Abstract
A new tinnitus syndrome is described: high-pitched, cardiac-synchronous tinnitus, whose pulsations are suppressed by strong contractions or compressions of the neck and jaw muscles (somatic testing). 14 cases, 6 non-lateralized and 8 unilateral, are reported. In the non-lateralized cases, onset was bilateral. In the one intermittent case, while her tinnitus was absent her pulsatile tinnitus could be induced by somatic testing. No etiology was found from physical examination, imaging, or ancillary testing. Because these cases of pulsatile tinnitus can be both induced and suppressed by activation of the somatosensory system of the head or upper lateral neck, we propose that this syndrome is occurring from (a) cardiac synchronous somatosensory activation of the central auditory pathway or (b) failure of the somatosensory-auditory central nervous system interactions to suppress cardiac somatosounds.
Keywords: tinnitus, pulsatile, somatosensory, cochlear nucleus
Tinnitus is common; estimates of its prevalence range up to 80% of all adults (Heller & Bergman, 1953). About 10% of adults complain of chronic tinnitus, and about 0.5% of all adults describe their tinnitus as interfering with their ability to lead a normal life (Coles, 1984). Tinnitus may have many different etiologies but, by some estimations, in about 50% of cases, no etiology can be determined (Coles, 1996).
One very distinct form of tinnitus is pulsatile tinnitus, which is described as having a rhythm that is synchronous with the heart beat. Known causes of pulsatile tinnitus are listed in Table 1. When we encounter patients with pulsatile tinnitus, we first check whether, in fact, their tinnitus is related to the cardiac cycle by comparing (a) the examiner's silent count of the patient's cardiac pulse at the wrist (radial artery) with (b) the patient's silent count of his or her tinnitus pulsations. The examiner indicates when the counting interval starts and stops and then the count of the examiner and the patient are compared. If the counts are virtually identical, then we conclude that the pulsatile tinnitus is cardiac related and launch an investigation to identify the source of this presumed somatosound (i.e., a sound generated by an acoustic source from within the body). In about 10% of our cases, a source for the pulsatile tinnitus can be found. But in the majority of people, no cause is found despite an exhaustive physical examination and ancillary investigation. Others have reported a much higher percentage of pulsatile tinnitus patients in whom a source can be determined (Sismanis, 1998). This difference probably relates to different referral patterns resulting in different patient populations. Patients seen in our clinic have usually already been screened for an objective acoustic source for their pulsatile tinnitus.
Table 1.
Known Causes of Pulsatile Tinnitus
| High cardiac output state: anemia, hyperthyroidism |
| Raised intracranial pressure: pseudotumor cerebri, brain tumor |
| Vascular anomalies (dural AVM, dehiscent jugular bulb, sigmoid sinus diverticulum, emissary vein, persistent stapedial artery, carotid-cavernous fistula, aberrant carotid artery; carotid artery dissection, stenosis, or fibromuscular dysplasia) |
| Increased vascularity of the middle ear, temporal bone (e.g., glomus jugulare tumor, Paget's disease, otosclerosis) |
| Superior semicircular canal dehiscence |
| Vascular compression of auditory nerve |
A major question then is, What is the cause of the tinnitus in people with idiopathic pulsatile tinnitus? We can begin to answer this question by asking whether or not the cases of idiopathic pulsatile tinnitus are on an acoustic basis (i.e., due to a somatosound). There is reason to believe that pulsatile tinnitus can occur on a nonacoustic basis, because there have been several reports that neurovascular decompression of the VIII nerve can abolish pulsatile tinnitus (Lesinski, Chambers, Komray, Keiser, & Khodadad, 1979; Ohashi, Yasumura, Nakagawa, Mizukoshi, & Kuze, 1992; Ryu, Yamamoto, Sugiyama, Uemura, & Nozue, 1998). For example, in patients with hemifacial spasm and ipsilateral pulsatile tinnitus, Ryu et al. (1998) reported that the pulsatile tinnitus could be abolished with repositioning of a blood vessel compressing the auditory nerve. We conclude that it is likely that all cases of pulsatile tinnitus are not due to somatosounds, but rather, some may have another mechanism, probably neural, such as compression of the auditory nerve by a blood vessel.
Herein, we report 13 cases, 6 of whom had nonlateralized pulsatile tinnitus, and the other 7 had unilateral pulsatile tinnitus, for which no etiology was found from the physical examination or ancillary testing. However, the pulsatile quality of their tinnitus could be suppressed by one or more strong contractions or compressions of the neck and jaw muscles, which we refer to as “somatic testing” (Abel & Levine, 2004; Levine, Abel, & Cheng, 2003). The maneuvers currently being used to test for somatic modulation are listed in Table 2. We provide evidence that this type of pulsatile tinnitus is not due to a somatosound but is related to the well-established phenomenon of somatosensory modulation of auditory perception within the central nervous system (CNS; Levine, 2004; Lewald, Karnath, & Ehrenstein, 1999).
Table 2.
