Abstract
Changes in brain electrical activity in response to cholinergic agonists, antagonists, or excitotoxic lesions of the basal forebrain may not be reflective entirely of changes in cholinergic tone, in so far as these interventions also involve noncholinergic neurons. We examined electrocortical activity in rats following bilateral intracerebroventricular administration of 192 IgG-saporin (1.8 μg/ventricle), a selective cholinergic immunotoxin directed to the low-affinity nerve growth factor receptor p75. The immunotoxin resulted in extensive loss of choline acetyl transferase (ChAT) activity in neocortex (80%–84%) and hippocampus (93%), with relative sparing of entorhinal-piriform cortex (42%) and amygdala (28%). Electrocortical activity demonstrated modest increases in 1- to 4-Hz power, decreases in 20- to 44-Hz power, and decreases in 4- to 8-Hz intra- and interhemispheric coherence. Rhythmic slow activity (RSA) occurred robustly in toxin-treated animals during voluntary movement and in response to physostigmine, with no significant differences seen in power and peak frequency in comparison with controls. Physostigmine significantly increased intrahemispheric coherence in lesioned and intact animals, with minor increases seen in interhemispheric coherence. Our study suggests that: (1) electrocortical changes in response to selective cholinergic deafferentation are more modest than those previously reported following excitotoxic lesions; (2) changes in cholinergic tone affect primarily brain electrical transmission within, in contrast to between hemispheres; and (3) a substantial cholinergic reserve remains following administration of 192 IgG-saporin, despite dramatic losses of ChAT in cortex and hippocampus. Persistence of a cholinergically modulated RSA suggests that such activity may be mediated through cholinergic neurons which, because they lack the p75 receptor, remain unaffected by the immunotoxin.
Keywords: Coherence, Entorhinal cortex, Cortex, Hippocampus, Amygdala, 192 IgG-saporin, Rat
Introduction
Past studies have demonstrated that changes in cholinergic function of the brain produce characteristic alterations of cortical electrical activity. Administration of cholinergic antagonists or excitotoxic lesions of cholinergic neurons in the basal forebrain increase slow-wave power and decrease high-frequency power (Buzsáki et al. 1983, 1988; Stewart et al. 1984; Riekkinen et al. 1990; Ray and Jackson 1991; Vanderwolf 1992; Holschneider et al. 1997), whereas cholinergic agonists result in a reversal of this phenomenon (Vanderwolf 1992). Furthermore, indirect cholinergic agonists such as physostigmine or eserine enhance the synchronization of theta activity, whereas cholinergic antagonists or excitotoxic lesions of the basal forebrain diminish it (Dickson et al. 1994; Leung et al. 1994; Holschneider et al. 1998).
Changes in brain electrical power and synchronization following administration of pharmacologic agents or excitotoxic lesions of the basal forebrain may not be reflective entirely of changes in cholinergic tone, in so far as these interventions have been shown to involve noncholinergic neurons also (Wenk et al. 1991; Lindefors et al. 1992; Muir et al. 1993; Waite and Thal 1996). 192 IgG-saporin, an immunotoxin linking an antibody to the low-affinity nerve growth factor receptor p75 to the ribosome inactivating protein saporin, has recently permitted specific lesioning of cholinergic neurons. When administered into the lateral ventricles, cholinergic neurons in the basal forebrain nuclei and some cerebellar Purkinje cells are destroyed (Heckers et al. 1994; Waite et al. 1994). The toxin spares GABAergic neurons in the basal forebrain and monoaminergic neurons because of its selectivity (Nilsson et al. 1992; Heckers et al. 1994; Waite et al. 1994).
Our study examines effects of intracerebroventricular (i.c.v.) administration of 192 IgG-saporin on brain electrocortical activity and cholinergic function of rats. We examined brain electrical power and signal synchronization (coherence) at baseline as well as in response to physostigmine or type I behavior (walking, rearing, turning, or large postural shifts), interventions that have previously been shown to elicit cholinergically mediated rhythmic slow activity (RSA).
Materials and methods
Animals
Fischer rats (344 male rats; Harlan Sprague-Dawley) of 260–280 g body mass were singly housed in Plexiglas cages with contact bedding in an environmentally controlled room on a 12-h light, 12-h dark cycle (lights on at 6:00 a.m.). The animals were given water and rodent laboratory chow ad libitum. The surgical and experimental procedures performed were in accordance with the Principles of laboratory animal care (NIH publication No. 86–23, revised 1985) and were reviewed and approved by the Animal Care and Use Committee at the West Los Angeles Veterans Administration Medical Center.
192 IgG-saporin administration
The rats were anesthetized with a mixture of 92 mg/kg ketamine, 0.92 mg/kg acepromazine, and 4.7 mg/kg xylazine in 0.9% saline, administered i.m. They were mounted in a stereotaxic apparatus in the skull-flat position. After exposing the skull, a hole was drilled over the left and right skull area (AP −1.1, L ±1.5), and a blunt-tipped 27-gauge needle was lowered into each cerebral ventricle to a depth of −4.2 below bregma. The experimental animals (TOX; n=11) each received an i.c.v. injection of 192 IgG-saporin (batch ATS-1–89; 1.8 μg/ventricle in 5 μl of phosphate-buffered saline, pH 7.4, over 15 s; Advanced Targeting Systems, Carlsbad, Calif.). Sham-lesioned control animals received infusions of vehicle (PBS, pH 7.4; n=12). Five minutes of diffusion time was allowed following each injection before the needle was retracted.
Implantation of epidural electrodes
Following a recovery period of 5 weeks, animals were reanesthetized with halothane (2.5% for induction, 1.5% for maintenance in 30% oxygen, 70% nitrous oxide), the skull was re-exposed, and eight further holes were drilled. Epidural recording electrodes (000–120×1/8 inch stainless steel machine screws) were placed, as previously reported (Holschneider et al. 1997, 1998), over the following left and right skull regions: frontal (F1, F2): AP 3.8 (distance to bregma, plus rostral and minus caudal), L (lateral to midline) ±2.0; frontoparietal (FP1, FP2): AP 0.3, L ±4.0; parieto-occipital (PO1, PO2): AP −4.3, L ±4.0; occipital (O1, O2): AP −7.8, L ±2.0. Two additional screws driven into the bone overlying the cerebellum served as ground and indifferent electrodes. The pins attached to the recording electrode screws were then placed into an Amphenol electrical connector, which was fastened to the skull with methacrylate cement.
Electroencephalographic recording: type I versus type II behavior
Eight days after implantation of the epidural electrodes, brain electrical activity (1–44 Hz) was recorded using a QND electroencephalograph (Neurodata; Pasadena, Calif.) and stored on hard disc. The digital sampling rate was 256 points/channel per second, with frequency filter settings of 0.5 Hz and 70 Hz. Electrical activity was referenced to the electrode overlying the cerebellum and recordings (TOX, n=6; PBS, n=6) were made before noon in an 8×8×15-inch Plexiglas cage. The animals were maintained in the alert state by tapping vigorously on the cage every 30 s. Electroencephalographic (EEG) activity was annotated on-line to mark episodes of type I behavior (includes walking, rearing, turning, or large postural shifts) or type II behavior (immobility), as well as grooming and drowsiness. In each session a cumulative 10 min of EEG activity was recorded. “TOX-I” and “TOX-II” refer to type I and II behaviors, respectively, in animals that received the immunotoxin; “PBS-I” and “PBS-II” refer to type I and II behaviors, respectively, in intact animals that received the vehicle (PBS).
