Abstract
A one-step transformation of heterocyclic N-oxides to 2-alkyl, aryl, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method for the late-stage modification of complex N-heterocycles is exemplified by facile syntheses of new structural analogs of several antimalarial, antimicrobial and fungicidal agents.
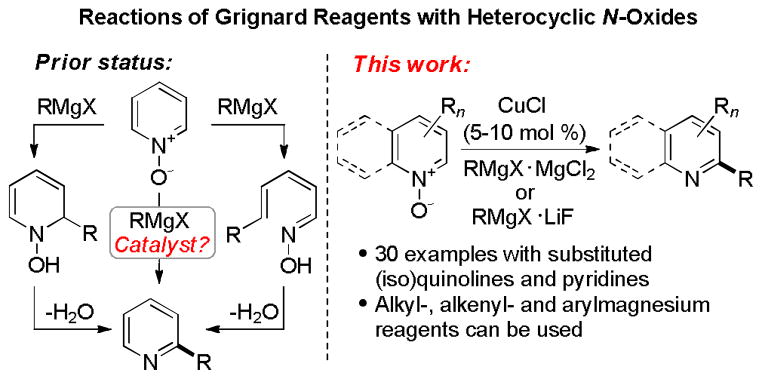
Substituted N-heterocycles are important structural motifs of bio-active compounds and advanced materials.1 Hence, methods that allow for regioselective construction of C–C-bonds to N-heterocycles have attracted continuous attention.2 In particular, introduction of substituents in the C2-position of pyridines, quinolines and related six-membered nitrogenous heterocycles is an important strategy in heterocyclic synthesis that represents a significant synthetic challenge.3 One of the most commonly used strategies is based on transition metal-catalyzed coupling reactions of organometallic reagents with 2-haloazines,4 which are prepared from the corresponding N-oxides5 (Scheme 1). However, despite its widespread use, this route has significant drawbacks. The synthesis of 2-haloazines from the N-oxides is low-yielding due to poor regioselectivity. In addition, although significant progress has been achieved in the development of catalytic coupling reactions of 2-haloazines, most of the methods focus on construction of aryl-heteroaryl bonds. A more recent strategy that has been developed by Fagnou and other groups entails Pd-catalyzed CH-functionalization of the heterocyclic N-oxides.6 However, a reduction of the N–O-bond is required to produce the corresponding 2-substituted N-heterocycles, and excess N-oxide is needed to achieve good yields. It would be, therefore, advantageous to devise a direct one-step method for regioselective conversion of heterocyclic N-oxides to 2-substituted N-heterocycles. We herein report a coupling reaction of heterocyclic N-oxides with Grignard reagents that directly affords 2-aryl, alkyl and alkenyl-substituted N-heterocycles regioselectively and in good to excellent yield.
Scheme 1.
Methods of synthesis of 2-substituted N-heterocycles from N-oxides.
Grignard reagents have previously been known to react with heterocyclic N-oxides to give predominantly, depending on reaction conditions and the structure of the heterocyclic core, variable ratios of addition and ring-opening products (Scheme 1). Based on the early work by Kellog, Shiess7 and Kato,8 Almqvist and Olsson,9 as well as Duan10 have significantly improved the versatility of the method, and showed that both products can subsequently be converted to 2-alkyl and aryl-substituted N-heterocycles on treatment with dehydrating reagents in a stepwise fashion.
We began our study by examining the reaction between quinoline N-oxide and isopropylmagnesium chloride (Table 1) in diethyl ether. While formation of product 3 was not observed upon reacting 1 and 2 in the absence of a catalyst, a yield of 53% was achieved in the presence of 10 mol% of copper(I) chloride. Iron(II) chloride was less effective, leading to complex reaction mixtures and a 36% yield of 3. Attempts to augment the activity of the copper and iron catalysts by using phosphine, NHC and amine ligands were not successful. Similarly, cobalt and nickel exhibited no appreciable catalytic activity (entries 5–7). Further experiments showed that copper(I) chloride was superior to other copper(I) and copper(II) salts (e.g. entries 8 and 9).