Somatic Testing: Maneuvers Currently Being Used to Test for Somatic Modulation (all use maximal force)
| Jaw Contractions | |
|---|---|
| 1 | clench teeth together |
| 2, 3 | open mouth, with and without restorative pressure |
| 4, 5 | protrude jaw, with and without restorative pressure |
| 6, 7 | slide jaw to left, with and without restorative pressure |
| 8, 9 | slide jaw to right, with and without restorative pressure |
| 10 | retract jaw |
|
Head and Neck Contractions—contractions were made to resist pressure applied by the examiner to: | |
| 11 | forehead, with the head in the neutral position |
| 12 | occiput, with the head in the neutral position |
| 13, 14 | temple, with the head in the neutral position (right and left) |
| 15, 16 | zygoma, with the head turned to the same side (right and left) |
| 17 | left temple, with the head turned to the right and tilted to the left (left sternocleidomastoid) |
| 18 | right temple, with the head turned to the left and tilted to the right (right sternocleidomastoid) |
|
Pressure on Muscles and Tendons | |
| 19, 20 | sternocleidomastoid, just below mastoid process (right and left) |
| 21, 22 | splenius capitis, just below occipital bone (right and left) |
| 23, 24 | mastoid process (right and left) |
| 25, 26 | posterior auricle at its attachment to the skull (right and left) |
| 27, 28 | pull auricle outward (right and left) |
All patients were seen in the Massachusetts Eye and Ear Infirmary Tinnitus Clinic between January 2004 and January 2008.
Nonlateralized Cases
A summary of the patient characteristics and findings from examination in the nonlateralized cases appears in Table 3. The three women and three men, ages 49 to 75, described their pulsatile tinnitus as high pitched, pulsating, and localized to both ears or in the head. No patient described the tinnitus onset as clearly occurring first in one ear, and at a later time in the other ear. One variant was patient B5, who had been seen at another clinic 3 months after his tinnitus onset; the medical record described that his tinnitus began after vigorous exercise as a “pulsating sensation on the right side of his scalp … now localized to the vertex of the scalp and at times behind the right ear.” At our clinic 3 years later, it was described as “at the bottom and back of the head.”
Table 3.
Six Cases With Continuous Bilateral Pulsatile Tinnitus
| Patient | Age | Sex | Tinnitus description | Onset | Audiogram | Jugular compression | Carotid Compression | Somatic Testing | Other |
|---|---|---|---|---|---|---|---|---|---|
| B1 | 58 | M | slightly lower in pitch than a teakettle | bilateral | normal | negative | negative | Multiple maneuvers abolish AS; AD decreased 70% & nonpulsatile from L SCM contraction | Arteriogram negative; ipsilateral tinnitus louder with upward ear canal pressure |
| B2 | 62 | M | pulsating, ringing | bilateral | HFSNHL (50 dB) | negative | negative | AU nonpulsatile with multiple maneuvers | |
| B3 | 49 | F | pinging | bilateral | normal | negative | R abolishes AU for a few seconds | R SCM pressure abolishes AU | |
| B4 | 59 | F | whooshing | bilateral | HFSNHL (40 dB) | negative | ipsilateral ear quieter | Retruding jaw abolishes AU; nonpulsatile AD & AS with backward flexion; nonpulsatile ipsilaterally with temple pressure | initially intermittent; treated for TMD for 2 years prior to tinnitus |
| B5 | 75 | M | beat with vibration | bilateral | HFSNHL (70 dB) | negative | R causes louder occipital “squishing” | Multiple maneuvers abolish AS & AD nonpulsatile; AU nonpulsatile with jaw protrusion | |
| B6 | 60 | F | whooshing | bilateral | HFSNHL (35 dB) | negative | negative | AU abolished with resisted neck forward flexion; AU louder with resisted jaw opening | onset with bruxism from extreme stress |
NOTES: M = male; F = female; HFSNHL = high frequency sensorineural hearing loss (numbers within brackets indicate the maximal threshold elevation); R = right; L = left; AD = right ear tinnitus; AS = left ear tinnitus; AU = right and left ear tinnitus; SCM = sternocleidomastoid; TMD = temporomandibular disorder.
In each case, the examiner's silent count of the cardiac pulse at the wrist and the patient's simultaneous silent count of his or her tinnitus pulsations were always within 5% of each other. Pure tone thresholds were symmetric in all patients; two had normal pure tone thresholds and four had a mild symmetric high frequency sensorineural hearing loss. Thyroid function tests and hematocrits were normal. Auscultation was normal in all. Jugular compression did not modulate any patient's tinnitus. Carotid artery compression was performed by palpation of the carotid pulse at the level of the upper border of the thyroid cartilage and pressing intensely in a ventral-dorsal direction on the overlying sternocleidomastoid. Carotid compression did suppress tinnitus in two patients, but a similar effect occurred with compression of the same sternocleidomastoid muscle at the same location but in a lateral to medial direction such that the pulsations were not felt and, presumably, the carotid was not occluded. In one individual, B5, right carotid compression increased the loudness of his pulsatile tinnitus. Imaging studies were all unrevealing, including cerebral angiography in B1. All could suppress the pulsatile nature of their tinnitus by either pressure on the sternocleidomastoid muscle or with various strong neck or jaw muscle contractions. In some, the tinnitus was abolished; in others, only the pulsatile quality of the tinnitus was suppressed, leaving behind residual high pitched nonpulsatile tinnitus. With some maneuvers, the tinnitus from only one ear was suppressed. A representative nonlateralized case follows.
Case 1 (patient B1)
A 58-year-old man reported having had an episode of thumping of his ears that was heard only upon awakening; it persisted less than a month. About 2 years later, he had the sudden onset of nonlateralized pulsatile tinnitus, 12 hours after clearing water from his right ear. It has persisted now for more than 5 years. He describes his tinnitus as constant, high pitched (“slightly lower in pitch than a teakettle”), tormenting, and bordering on pain. His tinnitus is associated with insomnia and is worse in the quiet, upon awakening, when lying down, and with nasal congestion. Tinnitus pitch and loudness vary with head and jaw position. He denied headaches, dizziness, or neck, jaw, or facial pain. A bite guard had provided no benefit.