Electroencephalographic recording: physostigmine versus normal saline infusion
The following day animals were reanesthetized with halothane and received cannulation of the bilateral femoral artery and vein. Following surgery, recovery from anesthesia was rapid, with animals being fully awake and exhibiting exploratory behavior of their environment within 5 min. A 2-h period was allowed prior to any EEG recordings while the animal was passively restrained in a Bollman Cage. In these cages the animal rests in the prone position with its limbs hanging to the sides. Acrylic, nontraumatic bars entrap the animal, preventing locomotion but allowing limb and head movements. Rectal temperature was recorded and maintained at 37°C with a BAT-12 thermocouple thermometer connected to a TCAT-1A temperature controller (Physitemp) and a source of radiant heat. A 10-min EEG recording was performed on the passively restrained animal. This was followed by initiation of a continuous intravenous infusion of either physostigmine (4.2 μg/kg per minute in normal saline) or normal saline (TOX+NS, n=5; PBS+NS, n=6; TOX+physostigmine, n=6; PBS+physostigmine, n=6) administered over 50 min by a motor-driven syringe pump at a rate of 1.98 cm3/h. EEG activity was continuously monitored over the period of 50 min and stored on hard disc. The animals were killed by administration of an intravenous bolus injection of 0.5 ml of 50 mg/kg pentobarbital in 3 M KCl, which caused circulatory arrest.
Biochemical analysis
The brain was rapidly removed, flash-frozen in methyl-butane chilled to −70ºC, and embedded in OCT compound. Cortical regions underlying each of the eight electrode sites, the cerebellum, the dorsal hippocampus, and the amygdaloid-entorhinal-piriform area were dissected from tissue blocks produced by serial coronal cuts of the frozen brain (180 μm, −20°C). For each region, tissue samples from the left and right hemispheres were pooled. The tissue was homogenized in 1 ml of 50 mM phosphate buffer (pH 7.4). After vortexing, half of the homogenate was added to tubes containing 5 μl of Triton X-100. These were measured for the determination of choline acetyl transferase (ChAT) activity according to the method of Fonnum (Fonnum 1975). They were left on ice for 1 h, after which 10-μl aliquots were added to tubes containing the following: 100 mM physostigmine; 280 mM NaCl; 0.26 mM [3H]acetyl-CoA; 8.8 mM choline chloride; 50 mM NaPO4; 1.0 mM EDTA. The reaction was started by adding 3H-labeled acetyl-CoA (ICN biochemicals) to a final specific activity of 12.3 mCi·mmol−1 and was stopped by adding 0.5 ml of ice-cold 10 mM phosphate buffer and transferring to an ice bath. The reaction tube contents were then transferred to scintillation vials to which 1 ml of tetraphenylboron in acetonitrile and 3.5 ml of spectrafluor-toluene (Amersham) were added and slowly mixed while maintaining phase separation. Difference in the solubilities of [3H]acetyl-CoA and [3H]acetylcholine provides the basis for the separation of these two compounds in the extraction step. Because [3H]acetylcholine and not [3H]acetyl-CoA enters the phase containing the scintillation fluid, the activity measured reflects the amount of tritium related to acetylcholine. After 1 h, the vials were transferred to a Beckman LS 5801 liquid-scintillation spectrometer for counting using a tritium window. The protein content of the homogenates was assayed according to the method of Smith et al. (Smith et al. 1985, 1987). Of the original homogenate, a volume diluted up to 0.1 ml was vortexed with 2.0 ml of the protein determination reagent (copper (II) sulfate pentahydrate 4% to bi-cinchoninic acid, 1:50). This was incubated at 37°C for 30 min. Absorbance at 562 nm was measured in a spectrophotometer (Spectronic 20, Milton Rowe).
ChAT activity in an additional group of animals that had received either immunotoxin (i.c.v., n=9) or PBS (i.c.v., n=7) but no recording electrodes was examined to determine whether the relative sparing of ChAT activity that was observed in the amygdaloid-entorhinal-piriform area differed between the amygdala and entorhinal-piriform cortex.
Data analysis of ChAT activity
For the analysis of ChAT activity, a univariate repeated-measures analysis of variance (ANOVA) was performed using “Toxin” as a between-subject factor and “Region” as a within-subject factor (SPSS 1990). Post hoc tests (SNK, P<0.05) were used to examine group differences between brain regions.
Data analysis of absolute power, relative power, and peak frequency
The first twenty 4-s epochs (a total of 80 s) of artifact-free EEG data was selected in nonrestrained animals during (1) type I behavior and (2) type II behavior. Additionally, EEG data was selected in passively restrained animal (1) at baseline and (2) 45 min following continuous infusion of either physostigmine or normal saline. Data was reformatted off-line to a bipolar montage (F1-FP1, FP1-PO1, PO1-O1, O1-Cb; F2-FP2, FP2-PO2, PO2-O2, O2-Cb). Spectral analysis of the EEG data was performed using a discrete Fourier transformation.
Electrocortical changes in response to the toxin were examined in passively restrained animals at baseline (TOX, n=11; PBS, n=12). Absolute power (ABS), relative power (REL, percentage total power) were calculated in the delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta-1 (12–20 Hz), and beta-2 (20–44 Hz) bands. Total power was calculated across the 1- to 44-Hz band. Significant group differences in response to toxin were tested separately for ABS and REL with a univariate repeated-measures ANOVA using “Toxin” as a between-subject factor and “Frequency” and “Electrode” as within-subject factors. Post hoc t-tests (1-tailed, Bonferroni P<0.05) were used to define differences in the regional electrocerebral response within specific frequency bands.
Changes in peak frequency of RSA in response to physostigmine or type I behavior were initially examined in the 4- to 12-Hz band with a univariate repeated-measures ANOVA using “Toxin” as a between-subject factor and “Electrode” and “Physostigmine” (or “Behavior”) as within-subject factors. ABS and REL during the physostigmine infusion were calculated in the 4- to 8-Hz band, which circumscribed the pharmacologic peak response; whereas ABS and REL during behavioral activation were calculated in the 6- to 10-Hz band, which circumscribed the peak response during type I behavior. Group differences in ABS and REL in response to pharmacologic (or behavioral) activation were tested with a univariate repeated-measures ANOVA using Toxin as a between-subject factor and Electrode and Physostigmine (or Behavior) as within-subject factors. Post hoc tests (SNK, P<0.05) were used to define differences in the regional electrocerebral response to physostigmine or type I behavior. Percentage changes in ABS or REL in response to pharmacologic challenge or behavioral activation were calculated as a percent change from baseline by electrode site for each animal [i.e., 100×(Postphysostigmine infusion − Preinfusion)/Preinfusion) or 100×(Type I-Type II)/Type II].
Data analysis of coherence
Coherence measures the correlation between pairs of EEG signals as a function of their frequency components. It quantifies the phase consistency of two signals, that is, the extent to which EEG signals have common, time-locked, frequency components. Unlike spectral power, coherency estimates are independent of amplitude (Shaw 1981, 1984; Bendat and Piersol 1986; Nunez 1995). In the past coherence has been used as an indirect measure of functional connectivity between different brain regions (O’Connor et al. 1979; Leuchter et al. 1992). Coherence has the dimensions of a correlation coefficient squared with values varying between 0 and 1. Analogous to a correlation coefficient, a value near 1 means that there is a great deal of shared activity, and a value near zero means that there is little shared activity.
Our calculations employed a fast Fourier transform of 1024 points. Since each data point was sampled at 3.91 ms (i.e., 256 samples/s), the duration of each EEG selection was 4.0 s and the frequency resolution was 0.25 Hz (i.e., 1/4 s). Coherence [Cxy (λ)] was defined as a function of the power spectral outputs for two electrode channel pairings (x,y) at any given frequency λ (Bendat and Piersol 1986):
or the square of the cross-spectrum of the two channels x and y [| Sxy(λ)|2] divided by the product of the spectra of the individual channels [Sx(λ) ×Sy(λ)]. To yield the final coherences, spectral intensities were averaged first over each of the above frequency bands using adjacent frequency components of width 0.25 Hz each, and second, across the selected, sequential 4-s electroencephalogram segments. The 95% confidence interval estimates in the 4- to 8-Hz band for sample coherences of 0.2, 0.5, 0.8 were, respectively, 0.16–0.28, 0.45–0.56, 0.77–0.83 (Nunez 1995).