Table 1.
Optimization of the Reaction Conditions.a
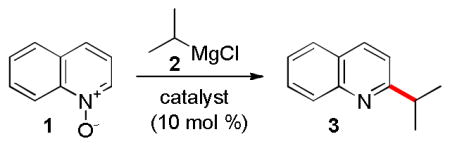
| |||||
|---|---|---|---|---|---|
| entry | catalyst | additiveb | solvent | temp (°C) | yield % |
| 1 | – | – | Et2O | 23 | 0 |
| 2 | CuCl | – | Et2O | 23 | 53 |
| 3 | FeCl2 | – | Et2O | 23 | 36 |
| 4c | FeCl2/SIMes | – | THF | 23 | 0 |
| 5 | CoCl2 | – | Et2O | 23 | 6 |
| 6 | Ni(PPh3)2Cl2 | – | THF | 23 | 3 |
| 7 | NiCl2·dme | – | Et2O | 23 | 0 |
| 8 | CuCl2 | – | Et2O | 23 | 17 |
| 9 | CuBr | – | Et2O | 23 | 21 |
| 10 | CuCl | – | THF | 23 | 45 |
| 11 | CuCl | – | PhMe | 23 | 38 |
| 12 | CuCl | – | Et2O | 0 | 44 |
| 13 | CuCl | – | Et2O | −20 | 25 |
| 14 | CuCl | – | Et2O | 50 | 42 |
| 15 | CuCl | LiCl | Et2O | 23 | 67 |
| 16 | CuCl | ZnBr2 | Et2O | 23 | 0 |
| 17 | CuCl | tmeda | Et2O | 23 | 2 |
| 18 | CuCl | MgCl2 | Et2O | 23 | 95 |
| 19 | CuCl | MgF2 | Et2O | 23 | 37 |
| 20 | CuCl | LiF | Et2O | 23 | 96 |
Reaction conditions: 1 (0.17 mmol), 2 (1.5 equiv), catalyst (10 mol %), in the solvent (1 mL) under Ar for 12 h.
1.5 equiv
SIMes = 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene.
Solvents were found to have a notable effect on the reaction efficiency. Ethereal solvents (diethyl ether and methyl tert-butyl ether) proved to be the optimal reaction media, while formation of substantial amounts of side-products was observed in tetrahydro-furan (THF), toluene (entries 10 and 11) and other non-ethereal solvents. Ambient temperature (23 °C) was found to be optimal, while diminished yields of coupling product 3 were observed at lower (−20 and 0 °C) and higher (50 °C, in a sealed vessel) temperatures (entries 13–14).
In an effort to improve the yield, we then turned our attention to additives, since it is known that the reactivity of organomagnesium11 and organocopper12 species can be modulated by Lewis acids and bases.
Encouragingly, addition of LiCl improved the yield to 67%. At the same time, zinc bromide and N,N,N′,N′-tetramethyl-ethylenediamine (tmeda) completely suppressed the coupling (entries 16 and 17). Surprisingly, when the reaction was run in the presence of magnesium chloride, a clean conversion of N-oxide 1 into product 3 took place (entry 18, 95% yield). It is interesting that other magnesium salts (e.g. fluoride, entry 19) were much less effective. On the other hand, lithium fluoride exhibited comparable efficiency to magnesium chloride (entry 20).