His silent count of his tinnitus pulsations and the examiner's simultaneous silent count of his radial pulse were identical at 19. No bruits were heard over the mastoid and jugular regions or over the globes and occipital regions at rest or following exercise. Jugular or carotid compression on either side did not affect his tinnitus. With intense left sternocleidomastoid contraction, his left tinnitus was abolished and his right ear tinnitus became 70% quieter and nonpulsatile but was never abolished with any maneuver. Multiple other intense muscle contractions of his neck and jaw abolished his left ear tinnitus. Upward pressure on either ear canal produced louder tinnitus on that side only. Valsalva did not change his tinnitus.
His audiogram was normal. Head and neck MRI and MRA scans were unremarkable. Cerebral angiography was normal. Microphone recordings from the ear canals were normal (see Figure 1). Trials of the following medications did not alter his tinnitus: clonazepam, lorazepam, alprazolam, and oxycodone. Right auricular electroacupuncture using P-Stim, a wearable device applied according to a previously described regimen (Sator-Katzenschlager & Michalek-Sauberer, 2007), quieted his tinnitus by 50% to the point that he is able to sleep without medication.
Figure 1.
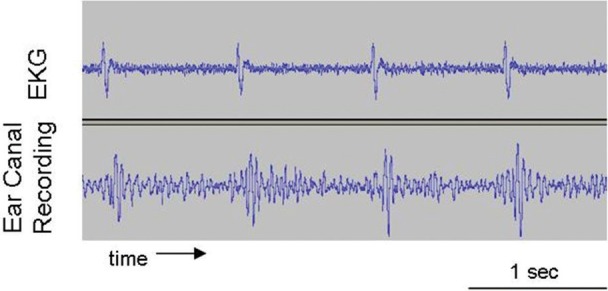
Simultaneous recordings of (a) sound from left ear canal and (b) EKG in patient B1.
Note: Ear canal recordings were made with an ER10c microphone whose tube was sealed into the external auditory canal. Patient was lying supine in a sound-attenuated room.
Lateralized Cases
Seven other pulsatile tinnitus patients have been seen with similar features to the nonlateralized cases but with tinnitus localized to just one ear; six had left ear tinnitus and one had right ear tinnitus. A summary of the patient characteristics and findings from examination in the lateralized cases appears in Table 4. The pulse count at the wrist and the patient's simultaneous silent count of the tinnitus pulsations were very similar for each patient. Like the nonlateralized group, all described their tinnitus as high pitched and pulsating. Six of the seven abolished their tinnitus with one or more strong neck or jaw muscle contractions. The pulsatile tinnitus of the other patient, M4, became nonpulsatile (“wind”) with such maneuvers. Audiometry was symmetric in all except M3, who had a conductive loss above 1 kHz and ipsilateral to her pulsatile tinnitus. Thyroid function tests and hematocrits were normal. Auscultation was normal in all. Jugular compression did not modulate any patient's tinnitus. Carotid compression altered the pulsatile tinnitus in patient M6 only. His tinnitus stopped briefly upon release of compression of his right carotid, as did release of his right sternocleidomastoid compression without carotid compression. In addition, focal right ear canal pressure at “2 o'clock” abolished his pulsations and, overall, quieted his tinnitus. A representative lateralized pulsatile tinnitus case follows.
Table 4.
Seven Cases With Continuous Unilateral Pulsatile Tinnitus
| Patient | Age | Sex | Tinnitus side | Tinnitus description | Audiogram | Jugular compression | Carotid Compression | Somatic Testing | Other |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 60 | F | L | hissing, swishing | HFSNHL (40 dB) | negative | negative | multiple maneuvers abolish tinnitus | |
| M2 | 48 | F | L | whooshing | normal | negative | negative | abolished by L SCM | |
| M3 | 48 | F | L | furnace | cond HL above 1kHz (35 dB) | negative | not done | abolished by L SCM | 32 years earlier, left carotid artery balloon obliteration for carotid cavernous fistula; arteriogram negative |
| M4 | 37 | M | L | high ringing | HFSNHL (40 dB) | negative | negative | multiple maneuvers replace pulsatile tinnitus with louder AS “wind”; AD “wind” from L jaw deviation | |
| M5 | 45 | F | L | very high pitched, “like hum of a computer” | normal | negative | negative | multiple maneuvers abolish tinnitus | initially intermittent; abolished by L tooth abscess drainage/anesthetic |
| M6 | 55 | M | R | whooshing | normal | negative | R SCM, tinnitus louder, but upon release not heard for seconds | jaw protrusion abolishes tinnitus | Q-tip at 2 o'clock in R ear canal suppresses pulsations & quiets tinnitus |
| M7 | 40 | M | L | high pitched | normal | negative | negative | R jaw deviation abolishes; multiple maneuvers increase AS loudness; R SCM induces slight pulsatile AD | L auricular electroacupuncture quiets tinnitus |
NOTES: M = male; F = female; HFSNHL = high frequency sensorineural hearing loss (numbers within brackets indicate the maximal threshold elevation); R = right; L = left; AD = right ear tinnitus; AS = left ear tinnitus; SCM = sternocleidomastoid; cond HL = conductive hearing loss.