Intrahemispheric coherence in each hemisphere was examined for adjacent electrode pairings (left hemisphere: F1-FP1/FP1-PO1, FP1-PO1/PO1-O1, PO1-O1/O1-Cb; right hemisphere: F2-FP2/FP2-PO2, FP2-PO2/PO2-O2, PO2-O2/O2-Cb). Interhemispheric coherence was examined for electrode pairings across the hemispheres (F1-FP1/F2-FP2, FP1-PO1/FP2-PO2, PO1-O1/PO2-O2, O1-Cb/O2-Cb). Analysis of intrahemispheric coherence and interhemispheric coherence proceeded separately in accordance with the methods described above for the analysis of ABS and REL.
Results
ChAT analysis
The immunotoxin induced a large drop in ChAT activity in neocortex (80%–84%) and dorsal hippocampus (93%; Table 1). A considerably smaller drop was found in entorhinal-piriform cortex (42%) and amygdala (28%). These changes were independent of whether animals subsequently received saline or physostigmine during the EEG recording. ChAT activity in cerebellum showed the lowest value of all regions and it was not affected by immunotoxin treatment.
Table 1.
Choline acetyl transferase (ChAT) activity (nanomoles of ACh formed per milligram of protein per hour, ±SEM) 6 weeks following i.c.v. administration of 192 IgG-saporin (TOX) or PBS. Depicted are group means of ChAT values in frontal, fronto-parietal, parieto-occipital, and occipital cortex, as well as cerebellum, dorsal hippocampus, entorhinal-piriform cortex and amygdala (NS normal saline, PHY physostigmine)
| PBS+NS
|
PBS+PHY
|
TOX+NS
|
TOX+PHY
|
All TOXIN
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SEM | n | Mean±SEM | n | Mean±SEM | n | Mean±SEM | n | %Decrease | |
| Frontal | 46.78±3.67 | 5 | 47.04±0.85 | 6 | 8.93±1.05** | 5 | 9.44±1.17**** | 6 | 80 |
| Fronto-parietal | 39.16±2.96 | 5 | 44.46±1.97 | 6 | 9.28±4.59** | 5 | 6.50±0.65**** | 6 | 82 |
| Parieto-occip. | 36.97±1.80 | 5 | 39.43±1.44 | 6 | 5.91±0.70** | 5 | 6.61±0.62**** | 6 | 84 |
| Occipital | 51.43±8.26 | 5 | 46.49±1.81 | 6 | 6.64±0.74** | 4 | 9.34±1.34**** | 6 | 83 |
| Cerebellum | 3.91±0.68 | 5 | 4.40±0.57 | 6 | 3.89±0.63 | 5 | 4.28±0.43 | 6 | 2 |
| Ent-pyrif-amyg. | 83.79±7.69 | 4 | 77.22±12.99 | 5 | 49.34±8.17* | 4 | 48.01±8.04*** | 5 | 39 |
| Dorsal hippoc. | 44.25±6.05 | 5 | 44.79±2.43 | 6 | 2.86±0.63** | 4 | 3.56±0.79**** | 6 | 93 |
| Ent-pyrif.a | 76.7±6.17 | 9 | 44.40±4.08* | 6 | 42 | ||||
| Amygdalaa | 90.4±3.41 | 7 | 65.00±7.42* | 5 | 28 | ||||
P<0.01,
P<0.001 for TOX+NS vs PBS+NS;
P<0.01 or
P<0.001 for TOX+PHY vs PBS+PHY
EEG recordings were not made from animals in these groups
Electrocortical power after 192 IgG-saporin
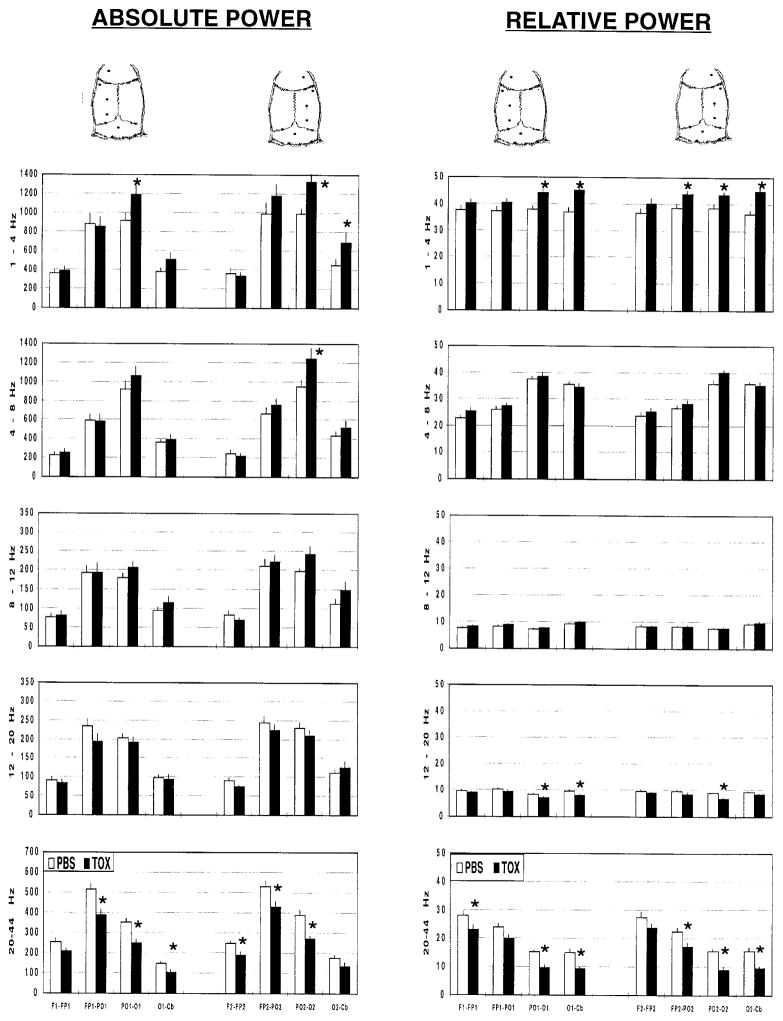
Administration of the toxin resulted in significant increases in ABS in the 1- to 4-Hz band and decreases in ABS in the 20- to 44-Hz band (TOX × Frequency: F4,84=6.46, P<0.0005) at specific electrode sites (Fig. 1; TOX × Frequency ×Electrode: F28,588=2.75, P<0.0005). Increases in the 1- to 4-Hz band were significant in the bilateral parieto-occipital and right occipital region, whereas significant decreases in the 20- to 44-Hz band were seen in a global distribution (Fig. 1). REL increased in the 1- to 4-Hz band and decreased in the 12-to 20-Hz and 20- to 44-Hz bands (TOX × Frequency: F4,84=12.81, P<0.0001). Changes were seen globally across the cortex (Fig. 1). Statistical significe was achieved in the 4- to 8-Hz band only at a single electrode site in the right posterior hemisphere for absolute power, with no significant difference in relative power. Total power (1–44 Hz) did not differ significantly between lesioned and intact animals.
Fig. 1.