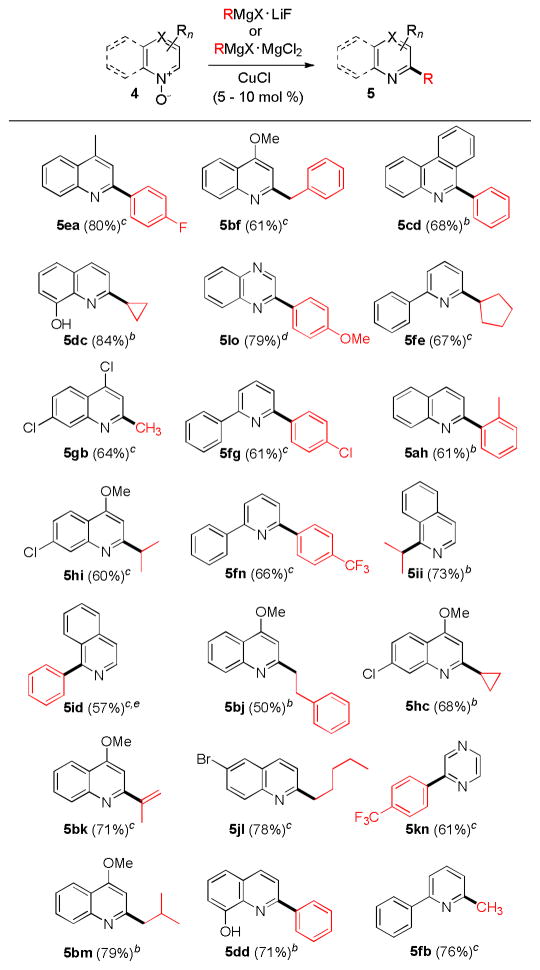
After completion of the optimization study we set out to explore the scope of the novel method with other Grignard reagents (16 reagents) and heterocyclic N-oxides (12 substrates) (Figure 1).
Figure 1. Scope of the Azine N-Oxides and Grignard Reagents.a.
a Reaction conditions: 4 (0.17–1.3 mmol), RMgX (1.5–4 equiv), MgCl2 or LiF (1.5–4 equiv), catalyst (5–10 mol %), Et2O (c=0.2 M), at 23 °C under Ar for 12 h. b RMgCl was used as a nucleophile. c The reaction was run with RMgBr. d RMgI was used. e The reaction was run with LiF as an additive.
The reaction generally shows a broad scope for both counterparts. Thus, both primary and secondary alkylmagnesium halides proved to be suitable nucleophiles for the reaction with substituted quinoline, pyridine and isoquinoline N-oxides giving rise to the corresponding products in a highly regioselective fashion.
Similarly, arylmagnesium halides afforded the desired products in good yields, e.g. o-tolyl group can be installed in 61% yield. 2-Propenylmagnesium bromide also proved to be a suitable nucleophile. With respect to the N-oxide counterparts, quinoline, isoquinoline, pyridine, phenanthridine, pyrazine and quinoxaline N-oxides are viable substrates.
Halogenated substrates undergo chemoselective reaction at C2-position with Grignard reagents under these conditions, including 4-chloroquinoline that is known to be prone to facile nucleophilic displacement of the 4-chloro group.13 Hydroxy group is also well tolerated, provided that an additional equivalent of a Grignard reagent is added. The reaction has been successfully carried out on preparative scale (e.g. 3 g 2-phenylquinoline from 1 and phenylmagnesium chloride) with a 94% yield.
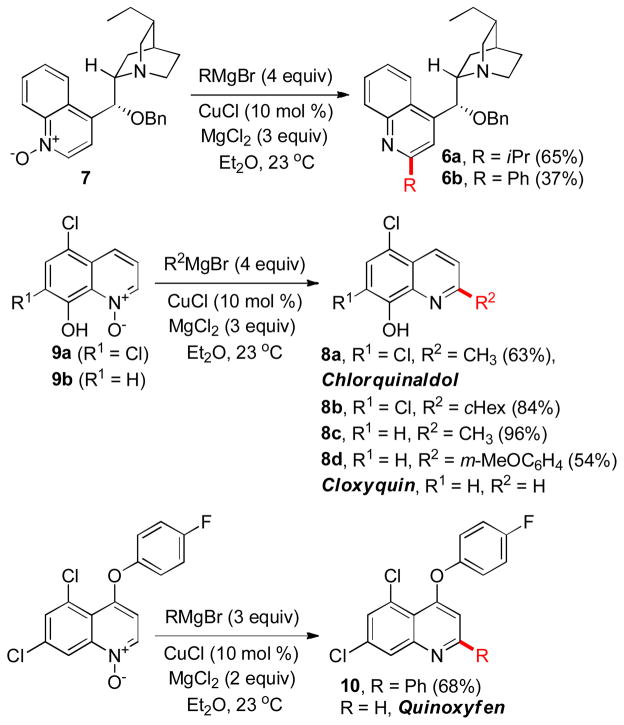
The new method has been used for the synthesis of several derivatives of biologically relevant heterocycles. 2′-Isopropyl and 2′-phenyl substituted dihydrocinchonidine derivatives 6a,b have been prepared from N-oxide 7 in 65 and 37% yields (Scheme 2).