Case 2 (patient M5)
A 45-year-old right-handed woman presented with almost 2 years of left ear pulsatile tinnitus (a very high-pitched pulsation “like the hum of a computer”) that she could stop for 1 to 2 seconds by clenching her teeth. Initially, it was heard only when in bed but became constant within weeks and was associated with left forehead, lateral cervical, and retro-orbital discomfort that could be relieved by lying in bed for 20 minutes.
Four months after the onset of her pulsatile tinnitus, she developed increased sensitivity to a left upper molar that had been painful for about 20 years. With administration of local anesthetic during a subsequent root canal procedure, her tinnitus was abolished for about 7 hours. Ultimately, the tooth was extracted. Soon after, when “white puss and blood came out” of the tooth's socket, her tinnitus stopped again for 24 hours. Her audiogram was normal, as were head and neck CT angiograms and MRI and MRA scans.
The patient's silent count of her tinnitus pulsations and the examiner's simultaneous silent count of her radial pulse were 16 and 17, respectively. No bruits were detected from the periauricular region, over the carotids, in the suboccipital region, or over the globes. Jugular or carotid compression on either side did not affect her tinnitus. With multiple intense muscle contractions of her neck and jaw, she could abolish her tinnitus. For some maneuvers, the degree of tinnitus suppression was related to the intensity of the maneuver. For example, turning her head to the right halved her tinnitus loudness, but her tinnitus was not abolished until the contraction was intensified by the examiner resisting her head turning with pressure against her right temple.
Over the 2 years following our initial assessment, she had recurrent episodes of left periorbital pain, intensification of her left pulsatile tinnitus, and purulent drainage of her left sinuses. Left sinus surgery was performed but had no effect on her tinnitus, which remained high pitched, pulsatile, and lateralized to the left ear.
Discussion
In this report are described 13 cases of cardiac-synchronous pulsatile tinnitus for which no apparent acoustic source of their tinnitus could be identified. All patients share the characteristic that their pulsatile tinnitus can be either abolished or transformed to nonpulsatile tinnitus by activation of their somatosensory system of the upper neck or jaw (i.e., with intense muscle contractions or pressure on muscles and tendons). We now attempt to answer the question of why their tinnitus is pulsatile. We start by considering the nonlateralized cases.
What Are the Possible Causes of Nonlateralized Pulsatile Tinnitus?
The localization of the pulsatile tinnitus to the center of the head in these six cases suggests several possible causes: (a) a high cardiac output state, such as anemia or hyperthyroidism, (b) bilateral independent extracranial or intracranial somatosounds such as bilateral carotid stenosis, (c) a central somatosound such as a carotid-cavernous fistula, (d) independent pulsatile modulation of neural activity of each side of the auditory CNS, such as bilateral auditory nerve vascular compression, or (e) pulsatile modulation of a single auditory CNS structure that can affect auditory perception on both sides or cause a centered sound percept. This last possibility would include the auditory pathway above the auditory decussation (trapezoid body) because the auditory pathway is both crossed and uncrossed rostral to the trapezoid body. Even a cochlear nucleus could be playing a role, because the cochlear nuclei have reciprocal connections (Cant & Gaston, 1982). Likewise, pulsatile modulation of neural activity at one location along the auditory pathway might project to higher or lower parts of the auditory pathway, thereby resulting in nonlateralized pulsatile tinnitus.
The first possibility has been eliminated by our laboratory studies. Somatosounds that are coming from two independent sources, one on each side of the head, can be eliminated on the basis that, in all but one of our cases (B5), we have no information to suggest that the tinnitus began on one side and later became bilateral or nonlateralized. In all our nonlateralized cases, the tinnitus was never perceived initially as unilateral, even though for some patients (B1 & B5), the spatial perception of their pulsatile tinnitus could be transformed from nonlateralized to unilateral with somatic testing. As such, two different independent somatosounds on the two sides either intracranially or extracranially beginning at the same time are extremely unlikely and so can be eliminated from consideration on that basis alone. The same arguments can be applied to possibility (d), bilateral pulsatile modulation of neural activity of the auditory CNS from two independent sources, one within each side of the auditory pathway. The unremarkable imaging studies likewise exclude such possibilities, as well as excluding a central somatosound. The lack of any eye signs such as pulsating exophthalmos or ocular bruits excludes carotid-cavernous fistula.
Exclusion of (a) thru (d) leaves, as the only possibilities for our cases of nonlateralized pulsatile tinnitus, vascular modulation of the auditory CNS either in one locus above the trapezoid body or from interactions between neural structures from both sides either above or below the trapezoid body, such as through the reciprocal connections between the cochlear nuclei of the two sides (Cant & Gaston, 1982).
How Might Central Pulsatile Modulation Occur? Is Somatic Modulation of the Auditory Pathway Accounting for These Cases?
Because (a) the dorsal cochlear nucleus (DCN) is implicated in playing a role in tinnitus from cochlear insults based on animal studies and (b) a combination of human studies and animal studies implicates the DCN in tinnitus from a somatosensory insult, consideration of pulsatile modulation of DCN activity as a cause for pulsatile tinnitus is reasonable and may serve as a model for pulsatile modulation of neural activity at other sites along the auditory pathway (Kaltenbach, Zhang, & Finlayson, 2005; Kanold & Young, 2001; Levine, 2004). Besides receiving deep layer inputs from type I myelinated auditory nerve fibers to the smooth dendrites of DCN fusiform and giant cells, as well as tuberculoventral neurons, the DCN granular cell layer receives inputs from multiple other CNS loci, including the somatosensory system of the head and upper neck, vestibular system, pontine nuclei, type II unmyelinated auditory nerve fibers, the octopus cell area of the ventral cochlear nucleus, the inferior colliculus, and the auditory cortex (Oertel & Young, 2004). Any one of these inputs to the DCN could be responsible for the pulsatile quality of these patients' tinnitus, if their neural activity had a pattern synchronous with the cardiac cycle. At present, there is no compelling reason to choose one of these inputs as the most likely candidate.