Electrocortical power (±SEM) in the passively restrained rat 6 weeks following i.c.v. administration of 192 IgG-saporin (TOX) or vehicle (PBS). Group means of absolute power (ABS; microvolts squared) and relative power (REL; percentage power) were determined in the 1–4, 4–8, 8–12, 12–20, and 20–44-Hz bands and are depicted on the vertical axis. The horizontal axis and the rat skull pictorials delineate brain regions from which cortical activity was recorded. Depicted are electrode pairs in left and right frontal (F1-FP1, F2-FP2), fronto-parietal (FP1-PO1, FP2-PO2), parieto-occipital (PO1-O1, PO2-O2), and occipital (O1-Cb, O2-Cb) areas. *P<0.05 for PBS versus TOX (Bonferroni). White bars PBS, black bars TOX
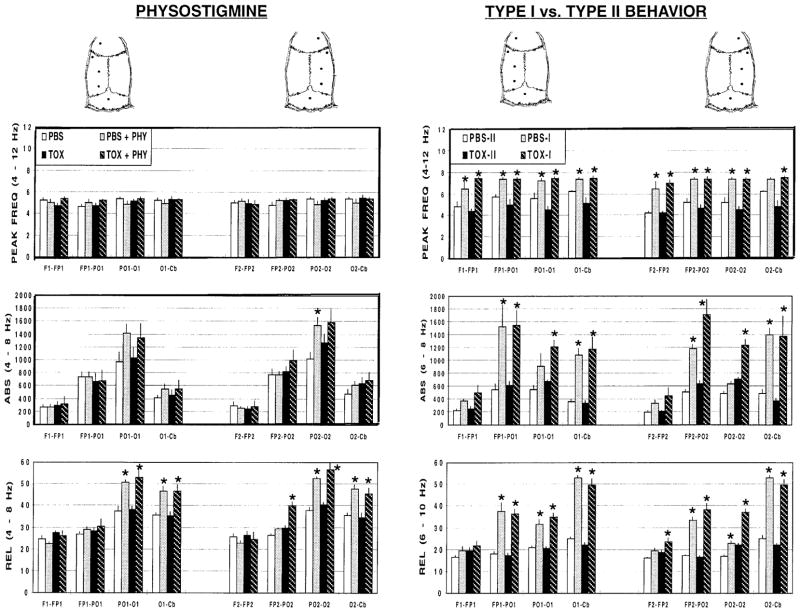
Peak frequency of rhythmic theta: effects of physostigmine and type I behavior
Neither lesioned nor intact animals showed a significant change in peak frequency in the 4- to 12-Hz band in response to physostigmine (Electrode mean: PBS 5.18±0.08 Hz; PBS+Physostigmine 5.06±0.04 Hz; TOX 4.97±0.07 Hz; TOX+Physostigmine 5.31±0.06 Hz; Fig. 2, column 1, row 1). In contrast to pharmacologic activation, behavioral activation resulted in a significant increase in peak frequency of RSA (Behavior: F1,10=75.37, P<0.0005). The increase in peak frequency was independent of the presence of an immunotoxic lesion and did not differ between electrode sites (Electrode mean: PBS-I 7.15±0.15 Hz; PBS-II 5.52±0.20 Hz; TOX-I 7.36±0.05 Hz; TOX-II 4.70±0.10 Hz; Fig. 2, column 2, row 1).
Fig. 2.
Electrocortical response (±SEM) of lesioned animals (TOX) or intact animals (PBS) to physostigmine, type I behavior (walking, rearing, turning, large postural shifts) or type II behaviors (immobility). Row 1 Peak frequencies (4–12 Hz); row 2 absolute power (ABS; microvolts squared; 4–8 Hz); row 3, relative power (REL; percentage power; 4–8 Hz) at baseline and during administration of physostigmine (PBS+PHY, TOX+PHY), or during type I behavior (PBS-I, TOX-I) or type II behaviors (PBS-II, TOX-II). *P<0.05 (SNK) for baseline versus physostigmine and type I versus type II behavior
Electrocortical power following physostigmine
Lesioned as well as intact animals showed a robust electrographic response to physostigmine (Fig. 3). Physostigmine increased ABS (Physostigmine: F1,10=9.75, P<0.01) at specific electrode sites (Physostigmine × Electrode: F7,70=18.25, P<0.0005) in the 4- to 8-Hz band, which circumscribed the peak physostigmine frequency (Fig. 2, row 2, column 1). Increases were independent of the presence of an immunotoxic lesion and occurred equivalently in intact animals (PBS electrode mean, 21±8%) and lesioned animals (TOX electrode mean, 12±4%).
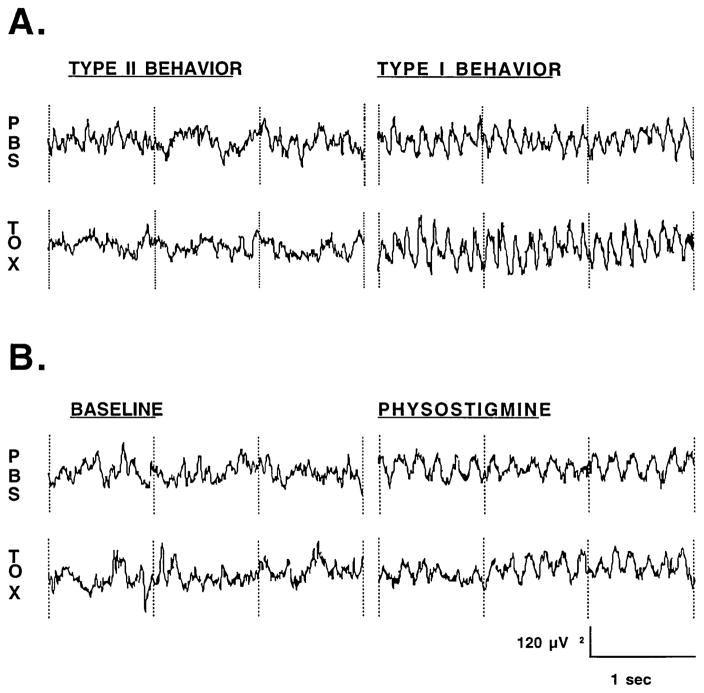
Fig. 3.
A, B Representative cortical EEG tracings from the parieto-occipital region (PO1-O1) of a representative lesioned animal (TOX) or intact animal (PBS): A during type I behavior (walking, rearing, turning, large postural shifts) and type II immobility), or B before and after continuous i.v. infusion of physostigmine
Significant changes were also seen in REL (Physostigmine: F1,10=40.01, P<0.0005) at specific electrode sites (Physostigmine × Electrode: F7,70=40.17, P<0.0005), with greatest significance in posterior brain regions (Fig. 2, row 3, column 1). There was no significant effect of the immunotoxin on REL during pharmacologic activation, with equivalent increases with respect to baseline in both intact animals (PBS electrode mean, 18±7%) and lesioned animals (TOX electrode mean, 22±7%). In the 8- to 12-Hz band, physostigmine resulted in a significant decrease from baseline in ABS (Electrode mean: PBS 28±3%, TOX 26±7%) and REL (Electrode mean: PBS 28±2%, TOX 21±4%, data not shown).
Electrocortical power after type I behavior
Lesioned as well as intact animals showed a robust electrographic response to behavioral activation (Fig. 3). Type I compared with type II behavior significantly increased ABS (Behavior: F1,10=44.08, P<0.0005) at specific electrode sites (Behavior × Electrode: F7,70=18.12, P<0.0005) in the 6- to 10-Hz band, which circumscribed the change in peak frequencies. Greatest significance was seen in posterior brain regions (Fig. 2, row 2, column 2). Increases were independent of the presence of an immunotoxic lesion and occurred equivalently in both lesioned animals (TOX electrode mean, 148±24%) and intact animals (PBS electrode mean, 116±22%).