Scheme 2.
Synthesis of 2-substituted derivatives of cinchonidine, chlorquinaldol, cloxyquin and quinoxyfen.
Similarly, antimicrobial drug chlorquinaldol 8a (63%) and the 2-cyclohexyl analog 8b (84%) have been accessed from N-oxide 9a, as well as 2-methyl and 2-(m-methoxyphenyl) derivatives 8c,d of an antimicrobial drug cloxyquin from N-oxide 9b. In addition, 2-phenyl derivative of selective agrochemical fungicide quinoxyfen (Quintec™) 10 has been prepared in 68% yield.
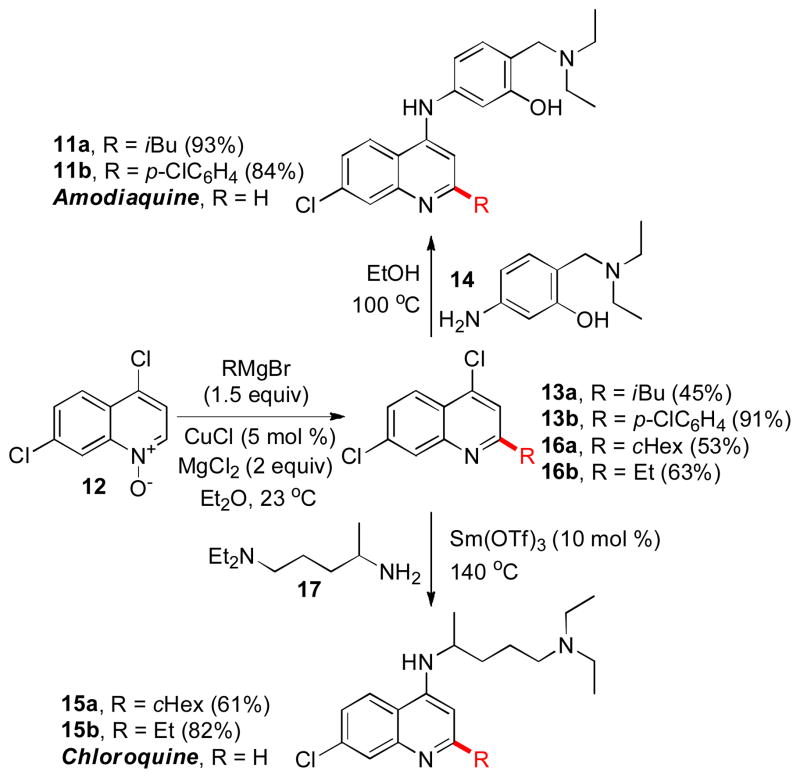
The chemoselective preference for the C2-addition in 4-chloroquinoline N-oxides has been exploited for a regioselective synthesis of new structural analogs of antimalarial drugs amodiaquine and chloroquine (Scheme 3). Both 4-aminoquinolines have long been considered as the most important drugs for the treatment and prophylaxis of malaria.14 However, the emergence of widespread parasite resistance to these drugs in every region, where P. falciparum is prevalent, has spurred interest in new 4-aminoquinolines with improved pharmacological profiles.15 In addition, chloroquine has recently demonstrated promising anti-tumor,16 antirheumatic17 and antiviral activities.18
Scheme 3.
Preparation of 2-substituted derivatives of amodiaquine and chloroquine.