What must be reconciled with any hypothesis is the fact that all these patients can suppress the pulsatile quality of their tinnitus with somatosensory activation, either strong neck or jaw muscle contractions or direct pressure on an upper neck muscle. All these maneuvers are activators of the somatosensory receptors of tendons and muscles.
In some of the patients, the tinnitus was not heard altogether with one or more of these maneuvers (B1 [left], B3 [right and left], B4 [right and left], B5 [left], and B6 [right and left]). With other maneuvers, only the pulsatile quality of the tinnitus could be suppressed, leaving behind residual high-pitched nonpulsatile tinnitus (B1 [right], B2 [right and left], B4 [right and left], and B5 [right]). For example, several maneuvers abolished patient B1's left ear pulsatile tinnitus, and his right ear tinnitus became nonpulsatile but was not abolished. Despite being unable to lateralize their tinnitus, with some maneuvers, half of the six patients (B1, B4, and B5) could suppress the tinnitus from only one ear. The other three (B2, B3, and B6) could suppress both but could not suppress just one side.
Because somatosensory innervation to the DCN is ipsilateral and the DCN is prior to the auditory decussation, the cases of ipsilateral suppression are consistent with the DCN being the site of somatosensory-auditory interaction. Moreover, one of the roles that has been suggested for DCN is suppression of self-generated sounds such as heart beats (Haenggeli, Pongstaporn, Doucet, & Ryugo, 2005). This suggests that somatic pulsatile tinnitus may be due to a failure of the DCN to suppress the normal self-generated sound of our heart beats. Suppression of pulsatile tinnitus with somatosensory activation from somatic testing may represent a temporary correction of this malfunction.
We conclude that the most likely causes for the pulsatile tinnitus of the nonlateralized cases are related to the somatosensory-auditory interactions in the CNS, possibly at the DCN level. We further speculate that it occurs in one of two ways. The first explanation is failure to suppress the normal self-generated sound of our heart beats. A second possibility is that the well-established somatosensory-auditory system interactions within the central auditory pathway are causing pulsatile tinnitus because the somatosensory inputs to the central auditory pathway are pulsatile in nature. As shown in Figure 2, we suggest that the head and neck somatosensory inputs, which are being continuously modulated by cardiac synchronous blood flow, lead to pulsatile disinhibition of the DCN. This increased activity in the output of the DCN projects to other centers and eventually leads to activation of the auditory perceptual machinery responsible for pulsatile tinnitus.
Figure 2.
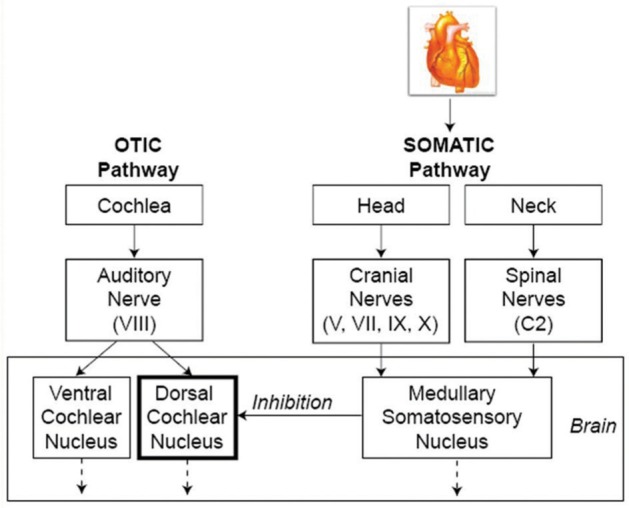
Schematic depiction of cardiac modulation of the somatosensory system leading to pulsatile tinnitus.
Note: The head and neck somatosensory inputs, which are being continuously modulated by cardiac synchronous blood flow, lead to pulsatile disinhibition of the DCN. This increased activity in the output of the DCN projects to other centers and eventually leads to activation of the auditory perceptual machinery responsible for pulsatile tinnitus.
In fact, our ear canal recordings support this second hypothesis. Figure 1 shows recordings from the ear canal of patient B1, along with his simultaneous EKG. These recordings are typical of all patients without pulsatile tinnitus; EKG synchronous sound pressure changes were occurring that have a dominant component of about 20 Hz, a frequency that is outside the range of normal hearing and so is generally not heard. However, just as the somatosensory receptors of the external auditory canal are being stimulated with each cardiac cycle, likewise, we suggest that the somatosensory receptors in muscles, tendons, and joints are receiving cardiac-synchronous somatosensory stimulation, which in these patients is leading to pulsatile tinnitus. In fact, note that patients B1 and M6 (Tables 3 and 4, respectively) can modulate their pulsatile tinnitus with ear canal stimulation and that relieving the pressure/inflammation or blocking the nerve impulses from the root of a tooth can sometimes abolish the pulsatile tinnitus, as in Case 2 (M5) above.
