditory cortex in tinnitus. Proceedings of the National Academy of Sciences of the United States of America, 95, 10340–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. W., Sandridge S. A., Jacobson G. P. (1998). Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. Journal of the American Academy of Audiology, 9(2), 153–160 [PubMed] [Google Scholar]
- Norena A. J., Eggermont J. (2005). Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. Journal of Neuroscience, 25(3), 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena A. J., Eggermont J. (2006). Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. NeuroReport, 17, 559–563 [DOI] [PubMed] [Google Scholar]
- Nuttall A., Miekle M. B., Trune D. R. (2004). Peripheral processes involved in tinnitus. In Snow J. B. (Ed.), Tinnitus: Theory and management (pp. 52–68). Hamilton, Ontario: BC Decker [Google Scholar]
- Searchfield G. D., Morrison-Low J., Wise K. (2007). Object identification and attention training for treating tinnitus. Progress in Brain Research, 166, 441–460 [DOI] [PubMed] [Google Scholar]
- Stoudenmire J. (1975). A comparison of muscle relaxation training and music in the reduction of state and trait anxiety. Journal of Clinical Psychology, 31, 490–492 [DOI] [PubMed] [Google Scholar]
- Stouffer J. L., Tyler R. S. (1990). Characterization of tinnitus by tinnitus patients. Journal of Speech and Hearing Disorders, 55, 439–453 [DOI] [PubMed] [Google Scholar]
- Tyler R. S. (1996, March). Tinnitus treatments modify behavior. Hearing Instruments, pp. 20–24 [Google Scholar]
- Tyler R. S. (2005). Neurophysiological models, psychological models, and treatments for tinnitus. In Tyler R. S. (Ed.), Tinnitus treatments: Clinical protocols (pp. 1–22). New York: Thieme [Google Scholar]
- Vernon J. A., Meikle M. B. (1988). Measurement of tinnitus. In Kitahara M. (Ed.), Tinnitus: Pathophysiology and management (pp. 36–52). Tokyo: Igaku-Shoin [Google Scholar]
- Wilson P. H., Henry J., Bowen M., Haralambous G. (1991). Tinnitus reaction questionnaire: Psychometric properties of a measure of distress associated with tinnitus. Journal of Speech and Hearing Research, 34, 197–201 [PubMed] [Google Scholar]