The amodiaquine derivatives 11a,b were prepared in 42 and 77% combined yields from N-oxide 12 in two steps. The copper-catalyzed reaction with a Grignard reagent and magnesium chloride was followed by a condensation of quinolines 13 with aniline 14. Similarly, chloroquine derivatives 15a,b that bear 2-cyclohexyl and 2-ethyl groups, have been synthesized in two steps by way of converting N-oxide 12 into quinolines 16, followed by a samarium triflate-catalyzed displacement reaction with amine 17.
In conclusion, we have developed a direct catalytic synthesis of 2-substituted nitrogenous heterocycles from Grignard reagents and N-oxides. Magnesium chloride and lithium fluoride have been identified as additives that drastically improve the chemoselectivity of the reaction. The reported transformation of heterocyclic N-oxides to 2-substituted heterocycles can be used to access heterocyclic scaffolds of practical synthetic and medicinal value as exemplified by facile late-stage modification of several antimalarial, antimicrobial and fungicidal agents.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (AX-1788), the Max and Minnie Tomerlin Voelcker Fund and the University of Texas at San Antonio for financial support. Mass spectroscopic analysis was supported by a grant from the National Institute on Minority Health and Health Disparities (G12MD007591).
Footnotes
Experimental procedures, as well as spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; (b) Vieth M, Siegel MG, Higgs RE, Watson IA, Robertson DH, Savin KA, Durst GL, Hipskind PA. J Med Chem. 2004;47:224–232. doi: 10.1021/jm030267j. [DOI] [PubMed] [Google Scholar]; (c) Welsch ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Yokoyama A, Nishiyama I, Yoshizawa A. Ferroelectrics. 1993;148:139. [Google Scholar]; (b) Skrypink YG, Doroshenko TF. Mater Sci. 1996;32:537. [Google Scholar]; (c) Tsutsumi H, Okada K, Oishi T. Electrochim Acta. 1996;41:2657. [Google Scholar]; (d) Bangcuyo CG, Rampey-Vaughn ME, Quan LT, Angel SM, Smith MD, Bunz UHF. Macromolecules. 2002;35:1563. [Google Scholar]; (e) Vetrichelvan M, Valiyaveettil S. Chem Eur J. 2005;11:5889. doi: 10.1002/chem.200500078. [DOI] [PubMed] [Google Scholar]; (e) Ji X, Huang H, Li Y, Chen H, Jiang H. Angew Chem Int Ed. 2012;51:7292–7296. doi: 10.1002/anie.201202412. [DOI] [PubMed] [Google Scholar]; (f) Hyodo I, Tobisu M, Chatani N. Chem Asian J. 2012;7:1357–1365. doi: 10.1002/asia.201100971. [DOI] [PubMed] [Google Scholar]; (g) Zhuo FF, Xie WW, Yang YX, Zhang L, Wang P, Yuan R, Da CS. J Org Chem. 2013;78:3243–3249. doi: 10.1021/jo400152f. [DOI] [PubMed] [Google Scholar]; (h) Nakao Y, Kanyiva KS, Hiyama T. J Am Chem Soc. 2008;130:2448. doi: 10.1021/ja710766j. [DOI] [PubMed] [Google Scholar]