Lateralized Pulsatile Tinnitus
Aside from the lateralization of their tinnitus, the seven cases of unilateral pulsatile tinnitus are very similar to the six nonlateralized cases. In fact, patient M7 of the lateralized group represents a transitional case that reinforces the similarity between the lateralized and nonlateralized cases. Although “99% of the time,” he localizes his pulsatile tinnitus to his left ear, on rare occasions, his pulsatile tinnitus is heard in the center of his head. In fact, when examined, he was hearing only his left ear pulsatile tinnitus, but intense right sternocleidomastoid contraction elicited pulsatile tinnitus in his right ear. This finding in patient M7 confirms that somatosensory activation can elicit pulsatile tinnitus. In this instance, somatosensory activation transforms his pulsatile tinnitus from lateralized to nonlateralized.
Like M7, another patient from our clinic could elicit lateralized pulsatile tinnitus from somatic activation. This case is not included in our tables, because she had no tinnitus when evaluated. However, her history has all the features of our cases of lateralized pulsatile tinnitus including suppression of her pulsations with somatic activation.
Case 3
After 18 months of orthodontia treatments, an audiometrically normal 57-year-old registered nurse developed intermittent left ear whistling tinnitus, which had a cardiac-synchronous pulsatile nature (“swishing”) about half the time. She noticed that jaw deviation to the right suppressed the pulsatile quality of her high-pitched tinnitus.
One month after her tinnitus onset, her bottom braces were removed, and within 3 months, her left ear tinnitus became constant. Head and neck MRI and MRA scans were normal. After another 4 months, her upper braces were removed.
Two months later, she received a dental appliance to correct her bite. Soon thereafter, her tinnitus became intermittent again.
At the time of her examination, she was experiencing no tinnitus. However, pressure against her left posterior auricle at its attachment to the skull transiently induced her left pulsatile tinnitus. Maximal opening or protruding of the jaw, as well as left sternocleidomastoid contraction, transiently elicited her left ear nonpulsatile tinnitus.
Several points are made by this case. The major point is that pulsatile tinnitus can be elicited by a somatosensory stimulus, in this case, pressure against the base of the auricle. This case also shows that (a) alterations of the bite may both induce tinnitus and relieve tinnitus and (b) pulsatile tinnitus can alternate with high-pitched continuous tinnitus.
The unilateral pulsatile tinnitus cases all described the pulsatile tinnitus as high pitched. Six of the seven could abolish their tinnitus with strong neck or jaw muscle contractions, whereas the other patient (M4) could suppress the pulsatile quality with multiple such contractions but could never abolish all tinnitus. Imaging studies were unrevealing, including a cerebral angiogram in patient M3. The similar properties of the lateralized and nonlateralized cases suggest that they all share a similar etiology. In fact, patient M7, as described above, could transform his lateralized tinnitus to nonlateralized with somatosensory activation.
Both patient M7 and case 3 could induce pulsatile tinnitus with somatosensory activation. For these two patients, it is straightforward that the somatosensory system is causing their pulsatile tinnitus. For the other patients, suppression of tinnitus with somatic testing implies that the somatosensory system is interacting somewhere along the tinnitus neural pathway to suppress the pulsations.
Other Possible Interpretations
Is the Pulsatile Tinnitus Due to a Vascular Somatosound?
An obvious consideration in these cases of pulsatile tinnitus, in particular when it is unilateral, is that the pulsatile tinnitus is due to a sound being generated by blood flow and this sound is being heard by the patient. One can then argue that the somatic testing is having its effect simply through diminishing blood flow.
Venous blood flow as the source of the sound can be excluded because jugular compression did not change the tinnitus in any of the 13 cases. On the other hand, an arterial source for the somatosound is possible, at least in the 11 patients who did not undergo cerebral angiography. Two patients, B1 and M3, had cerebral arteriograms that did not identify a source of a somatosound.
The vertebral arteries are inaccessible on the physical examination. Because the vertebral arteries cannot be tested with compression, they are possible sources for a somatosound. However, just as the vertebral-basilar system is inaccessible on examination to compression because it is protected by the skeletal system, so too, blood flow within the vertebral-basilar arterial system is unlikely to be affected by strong muscle contractions of the head and neck that have been performed on all of these patients as part of our somatic testing. Therefore, the suppression of the pulsatile tinnitus by somatic testing in all of these cases is unlikely to be due to an effect on vertebral-basilar blood flow per se.
On physical examination, the carotid arteries can be compressed and, likewise, their compression might be accounting for some of the changes in pulsatile tinnitus that occurred with strong muscle contraction of the neck and compression of neck muscles. In fact, carotid compression did have an effect in four of the cases, so this raises the possibility that (a) arterial blood flow from the carotid system is generating a somatosound in these four cases and (b) suppression of the pulsatile tinnitus with somatic testing is due to diminished carotid blood flow and not interactions between the somatosensory and the auditory system within the CNS.
We digress now to examine in detail these four cases where carotid compression affects their pulsatile tinnitus.
In two of the six nonlateralized cases, B3 and B4, all tinnitus was suppressed with carotid compression. For patient B3, bilateral pulsatile tinnitus was suppressed by a few seconds of right carotid compression at the level of the upper border of the thyroid cartilage, but left carotid compression caused no change. Against this effect being a result of diminished right carotid blood flow in this patient with a normal MRA scan of her carotid arteries are two findings: (a) When the right sternocleidomastoid, which overlies the carotid artery, was given other firm pressure such that it did not appear to affect the artery, it likewise quieted her tinnitus, and (b) another maneuver that required strong right-sided neck muscle contractions (from the neutral head position, isometric forceful tilt to the right) reduced her bilateral pulsatile tinnitus loudness by 60% on a visual analog scale.