Significant increases during type I behavior were also seen in REL (Behavior: F1,10=188.06, P<0.0005) at specific electrode sites (Behavior ×Electrode: F7,70=46.41, P<0.0005), with significance in most brain regions (Fig. 2, row 3, column 2). Increases were independent of the presence of an immunotoxic lesion and occurred equivalently in both lesioned animals (TOX electrode mean, 86±17%) and intact animals (PBS electrode mean, 69±15%).
Coherence following 192 IgG-saporin
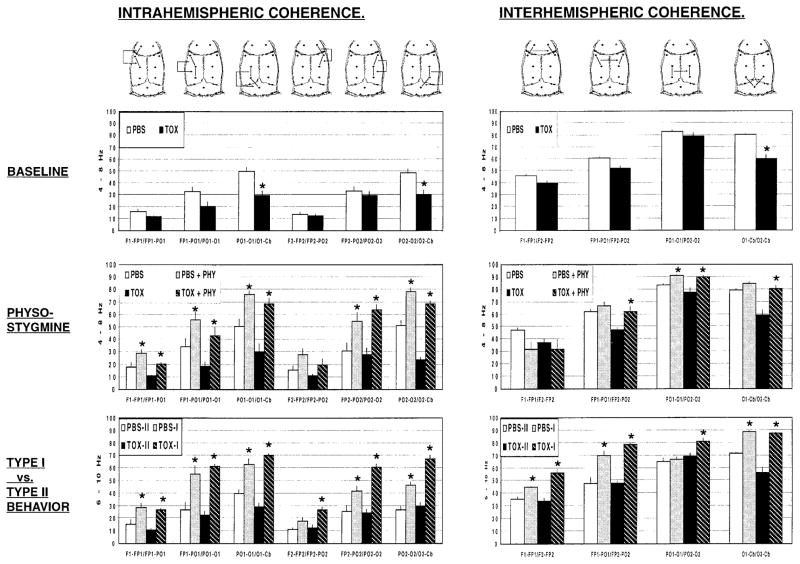
Administration of the immunotoxin resulted in modest decreases in intrahemispheric coherence within specific frequency bands (TOX × Frequency: F4,84=12.05, P<0.0005) and at specific electrode pairings (TOX × Frequency × Electrode pairing: F20,420=5.18, P<0.0005). Similarly, interhemispheric coherence decreased in response to the immunotoxin (TOX: F1,21=11.72, P<0.003) at specific electrode pairings (TOX × Electrode pairing: F3,63=8.09, P<0.0005) and within specific frequency bands (TOX × Frequency × Electrode pairing: F12,252=1.93, P<0.03). Post hoc t-tests (2-tailed, Bonferroni P<0.05) showed changes to be most significant at posterior electrode sites in the 4- to 8-Hz band for intra-hemispheric coherence, as well as interhemispheric coherence (Fig. 4, row 1). Immunotoxin lesions resulted in a larger decrease in intrahemispheric coherence (Electrode pairing mean, 28±6%) than interhemispheric coherence (Electrode pairing mean, 14±4%). Effects of the immunotoxin within the 4- to 8-Hz band are depicted in Fig. 4 (row 1).
Fig. 4.
Changes in intrahemispheric coherence (column 1) and interhemispheric coherence (column 2) to physostigmine, type I behavior (walking, rearing, turning, large postural shifts) or type II behaviors (immobility). Depicted are group means (±SEM) of lesioned animals (TOX) or intact animals (PBS). Row 1 at baseline; row 2 in response to physostigmine (PBS+PHY, TOX+PHY); or row 3 during type I behavior (PBS-I, TOX-I) or type II behaviors (PBS-II, TOX-II). *P<0.05 (SNK) for PBS versus TOX (row 1), baseline versus physostigmine (row 2) and type I versus type II behavior (row 3)
Coherence following physostigmine
Changes in coherence in response to physostigmine were examined in the 4- to 8-Hz band, which circumscribed the peak frequencies determined earlier for this pharmacologic challenge (Fig. 4, row 2). Physostigmine increased intrahemispheric coherence (Physostigmine: F1,10=259.73, P<0.0005) at specific electrode pairings (Physostigmine × Electrode pairing: F5,50=34.74, P<0.0005), with significance at most cortical sites (Fig. 4, row 2). Lesioned animals, compared with intact animals, showed a greater intrahemispheric coherence response to physostigmine at the PO1-O1/O1-Cb and PO2-O2/O2-Cb posterior sites (TOX × Physostigmine × Electrode pairing: F5,50=5.23, SNK P<0.001). This was due largely to lower intrahemispheric coherence of lesioned animals at these sites prior to pharmacologic activation.
Increases in intrahemispheric coherence in response to physostigmine exceeded increases in interhemispheric coherence in lesioned as well as nonlesioned animals (mean intrahemispheric coherence: PBS 77±7%, TOX 141±20%; mean interhemispheric coherence: PBS 18±13%; TOX –1±14%). Interhemispheric coherence increased modestly in response to physostigmine (Physostigmine: F1,10=16.75, P<0.002) in specific electrode pairings (Physostigmine × Electrode pairing: F3,30= 17.36, P<0.0005), with significance at several sites (Fig. 4, row 2). Changes were independent of the presence of an immunotoxic lesion.
Coherence following type I behavior
Changes in coherence in response to type I behavior were examined in the 6- to 10-Hz band, which circumscribed the peak frequencies determined earlier for this behavioral challenge (Fig. 4, row 3). Type I behavior increased intrahemispheric coherence (Behavior: F1,10= 278.27, P<0.0005) at specific electrode pairings (Behavior × Electrode pairing: F5,50=36.88, P<0.0005), with significance at most sites across the cortex (Fig. 4, row 3, column 1). Lesioned animals, compared with intact animals, showed a greater intrahemispheric coherence response to behavioral activation at specific electrode pairings (TOX × Behavior × Electrode pairing: F5,50=4.59, P<0.002), most significantly in the right hemisphere (F2-FP2/FP2-PO2, FP2-PO2/PO2-O2, PO2-O2/O2-Cb, SNK P<0.05).
Increases in intrahemispheric coherence in response to type I behavior exceeded increases in interhemispheric coherence in lesioned as well as nonlesioned animals (mean intrahemispheric coherence: PBS, 87±12%, TOX 167±9%; mean interhemispheric coherence: PBS 26± 9%, TOX 56±13%). Interhemispheric coherence increased in response to type I behavior (Behavior: F1,10= 134.07, P<0.0005) in specific electrode pairings (Behavior × Electrode pairing: F3,30=18.76, P<0.0005), with significance at several sites (Fig. 4, row 3, column 2). Lesioned animals, compared with intact animals, showed greater interhemispheric coherence response to behavioral activation (TOX × Behavior: F1,10=13.27, P<0.005) at two sites (FP2-PO2/PO2-O2, PO2-O2/O2-Cb, SNK P<0.05).
Discussion
Administration of 192 IgG-saporin resulted in significant changes in brain electrical activity, though these were surprisingly modest (7%–25% increase in delta and 13%–42% decrease in beta relative power) given the large depletion of ChAT in the cortex (80%–84%). By comparison, excitotoxic lesions of the basal forebrain, which result in lower levels of cortical ChAT depletion (22%–50%; Scremin et al. 1991; Waite and Thal 1996; Holschneider et al. 1997), demonstrate a greater increase in cortical slow-wave power (15%–81%) and a greater decrease in high-frequency power (20%–58%; Riekkinen et al. 1990, 1991; Holschneider et al. 1997). This suggest that some of the electrocortical changes following excitotoxic lesions of the basal forebrain may be due to the added damage of noncholinergic fibers. Surprisingly, unilateral injection of small doses of IgG-saporin (10 ng) directly into the nucleus basalis results in no change in electrocortical activity, despite a 50% decrease in cortical ChAT (Wenk et al. 1994). Our study suggests that larger decreases in cortical ChAT are needed to demonstrate brain electrical changes at the cortical level.