- 3.(a) Fujiwara Y, Dixon JA, O’Hara F, Funder ED, Dixon DD, Rodriguez RA, Baxter RD, Herle B, Sach N, Collins MR, Ishihara Y, Baran PS. Nature. 2012;492:95–99. doi: 10.1038/nature11680. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu B, Huang Y, Lan J, Songa F, You J. Chem Sci. 2013;4:2163–2167. [Google Scholar]; (c) Yao B, Song RJ, Liu Y, Xie YX, Li JH, Wang MK, Tang RY, Zhang XJ, Deng CL. Adv Synth Catal. 2012;354:1890–1896. [Google Scholar]; (d) Ren X, Wen P, Shi X, Wang Y, Li J, Yang S, Yan H, Huang G. Org Lett. 2013;15:5194–5197. doi: 10.1021/ol402262c. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bonnet V, Mongin F, Trecourt F, Queguiner G, Knochel P. Tetrahedron. 2002;58:4429–4438. [Google Scholar]; (b) Lutzen A, Hapke M. Eur J Org Chem. 2002;14:2292–2297. [Google Scholar]; (c) Slagt VF, de Vries AHM, de Vries JG, Kellogg RM. Org Proc Res Dev. 2010;14:30–47. [Google Scholar]; (d) Iglesias MJ, Prieto A, Nicasio MC. Org Lett. 2012;14:4318–4321. doi: 10.1021/ol302112q. [DOI] [PubMed] [Google Scholar]; (e) Kuzmina OM, Steib AK, Flubacher D, Knochel P. Org Lett. 2012;14:4818–4821. doi: 10.1021/ol302136c. [DOI] [PubMed] [Google Scholar]; (f) Kuzmina OM, Steib AK, Markiewicz JT, Flubacher D, Knochel P. Angew Chem Int Ed. 2013;52:4945–4949. doi: 10.1002/anie.201210235. [DOI] [PubMed] [Google Scholar]; (g) Steib AK, Kuzmina OM, Fernandez S, Flubacher D, Knochel P. J Am Chem Soc. 2013;135:15346–15349. doi: 10.1021/ja409076z. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ash ML, Pew RG. J Heterocyclic Chem. 1981;18:939–940. [Google Scholar]; (b) Mittelbach M. Synthesis. 1988:479–480. [Google Scholar]; (c) Singh B, Lesher GY, Pennock PO. J Heterocyclic Chem. 1990;27:1841–1842. [Google Scholar]; (d) Hurst DT. Adv Heterocyclic Chem. 1993;58:227–269. [Google Scholar]; (e) Constable EC, Seddon KR. Tetrahedron. 1983;39:291–295. [Google Scholar]; (f) Koenig T, Wieczorek JS. J Org Chem. 1968;33:1530–1532. [Google Scholar]; (g) Vozza J. J Org Chem. 1962;27:3856–3860. [Google Scholar]; (h) Yamanaka H, Arak T, Sakamoto T. Chem Pharm Bull. 1988;36:2244–2247. [Google Scholar]; (i) Wengryniuk SE, Weickgenannt A, Reiher C, Strotman NA, Chen K, Eastgate MD, Baran PS. Org Lett. 2013;15:792–795. doi: 10.1021/ol3034675. [DOI] [PubMed] [Google Scholar]
- 6.(a) Campeau LC, Rousseaux S, Fagnou K. J Am Chem Soc. 2005;127:18020. doi: 10.1021/ja056800x. [DOI] [PubMed] [Google Scholar]; (b) Leclerc JP, Fagnou K. Angew Chem, Int Ed. 2006;45:7781–7786. doi: 10.1002/anie.200602773. [DOI] [PubMed] [Google Scholar]; (c) Kanyiva KS, Nakao Y, Hiyama T. Angew Chem, Int Ed. 2007;46:8872. doi: 10.1002/anie.200703758. [DOI] [PubMed] [Google Scholar]; (d) Cho SH, Hwang SJ, Chang S. J Am Chem Soc. 2008;130:9254–9256. doi: 10.1021/ja8026295. [DOI] [PubMed] [Google Scholar]; (e) Wu J, Cui X, Chen L, Jiang G, Wu Y. J Am Chem Soc. 2009;131:13888–13889. doi: 10.1021/ja902762a. [DOI] [PubMed] [Google Scholar]; (f) Xiao B, Liu ZJ, Liu L, Fu Y. J Am Chem Soc. 2013;135:616–619. doi: 10.1021/ja3113752. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kellogg RM, Van Bergen TJ. J Org Chem. 1971;36:1705–1708. [Google Scholar]; (b) Schiess P, Ringele P. Tetrahedron Lett. 1972;311–312 [Google Scholar]
- 8.Kato T, Yamanaka H. J Org Chem. 1965;30:910–913. doi: 10.1021/jo01014a061. [DOI] [PubMed] [Google Scholar]