For the second nonlateralized patient, B4, carotid compression of either side abolished her ipsilateral tinnitus, but the pulsatile tinnitus of the other side remained unchanged. With firm pressure on the mastoid where the sternocleidomastoid inserts, her ipsilateral pulsatile tinnitus was also fully suppressed. Moreover, B4 could abolish her tinnitus of both sides by active jaw retrusion. Passive retrusion made very little change and graded active retrusion did not lead to graded attenuation of her tinnitus; rather, at one point during jaw retrusion, her tinnitus abruptly ceased bilaterally. Although the observations with B3 do not definitively exclude carotid compression stopping her tinnitus, B4's observations clearly cannot be accounted for by carotid occlusion. Because jaw retrusion is not compressing the carotid arteries of B4, retrusion must be affecting her tinnitus in some other way.
Six of the seven unilateral pulsatile tinnitus cases underwent carotid compression; M3 was not tested due to safety concerns because she had only one patent carotid artery. Ipsilateral carotid compression and other head and neck maneuvers caused patient M4's left pulsatile tinnitus to be replaced by a nonpulsatile sound (described as “wind”) that was twice as loud.
Patient M6, in a way, suppressed his pulsatile tinnitus from ipsilateral carotid compression. Synchronous with initiating carotid compression, his pulsatile tinnitus actually became louder followed by 1 to 2 seconds of no tinnitus perception. Moreover, other jaw and neck contractions would also transiently abolish his tinnitus, as part of a sequence of variations in tinnitus loudness lasting a few seconds.
Taken together, all of the above provide little or no support for the notion that (a) some of these patients' pulsatile tinnitus is from a sound generated by arterial blood flow and (b) somatic testing is diminishing arterial blood flow, which leads to quieting of the pulsations. On the contrary, these findings implicate the soft tissues nearby the internal carotid artery as a site particularly involved in somatic modulation of tinnitus in general and pulsatile tinnitus in particular.
Modulation of Pulsatile Tinnitus by Somatosensory Activation
Somatic modulation of auditory perception from interactions between the somatosensory and the auditory system within the CNS is a well-established fact (Levine et al., 2003; Lewald et al., 1999; Moller & Rollins, 2002). Several lines of evidence weigh against the idea that our observations in this group of patients with pulsatile tinnitus are simply the usual effect of the somatosensory system activation on auditory perception. For this analysis, we need to consider two possibilities, namely, that the pulsatile tinnitus is (a) due to a somatosound or (b) neurally based.
If we assume for the moment that these cases of pulsatile tinnitus are due to a vascular somatosound, then we now consider the possibility that the modulation of pulsatile tinnitus by somatosensory activation in these cases is just another example of this general phenomenon applied to this vascular somatosound.
In the case of a somatosound, the study of Moller and Rollins (2002), using median nerve electrical stimulation at the wrist as the somatosensory activator, is relevant. They found that somatosensory modulation of the perception of an external sound occurred only in people younger than age 20. Moreover, the effect was predominantly to intensify the loudness of the external sound; of those who could modulate, the effect of somatosensory stimulation was to increase the loudness in more than 80%. Because (a) none of our patients were younger than age 37 and (b) the overwhelming effect in our patients is a quieting of the tinnitus percept, from this clinical analysis, we reject the possibility that somatic modulation of a somatosound is accounting for suppression of the pulsatile quality of our patients' tinnitus. As a corollary, we reject the notion that a somatosound is accounting for our cases of pulsatile tinnitus.
We now consider the other possibility, namely, that (a) the pulsatile tinnitus is not a somatosound but is neurally based and (b) our findings are due to “routine” somatic modulation. In the case of neural tinnitus being somatically modulated, our prior studies using somatic testing as the somatosensory activator are pertinent (Levine et al., 2003). These studies have shown that in about 80% of people with neural tinnitus, strong muscle contractions of the head and neck can modulate their tinnitus; by contrast, 100% of our pulsatile tinnitus patients can somatically modulate their tinnitus (see Table 5). Furthermore, of the nonpulsatile tinnitus patients, 72% could make their tinnitus louder with one or more strong head or neck muscle contractions (Table 5, combining columns 2 and 4), whereas in only about 25% did their tinnitus become softer (Table 5, combining columns 3 and 4). Again, this contrasts with our pulsatile tinnitus patients. Although 69% did increase their tinnitus loudness, 77% decreased their tinnitus loudness and 69% could abolish their tinnitus, as compared with only 14% of nonpulsatile tinnitus patients (Table 5). Most important, all 13 of our patients could suppress the pulsatile nature of their tinnitus, an observation that has not been previously described to the best of our knowledge. These differences are significant at the p = .01 confidence level (Pearson chi-square test). Because the effect of somatic testing in the pulsatile tinnitus patients of this study is overwhelmingly in the direction of decreasing tinnitus loudness, it is clear that the results from these previous studies argue against the pulsatile tinnitus of our patients being due to routine somatic modulation.
Table 5.
Comparison of Somatic Modulation in 28 Nonpulsatile and 13 Somatic Pulsatile Tinnitus Patients
| Increase Only | Decrease Only | Increase and Decrease | No Change | Abolish | |
|---|---|---|---|---|---|
| Nonpulsatile | 15 (54%) | 2 (7%) | 5 (18%) | 6 (21%) | 4 (14%) |
| Somatic Pulsatile | 3 (23%) | 4 (31%) | 6 (46%) | 0 (0%) | 9 (69%) |
Conclusions
A pulsatile tinnitus syndrome has been described, which is characterized by cardiac synchronous tinnitus that is high pitched and can be suppressed by activation of the somatosensory system of the jaw or upper lateral neck. The tinnitus percept may be lateralized or nonlateralized and is not affected by jugular compression. Imaging studies are negative. The syndrome likely occurs from (a) failure of the somatosensory-auditory CNS interactions to suppress cardiac somatosounds or (b) cardiac synchronous somatosensory activation of the central auditory pathway.