Changes in cholinergic tone through immunotoxic lesions or through administration of physostigmine resulted in a greater change of coherence within as compared to between hemispheres, with changes most significant in the theta (4–8 Hz) band. Disconnection of cortical neurons from their cholinergic afferents may have resulted in a decrease in responsiveness of corticocortical circuits to the synchronizing effects of their subcortical rhythmic input. Disruption of rhythmic slow-wave activity is likely to have occurred also at the level of the subcortical pacemaker cells themselves. The fact that both lesions and physostigmine resulted in a greater change of intrahemispheric as compared to interhemispheric coherence may reflect the underlying neuroanatomy of the cholinergic system. Cholinergic projections from the basal forebrain to the cortex remain largely ipsilateral, whereas virtually all fibers connecting the hemispheres through the corpus callosum or hippocampal commissures are noncholinergic, employing glutamate, aspartate, or GABA as their transmitters (Conti and Manzoni 1994). Thus, it might be expected that decreases or increases in cholinergic tone would result in greater changes in brain electrical synchronization within as compared to between hemispheres. Recently we reported similar findings in rats with excitotoxic nucleus basalis lesions in response to administration of the cholinergic neurotrophin nerve growth factor (Holschneider et al. 1998). Type I behavior also resulted in robust increases in intra-hemispheric coherence. Increases in interhemispheric coherence in response to behavioral activation were greater than those seen in response to physostigmine administration. A possible explanation for this may lie in the fact that RSA generated during type I behavior has been shown to depend not only on a cholinergic component but also on a noncholinergic one (Vanderwolf and Baker 1986; Lee et al. 1994).
The smaller drop in ChAT noted in our study in the amygdala (28%) and entorhinal-piriform cortex (42%) suggest that these brain regions were less susceptible to the immunotoxin than either cortex or hippocampus. Proximity to the lateral ventricles may affect penetration of the toxin and hence loss of ChAT activity in a specific region (Schweitzer 1987; Waite et al. 1995); however, differences in ChAT losses between neocortex and amygdala or entorhinal-piriform cortex are more difficult to reconcile on the basis of simple accessibility of the toxin to these structures. Immunohistochemical work in rats and humans has demonstrated that, although the p75 receptor is found on cholinergic cells, not all cholinergic neurons carry p75 (Gage et al. 1989; Yan and Johnson 1989; Heckers et al. 1994; Rossner et al. 1995b). In particular, ChAT-positive, p75-negative neurons have been reported in the nucleus basalis-substantia innominata complex, whose fibers have been surmised to project to the amygdala and parts of the rhinal paralimbic areas (Heckers et al. 1994). An absence or decrease in the p75 receptor has been demonstrated in the amygdala of rodents (Yan and Johnson 1988) as well as in the entorhinal cortex of humans (Kordower and Mufson 1992; Chen et al. 1996). This may explain the relative sparing of ChAT observed in immunotoxin-treated animals in the entorhinal-piriform cortex and the amygdala (Heckers et al. 1994; Schliebs et al. 1996).
Despite extensive losses in hippocampal ChAT (93%) and cortical ChAT (80%–84%), toxin-treated animals retained the ability to generate RSA. In the rat the major source of RSA is felt to reside in the hippocampus, though this appears to depend on external pacemaker cells found within the medial septum and diagonal band of Broca (Stumpf 1965; Stewart and Fox 1989; Vander-wolf 1992; Dickson et al. 1994; Barrenechea et al. 1995). In the original formulation of the hypothesis of the origin of RSA, cholinergic cells of the medial septum were hypothesized to rhythmically excite hippocampal neurons, which, because of the parallel nature of their cellular arrays and the synchrony of their cells, were able to generate a coherent signal of substantial amplitude detectable at the hippocampal or cortical level. Subsequent work demonstrated an atropine-sensitive and an atropine-resistant component to RSA, with evidence suggesting that the median raphe serotonergic system (Vanderwolf and Baker 1986), as well as GABAergic septohippocampal projections (Lee et al. 1994), may play a role in the latter. In our study toxin-treated animals, in comparison with controls, showed no significant difference in ABS, REL, peak frequency, or coherence in response to behavioral activation. Our cortical recordings support work by others from hippocampal recordings that demonstrates a persistence of RSA in animals treated with the immunotoxin (Lee et al. 1994) or those administered i.c.v. colchicine, which has been reported to be selectively toxic to cholinergic neurons in the medial septum (Gilbert and Peterson 1991). Although it might appear that such findings can be explained solely on the basis of noncholinergic mechanisms, the robust response in RSA seen in our study in response to the acetylcholinesterase inhibitor physostigmine suggests otherwise. Animals administered the toxin showed a peak frequency, ABS, REL, and coherence response to physostigmine equivalent to that of intact animals. This suggests that such activity may be mediated by cholinergic neurons that remained unaffected by the toxin, and that subpopulations of p75-negative cholinergic cells may continue to play a role in the generation of cholinergically sensitive RSA.
A previous study employing intraseptal doses of the immunotoxin demonstrated no change in RSA in the 4-to 12-Hz band in rats following intraperitoneal administration of physostigmine (Lee et al. 1994). Our results suggests that averaging power over the broad 4- to 12-Hz frequency range may mask this pharmacologic response, in so far as in our study physostigmine increased power in the 4- to 8-Hz band but decreased power in the 8- to 12-Hz band.
The location of p75-negative cholinergic cells that participate in the generation of RSA remains unclear. That such cells lie outside of the septohippocampal system is suggested by our findings (Waite et al. 1995), as well as those of others (Rossner et al. 1995a; Zhang et al. 1996) which show that 192 IgG-saporin administered i.c.v. at doses of 3.4–4.0 μg results in a near complete loss of cholinergic function in the medial septum and hippocampus as measured by ChAT assay, ChAT immunoreactivity, acetylcholinesterase, or p75 staining. Neurons with voltage-dependent oscillatory properties at the theta frequency have been described in the entorhinal cortex. It has been proposed that such neurons may function as extraseptal pacemakers of RSA (Mitchell and Ranck 1980; Alonso and Garcia-Austt 1987a, 1987b; Stewart et al. 1992; Dickson et al. 1994).
The entorhinal cortex has many reciprocal connections with subcortical and cortical regions (Alonso and Kohler 1984; Swanson and Kohler 1986). Best known of the entorhinal connections is the perforant pathway, a massive association projection via the medial septum/diagonal band to the molecular layer of the dentate gyrus and Ammon’s horn. The findings of reciprocal connections between the septum and the entorhinal area extend previous investigations showing prominent connections between the septum and the hippocampal formation (Meibach and Siegel 1977; Swanson and Cowan 1977). It has been proposed that septal modulation of hippocampal activity can occur at different levels within the hippocampal region, including the entorhinal area (Alonso and Kohler 1984; Ylinen et al. 1995). Such pathways, if they contain cholinergic cells that remain unaffected by the immunotoxin, may continue to mediate the local effects of acetylcholine on the basal fore-brain/septohippocampal system, acting possibly through GABAergic systems (Lee et al. 1994).