- 9.(a) Hussain M, Banchelin TSL, Andersson H, Olsson R, Almqvist F. Org Lett. 2013;15:54–57. doi: 10.1021/ol303085q. [DOI] [PubMed] [Google Scholar]; (b) Andersson H, Olsson R, Almqvist F. Org Biomol Chem. 2011;9:337–346. doi: 10.1039/c0ob00336k. [DOI] [PubMed] [Google Scholar]; (c) Andersson H, Banchelin TSL, Das S, Gustafsson M, Olsson R, Almqvist F. Org Lett. 2010;12:284–286. doi: 10.1021/ol902619h. [DOI] [PubMed] [Google Scholar]; (d) Andersson H, Banchelin TSL, Das S, Olsson R, Almqvist F. Chem Commun. 2010;46:3384–3386. doi: 10.1039/c000748j. [DOI] [PubMed] [Google Scholar]; (e) Andersson H, Gustafsson M, Bostrom D, Olsson R, Almqvist F. Angew Chem Intl Ed. 2009;48:3288–3291. doi: 10.1002/anie.200900189. [DOI] [PubMed] [Google Scholar]; (f) Andersson H, Wang X, Bjorklund M, Olsson R, Almqvist F. Tetrahedron Lett. 2007;48:6941–6944. [Google Scholar]; (g) Andersson H, Almqvist F, Olsson R. Org Lett. 2007;9:1335–1337. doi: 10.1021/ol070184n. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zhang S, Liao LY, Zhang F, Duan XF. J Org Chem. 2013;78:2720–2725. doi: 10.1021/jo302511s. [DOI] [PubMed] [Google Scholar]; (b) Zhang F, Zhang S, Duan XF. Org Lett. 2012;14:5618–5620. doi: 10.1021/ol3026632. [DOI] [PubMed] [Google Scholar]; (c) Zhang F, Duan XF. Org Lett. 2011;13:6102–6105. doi: 10.1021/ol202597b. [DOI] [PubMed] [Google Scholar]
- 11.(a) Farkas JJ, Stoudt SJ, Hanawalt EM, Pajerski AD, Richey HGJ. Organometallics. 2004;23:423–427. [Google Scholar]; (b) Hanawalt EM, Farkas JJ, Richey HGJ. Organometallics. 2004;23:416–422. [Google Scholar]; (c) Westerhausen M. Dalton Trans. 2006;40:4755–4768. doi: 10.1039/b609040k. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kleijn H, Elsevier CJ, Westmijze H, Meijer J, Vermeer P. Tetrahedron Lett. 1979:3101–3102. [Google Scholar]; (b) Marfat A, McGuirk PR, Helquist P. J Org Chem. 1979;44:3888–3901. [Google Scholar]; (c) Davies RP. Coord Chem Rev. 2011;255:11–12. [Google Scholar]
- 13.(a) Pino P, Piccolo O, Straub B, Consiglio G, Dich CT. Helv Chim Acta. 1982;65:2102–2109. [Google Scholar]; (b) Wendeborn S, Winkler T, Foisy I. Tetrahedron Lett. 2000;41:6387–6391. [Google Scholar]; (c) Matsumoto K, Kannami M, Inokuchi D, Kurata H, Kawase T, Oda M. Org Lett. 2007;9:2903–2906. doi: 10.1021/ol071189n. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill PM, Barton VE, Ward SA, Chadwick J. In: Treatment and Prevention of Malaria. Staines HM, Krishna S, editors. Springer; Basel: 2012. pp. 19–44. [Google Scholar]
- 15.(a) Winstanley PA, Ward SA, Snow RW. Microb Infect. 2002;4:157–164. doi: 10.1016/s1286-4579(01)01523-4. [DOI] [PubMed] [Google Scholar]; (b) Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 16.Solomon VR, Lee H. Eur J Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Augustijns P, Geusens P, Verbeke N. Eur J Clin Pharmacol. 1992;42:429–433. doi: 10.1007/BF00280130. [DOI] [PubMed] [Google Scholar]
- 18.Savarino A, Di TL, Donatelli I, Cauda R, Cassone A. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.