Further Directions
Based on our hypothesis that these patients' pulsatile tinnitus is probably occurring on a somatosensory basis, and evidence that somatosensory-based tinnitus can benefit from somatosensory-based treatment modalities such as acupuncture (Levine, Nam, Oron, & Melcher, 2007), we have begun treating some of our patients (B1, B4, M3, and M7) with auricular electroacupuncture. They are treated with a device that stimulates three points on the auricle continuously for 4 days. This same electroacupuncture treatment protocol is repeated weekly for a minimum of 3 weeks or until benefit plateaus. B1 and M7 plateaued with about a 50% improvement in their tinnitus loudness after 14 and 6 weeks, respectively. The improvement has persisted for more than 2 months with no additional electroacupuncture. B4 and M3 have recently begun their electroacupuncture treatments, and both have improved after 2 and 4 weeks, respectively. More trials are planned.
References
- Abel M. D., Levine R. A. (2004). Muscle contractions and auditory perception in tinnitus patients and non-clinical subjects. Cranio, 22(3), 181–191 [DOI] [PubMed] [Google Scholar]
- Cant N. B., Gaston K. C. (1982). Pathways connecting the right and left cochlear nuclei. Journal of Comparative Neurology, 212(3), 313–326 [DOI] [PubMed] [Google Scholar]
- Coles R. R. (1984). Epidemiology of tinnitus: (1) prevalence. Journal of Laryngology and Otology: Supplement, 9, 7–15 [DOI] [PubMed] [Google Scholar]
- Coles R.R.A. (1996). Tinnitus. In Stephens D. (Ed.), Adult audiology, 368–414 Newton, MA: Butterworth-Heinemann [Google Scholar]
- Haenggeli C. A., Pongstaporn T., Doucet J. R., Ryugo D. K. (2005). Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. Journal of Comparative Neurology, 484(2), 191–205 [DOI] [PubMed] [Google Scholar]
- Heller M. F., Bergman M. (1953). Tinnitus aurium in normally hearing persons. Annals of Otology, Rhinology, and Laryngology, 62(1), 73–83 [DOI] [PubMed] [Google Scholar]
- Kaltenbach J. A., Zhang J., Finlayson P. (2005). Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hearing Research, 206(1–2), 200–226 [DOI] [PubMed] [Google Scholar]
- Kanold P. O., Young E. D. (2001). Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. Journal of Neuroscience, 21(19), 7848–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesinski S. G., Chambers A. A., Komray R., Keiser M., Khodadad G. (1979). Why not the eighth nerve? Neurovascular compression—probable cause for pulsatile tinnitus. Otolaryngology—Head and Neck Surgery, 87(1), 89–94 [DOI] [PubMed] [Google Scholar]
- Levine R. A. (2004). Somatic tinnitus. In Snow J. (Ed.), Tinnitus: Theory and management (pp. 108–204). Hamilton, Ontario: BC Decker [Google Scholar]
- Levine R. A., Abel M., Cheng H. (2003). CNS somatosensory-auditory interactions elicit or modulate tinnitus. Experimental Brain Research, 153(4), 643–648 [DOI] [PubMed] [Google Scholar]
- Levine R. A., Nam E. C., Oron Y., Melcher J. R. (2007). Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Progress in Brain Research, 166, 195–207 [DOI] [PubMed] [Google Scholar]
- Lewald J., Karnath H. O., Ehrenstein W. H. (1999). Neck-proprioceptive influence on auditory lateralization. Experimental Brain Research, 125(4), 389–396 [DOI] [PubMed] [Google Scholar]
- Moller A. R., Rollins P. R. (2002). The non-classical auditory pathways are involved in hearing in children but not in adults. Neuroscience Letters, 319(1), 41–44 [DOI] [PubMed] [Google Scholar]
- Oertel D., Young E. D. (2004). What's a cerebellar circuit doing in the auditory system? Trends in Neurosciences, 27(2), 104–110 [DOI] [PubMed] [Google Scholar]
- Ohashi N., Yasumura S., Nakagawa H., Mizukoshi K., Kuze S. (1992). Vascular cross-compression of the VIIth and VIIIth cranial nerves. Journal of Laryngology and Otology, 106(5), 436–439 [DOI] [PubMed] [Google Scholar]
- Ryu H., Yamamoto S., Sugiyama K., Uemura K., Nozue M. (1998). Neurovascular decompression of the eighth cranial nerve in patients with hemifacial spasm and incidental tinnitus: An alternative way to study tinnitus. Journal of Neurosurgery, 88(2), 232–236 [DOI] [PubMed] [Google Scholar]
- Sator-Katzenschlager S. M., Michalek-Sauberer A. (2007). P-Stim auricular electroacupuncture stimulation device for pain relief. Expert Review of Medical Devices, 4(1), 23–32 [DOI] [PubMed] [Google Scholar]
- Sismanis A. (1998). Pulsatile tinnitus. A 15-year experience. American Journal of Otology, 19(4), 472–477 [PubMed] [Google Scholar]