Considering the prominence of cholinergic deafferentation in Alzheimer’s disease (AD), it is not surprising that decreases in coherence are prominent in this disorder (Leuchter et al. 1992, 1994; Besthorn et al. 1994; Dunkin et al. 1994; Jelic et al. 1997). Decreases in coherence in AD have been reported mostly in alpha (Leuchter et al. 1992; Besthorn et al. 1994; Sloan et al. 1994; Jelic et al. 1997) but also in beta bands (Leuchter et al. 1992; Besthorn et al. 1994), similar to those seen following scopolamine administration in normal subjects (Sloan et al. 1992). Decreases have been reported at least in one study to be more significant for intrahemispheric than for interhemispheric coherence (Calderon et al. 1997), with greatest decreases noted in long corticocortical fiber tracts (Leuchter et al. 1992). No study to date has examined whether or not cholinergic agonists in Alzheimer’s disease are able to reverse such losses in coherence. RSA activity seen in rodents is not detectable in human subjects and extrapolation of coherence changes in rat to those of humans requires caution. However, our study suggests that acetylcholinesterase inhibitors may normalize the impairment in brain electrical coherence resulting from cholinergic deafferentation, in particular intrahemispheric coherence.
In summary, administration of a specific cholinergic immunotoxin resulted in significant changes in brain electrocortical power. Despite marked losses in cortical and hippocampal ChAT, such changes were more modest than those reported previously following excitotoxic lesions of the basal forebrain. Cholinergic deafferentation resulted in losses in intrahemispheric coherence in the 4-to 8-Hz band that were restored following administration of physostigmine. Consistent with the largely ipsilateral projections of cholinergic nerve fibers, intrahemispheric compared with interhemispheric coherence changed to a greater extent in response to changes in cholinergic tone. The persistence of a cholinergically modulated RSA, despite dramatic losses of ChAT in the cortex and hippocampus, suggests that such activity may be mediated through cholinergic neurons that, because they lack the p75 receptor, remain unaffected by the immunotoxin.
Acknowledgments
Supported by: a Mentored Clinical Science Development Program Award (5-K12-AG-00521; Dr. Holschneider); the Alzheimer’s Association (FSA 94019; Dr. Waite), a research grant (NS33371) from the NIH (Dr. Waite); the Department of Veterans Affairs Medical Research (Drs. Walton and Scremin); a research grant (MH40705) and Research Scientist Development Award (MH01165) from the NIMH (Dr. Leuchter) and the UCLA Alzheimer’s Disease Center; and a research grant from the NIA (P30 AG10123-05; Dr. Leuchter).
Contributor Information
Daniel P. Holschneider, Departments of Neurology and Psychiatry and the Behavioral Sciences, University of Southern California, School of Medicine, Los Angeles, CA, USA
Jerene J. Waite, Department of Neurosciences, University of California at San Diego, USA
Andrew F. Leuchter, Department of Psychiatry and the Biobehavioral Sciences, University of California at Los Angeles, School of Medicine, USA
Nancy Y. Walton, Department of Neurology, University of California at Los Angeles, School of Medicine, USA. Neurology Research Services, Department of Veterans Affairs Medical Center, West Los Angeles, CA, USA
Oscar U. Scremin, Department of Physiology, University of California at Los Angeles, School of Medicine, USA
References
- Alonso A, Kohler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J Comp Neurol. 1984;225:327–343. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Exp Brain Res. 1987a;67:493–501. doi: 10.1007/BF00247282. [DOI] [PubMed] [Google Scholar]
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Exp Brain Res. 1987b;67:502–509. doi: 10.1007/BF00247283. [DOI] [PubMed] [Google Scholar]
- Barrenechea C, Pedemonte M, Nunez A, Garcia-Austt E. In vivo intracellular recordings of medial septal and diagonal band of Broca neurons: relationships with theta rhythm. Exp Brain Res. 1995;103:31–40. doi: 10.1007/BF00241962. [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random data: analysis and measurement procedures. Wiley; New York: 1986. [Google Scholar]
- Besthorn C, Forstl H, Geiger-Kabisch C, Sattel H, Gasser T, Schreiter-Gasser U. EEG coherence in Alzheimer disease. Electroencephalogr Clin Neurophysiol. 1994;90:242–245. doi: 10.1016/0013-4694(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon PL, Parra M, Llibre JJ, Fernandez AE, Gongora EM. The role of the cerebral coherence in the progress of the patient with Alzheimer’s disease. Rev Neurol. 1997;25:1393–1398. [PubMed] [Google Scholar]
- Chen EY, Mufson EJ, Kordower JH. TRK and p75 neurotrophin receptor systems in the developing human brain. J Comp Neurol. 1996;369:591–618. doi: 10.1002/(SICI)1096-9861(19960610)369:4<591::AID-CNE8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Conti F, Manzoni T. The neurotransmitters and postsynaptic actions of callosally projecting neurons. Behav Brain Res. 1994;64:37–53. doi: 10.1016/0166-4328(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Trepel C, Bland BH. Extrinsic modulation of theta field activity in the entorhinal cortex of the anesthetized rat. Hippocampus. 1994;4:37–51. doi: 10.1002/hipo.450040106. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter AF, Newton TF, Cook IA. Reduced EEG coherence in dementia: state or trait marker? Biol Psychiatry. 1994;35:870–9. doi: 10.1016/0006-3223(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975;24:407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Gage FH, Batchelor P, Chen KS, Chin D, Higgins GA, Koh S, Deputy S, Rosenberg MB, Fischer W, Bjorklund A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron. 1989;2:1177–1184. doi: 10.1016/0896-6273(89)90184-0. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Peterson GM. Colchicine-induced deafferentation of the hippocampus selectively disrupts cholinergic rhythmical slow-wave activity. Brain Res. 1991;564:117–126. doi: 10.1016/0006-8993(91)91360-d. [DOI] [PubMed] [Google Scholar]
- Heckers S, Ohtake T, Wiley RG, Lappi DA, Geula C, Mesulam MM. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J Neurosci. 1994;14:1271–1289. doi: 10.1523/JNEUROSCI.14-03-01271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Leuchter AF, Walton NY, Scremin OU, Treiman DM. Changes in cortical EEG and cholinergic function in response to NGF in rats with nucleus basalis lesions. Brain Res. 1997;765:228–237. doi: 10.1016/s0006-8993(97)00523-4. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Leuchter AF, Scremin OU, Treiman DM, Walton NY. Changes in brain electrical coherence in response to NGF in rats with nucleus basalis lesions. Brain Res Bull. 1998;45:531–541. doi: 10.1016/s0361-9230(97)00446-2. [DOI] [PubMed] [Google Scholar]
- Jelic V, Julin P, Shigeta M, Nordberg A, Lannfelt L, Winblad B, Wahlund LO. Apolipoprotein E epsilon-4 allele decreases functional connectivity in Alzheimer’s disease as measured by EEG coherence. J Neurol Neurosurg Psychiatry. 1997;63:59–65. doi: 10.1136/jnnp.63.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Mufson EJ. Nerve growth factor receptor-immunoreactive neurons within the developing human cortex. J Comp Neurol. 1992;323:25–41. doi: 10.1002/cne.903230104. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Newton TF, Cook IA, Walter DO, Rosenberg-Thompson S, Lachenbruch PA. Changes in brain functional connectivity in Alzheimer-type and multi-infarct dementia. Brain. 1992;115:1543–1561. doi: 10.1093/brain/115.5.1543. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Dunkin JJ, Lufkin RB, Anzai Y, Cook IA, Newton TF. Effect of white matter disease on functional connections in the aging brain. J Neurol Neurosurg Psychiatry. 1994;57:1347–1354. doi: 10.1136/jnnp.57.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Martin LA, Stewart DJ. Hippocampal theta rhythm in behaving rats following ibotenic acid lesion of the septum. Hippocampus. 1994;4:136–147. doi: 10.1002/hipo.450040204. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Boatell ML, Mahy N, Persson H. Widespread neuronal degeneration after ibotenic acid lesioning of cholinergic neurons in the nucleus basalis revealed by in situ hybridization. Neurosci Lett. 1992;135:262–264. doi: 10.1016/0304-3940(92)90451-c. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain Res. 1977;119:1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Ranck JB., Jr Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Res. 1980;189:49–66. doi: 10.1016/0006-8993(80)90006-2. [DOI] [PubMed] [Google Scholar]
- Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993;57:123–131. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Leanza G, Rosenblad C, Lappi DA, Wiley RG, Bjorklund A. Spatial learning impairments in rats with selective immunolesion of the forebrain cholinergic system. Neuroreport. 1992;3:1005–1008. doi: 10.1097/00001756-199211000-00015. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Neocortical dynamics and human EEG rhythms. Oxford University Press; New York: 1995. [Google Scholar]
- O’Connor KP, Shaw JC, Ongley CO. The EEG and differential diagnosis in psychogeriatrics. Br J Psychiatry. 1979;135:156–162. doi: 10.1192/bjp.135.2.156. [DOI] [PubMed] [Google Scholar]
- Ray PG, Jackson WJ. Lesions of nucleus basalis alter ChAT activity and EEG in rat frontal neocortex. Electroencephalogr Clin Neurophysiol. 1991;79:62–68. doi: 10.1016/0013-4694(91)90157-y. [DOI] [PubMed] [Google Scholar]
- Riekkinen PJ, Sirviö J, Riekkinen P. Relationship between the cortical choline acetyltransferase content and EEG delta-power. Neurosci Res. 1990;8:12–20. doi: 10.1016/0168-0102(90)90052-g. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Jr, Jakala P, Sirvio J, Koivisto E, Miettinen R, Riekkinen P. The effects of THA on scopolamine and nucleus basalis lesion-induced EEG slowing. Brain Res Bull. 1991;26:633–637. doi: 10.1016/0361-9230(91)90107-u. [DOI] [PubMed] [Google Scholar]
- Rossner S, Hartig W, Schliebs R, Bruckner G, Brauer K, Perez-Polo JR, Wiley RG, Bigl V. 192 IgG-saporin immunotoxin-induced loss of cholinergic cells differentially activates microglia in rat basal forebrain nuclei. J Neurosci Res. 1995a;41:335–346. doi: 10.1002/jnr.490410306. [DOI] [PubMed] [Google Scholar]
- Rossner S, Schliebs R, Perez-Polo JR, Wiley RG, Bigl V. Differential changes in cholinergic markers from selected brain regions after specific immunolesion of the rat cholinergic basal forebrain system. J Neurosci Res. 1995b;40:31–43. doi: 10.1002/jnr.490400105. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Rossner S, Bigl V. Immunolesion by 192 IgG-saporin of rat basal forebrain cholinergic system: a useful tool to produce cortical cholinergic dysfunction. Prog Brain Res. 1996;109:253–264. doi: 10.1016/s0079-6123(08)62109-3. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB. Nerve growth factor receptor-mediated transport from cerebrospinal fluid to basal forebrain neurons. Brain Res. 1987;423:309–317. doi: 10.1016/0006-8993(87)90854-7. [DOI] [PubMed] [Google Scholar]
- Scremin OU, Torres C, Scremin AM, O’Neal M, Heuser D, Blisard KS. Role of nucleus basalis in cholinergic control of cortical blood flow. J Neurosci Res. 1991;28:382–390. doi: 10.1002/jnr.490280310. [DOI] [PubMed] [Google Scholar]
- Shaw JC. An introduction to the coherence function and its use in EEG signal analysis. J Med Eng Technol. 1981;5:279–288. doi: 10.3109/03091908109009362. [DOI] [PubMed] [Google Scholar]
- Shaw J. Correlation and coherence analysis of the EEG: a selective tutorial review. Int J Psychophysiol. 1984;1:255–266. doi: 10.1016/0167-8760(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Sloan EP, Fenton GW, Standage KP. Anticholinergic drug effects on quantitative electroencephalogram, visual evoked potential, and verbal memory. Biol Psychiatry. 1992;31:600–606. doi: 10.1016/0006-3223(92)90246-v. [DOI] [PubMed] [Google Scholar]
- Sloan EP, Fenton GW, Kennedy NS, MacLennan JM. Neurophysiology and SPECT cerebral blood flow patterns in dementia. Electroencephalogr Clin Neurophysiol. 1994;91:163–170. doi: 10.1016/0013-4694(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochemistry. 1987;163:279. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- SPSS. Release 4.0. SPSS; Chicago, Ill: 1990. [Google Scholar]
- Stewart DJ, MacFabe DF, Vanderwolf CH. Cholinergic activation of the electrocorticogram: role of the substantia innominata and effects of atropine and quinuclidinyl benzilate. Brain Res. 1984;322:219–32. doi: 10.1016/0006-8993(84)90112-4. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Two populations of rhythmically bursting neurons in rat medial septum are revealed by atropine. J Neurophysiol. 1989;61:982–993. doi: 10.1152/jn.1989.61.5.982. [DOI] [PubMed] [Google Scholar]
- Stewart M, Quirk GJ, Barry M, Fox SE. Firing relations of medial entorhinal neurons to the hippocampal theta rhythm in urethane anesthetized and walking rats. Exp Brain Res. 1992;90:21–28. doi: 10.1007/BF00229252. [DOI] [PubMed] [Google Scholar]
- Stumpf C. Drug action on the electrical activity of the hippocampus. Int Rev Neurobiol. 1965;8:77–138. doi: 10.1016/s0074-7742(08)60756-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 1986;6:3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. The electrocorticogram in relation to physiology and behavior: a new analysis. Electroencephalogr Clin Neurophysiol. 1992;82:165–175. doi: 10.1016/0013-4694(92)90164-d. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Baker GB. Evidence that serotonin mediates non-cholinergic neocortical low voltage fast activity, non-cholinergic hippocampal rhythmical slow activity and contributes to intelligent behavior. Brain Res. 1986;374:342–356. doi: 10.1016/0006-8993(86)90428-2. [DOI] [PubMed] [Google Scholar]
- Waite JJ, Thal LJ. Lesions of the cholinergic nuclei in the rat basal forebrain: excitotoxins vs an immunotoxin. Life Sci. 1996;58:1947–1953. doi: 10.1016/0024-3205(96)00184-1. [DOI] [PubMed] [Google Scholar]
- Waite JJ, Wardlow ML, Chen AC, Lappi DA, Wiley RG, Thal LJ. Time course of cholinergic and monoaminergic changes in rat brain after immunolesioning with 192 IgG-saporin. Neurosci Lett. 1994;169:154–158. doi: 10.1016/0304-3940(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Waite JJ, Chen AD, Wardlow ML, Wiley RG, Lappi DA, Thal LJ. 192 immunoglobulin G-saporin produces graded behavioral and biochemical changes accompanying the loss of cholinergic neurons of the basal forebrain and cerebellar Purkinje cells. Neuroscience. 1995;65:463–476. doi: 10.1016/0306-4522(94)00479-o. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal fore-brain of rats. J Neurosci. 1994;14:5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk H, Bigl V, Meyer U, Biesold D. Cholinergic pathways to the cerebral cortex in rats. In: Pepeu G, Ladinsky H, editors. Cholinergic mechanisms: phylogenetic aspects, central and peripheral synapses, and clinical significance. Plenum Press; New York: 1991. pp. 673–683. [Google Scholar]
- Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8:3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Johnson EM., Jr Immunohistochemical localization and biochemical characterization of nerve growth factor receptor in adult rat brain. J Comp Neurol. 1989;290:585–598. doi: 10.1002/cne.902900411. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Berbos TG, Wrenn CC, Wiley RG. Loss of nucleus basalis magnocellularis, but not septal, cholinergic neurons correlates with passive avoidance impairment in rats treated with 192-saporin. Neurosci Lett. 1996;203:214–218. doi: 10.1016/0304-3940(95)12282-6. [DOI] [PubMed] [Google Scholar]